 Found 98207 hits with Last Name = 'ma' and Initial = 'g'
Found 98207 hits with Last Name = 'ma' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045797
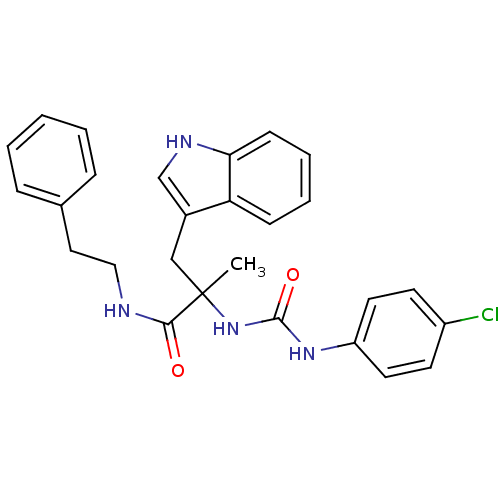
(2-[3-(4-Chloro-phenyl)-ureido]-3-(1H-indol-3-yl)-2...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(Cl)cc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C27H27ClN4O2/c1-27(17-20-18-30-24-10-6-5-9-23(20)24,25(33)29-16-15-19-7-3-2-4-8-19)32-26(34)31-22-13-11-21(28)12-14-22/h2-14,18,30H,15-17H2,1H3,(H,29,33)(H2,31,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50099843
((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...)Show SMILES CC(C)[C@H](N1CCC(O)NC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H48N4O6/c1-24(2)34(41-19-18-32(43)40-37(41)46)36(45)38-29(20-27-14-7-5-8-15-27)22-31(42)30(21-28-16-9-6-10-17-28)39-33(44)23-47-35-25(3)12-11-13-26(35)4/h5-17,24,29-32,34,42-43H,18-23H2,1-4H3,(H,38,45)(H,39,44)(H,40,46)/t29-,30-,31-,32?,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50099842

((S)-N-[(S)-4-[2-(2,6-Dimethyl-phenoxy)-acetylamino...)Show SMILES CC(C)[C@H](N1CCC(=O)NC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 Show InChI InChI=1S/C37H46N4O6/c1-24(2)34(41-19-18-32(43)40-37(41)46)36(45)38-29(20-27-14-7-5-8-15-27)22-31(42)30(21-28-16-9-6-10-17-28)39-33(44)23-47-35-25(3)12-11-13-26(35)4/h5-17,24,29-31,34,42H,18-23H2,1-4H3,(H,38,45)(H,39,44)(H,40,43,46)/t29-,30-,31-,34-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease |
Bioorg Med Chem Lett 11: 1351-3 (2001)
BindingDB Entry DOI: 10.7270/Q2HX1BX6 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl-carrier protein reductase
(Plasmodium falciparum) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Immunology
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ENR in presence of EGCG by dilution assay |
J Med Chem 50: 765-75 (2007)
Article DOI: 10.1021/jm061154d
BindingDB Entry DOI: 10.7270/Q2QJ7J4Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine deaminase
(Bos taurus (bovine)) | BDBM22925

((8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxol...)Show SMILES OC[C@H]1O[C@H](C[C@@H]1O)n1cnc2[C@H](O)CNC=Nc12 |c:17| Show InChI InChI=1S/C11H16N4O4/c16-3-8-6(17)1-9(19-8)15-5-14-10-7(18)2-12-4-13-11(10)15/h4-9,16-18H,1-3H2,(H,12,13)/t6-,7+,8+,9+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PubMed
| 0.00250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rhode Island
Curated by ChEMBL
| Assay Description
Binding affinity (Ki) at calf intestinal adenosine deaminase. |
J Med Chem 35: 4180-4 (1992)
Checked by Author
BindingDB Entry DOI: 10.7270/Q2SQ9118 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM578

((2S)-N-[(2S,4S,5S)-5-[2-(2,6-dimethylphenoxy)aceta...)Show SMILES CC(C)[C@H](N1CCCNC1=O)C(=O)N[C@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cccc1C)Cc1ccccc1 |r| Show InChI InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
| Assay Description
Inhibition assay using HIV protease and Sulfonamide compounds. |
Chem Biol Drug Des 69: 298-313 (2007)
Article DOI: 10.1111/j.1747-0285.2007.00514.x
BindingDB Entry DOI: 10.7270/Q2TQ6011 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537072

(CHEMBL440072)Show SMILES C[C@@H](O)[C@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N Show InChI InChI=1S/C63H88N16O16S2/c1-34(66)53(84)69-30-51(83)70-48-32-96-97-33-49(63(94)95)78-60(91)47(31-80)77-62(93)52(35(2)81)79-55(86)42(22-12-14-24-65)71-58(89)45(27-38-29-68-40-20-10-9-19-39(38)40)75-57(88)44(26-37-17-7-4-8-18-37)73-56(87)43(25-36-15-5-3-6-16-36)74-59(90)46(28-50(67)82)76-54(85)41(72-61(48)92)21-11-13-23-64/h3-10,15-20,29,34-35,41-49,52,68,80-81H,11-14,21-28,30-33,64-66H2,1-2H3,(H2,67,82)(H,69,84)(H,70,83)(H,71,89)(H,72,92)(H,73,87)(H,74,90)(H,75,88)(H,76,85)(H,77,93)(H,78,91)(H,79,86)(H,94,95)/t34-,35+,41+,42-,43+,44-,45+,46-,47-,48+,49-,52+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50466554
(CHEMBL4286615)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCC(N)=O)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C187H291N53O56S2/c1-21-93(10)147(176(288)229-126(85-139(193)251)166(278)218-117(65-75-298-20)161(273)223-122(78-91(6)7)169(281)234-148(99(16)241)177(289)221-118(33-25-69-205-187(200)201)179(291)236(18)105(46-59-135(189)247)83-140(252)211-110(30-22-66-202-184(194)195)155(267)231-130(183(295)296)82-104-44-53-109(246)54-45-104)233-170(282)124(80-102-40-49-107(244)50-41-102)226-157(269)112(32-24-68-204-186(198)199)214-156(268)111(31-23-67-203-185(196)197)215-164(276)120(76-89(2)3)224-168(280)128(87-145(260)261)222-152(264)96(13)207-150(262)95(12)209-162(274)123(79-101-38-47-106(243)48-39-101)225-160(272)113(55-60-136(190)248)213-151(263)97(14)208-154(266)116(64-74-297-19)217-158(270)114(56-61-137(191)249)216-159(271)115(57-62-142(254)255)219-172(284)133-36-29-73-240(133)182(294)149(100(17)242)235-153(265)98(15)210-163(275)125(84-138(192)250)227-167(279)127(86-144(258)259)212-141(253)88-206-171(283)131-34-26-71-238(131)181(293)129(81-103-42-51-108(245)52-43-103)230-175(287)146(92(8)9)232-174(286)134-37-28-72-239(134)180(292)119(58-63-143(256)257)220-165(277)121(77-90(4)5)228-173(285)132-35-27-70-237(132)178(290)94(11)188/h38-45,47-54,89-100,105,110-134,146-149,241-246H,21-37,46,55-88,188H2,1-20H3,(H2,189,247)(H2,190,248)(H2,191,249)(H2,192,250)(H2,193,251)(H,206,283)(H,207,262)(H,208,266)(H,209,274)(H,210,275)(H,211,252)(H,212,253)(H,213,263)(H,214,268)(H,215,276)(H,216,271)(H,217,270)(H,218,278)(H,219,284)(H,220,277)(H,221,289)(H,222,264)(H,223,273)(H,224,280)(H,225,272)(H,226,269)(H,227,279)(H,228,285)(H,229,288)(H,230,287)(H,231,267)(H,232,286)(H,233,282)(H,234,281)(H,235,265)(H,254,255)(H,256,257)(H,258,259)(H,260,261)(H,295,296)(H4,194,195,202)(H4,196,197,203)(H4,198,199,204)(H4,200,201,205)/t93-,94-,95-,96-,97-,98-,99+,100+,105-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,146-,147-,148-,149-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay |
J Med Chem 61: 10519-10530 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01046
BindingDB Entry DOI: 10.7270/Q2ZC85JC |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049750

((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...)Show InChI InChI=1S/C9H12N2O/c1-2-9(6-10-4-1)12-7-8-3-5-11-8/h1-2,4,6,8,11H,3,5,7H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50066789
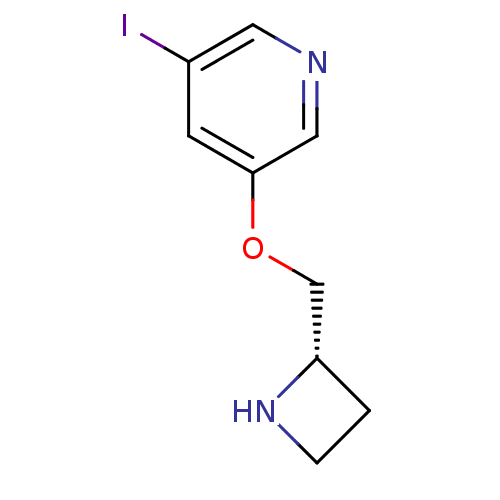
(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110121
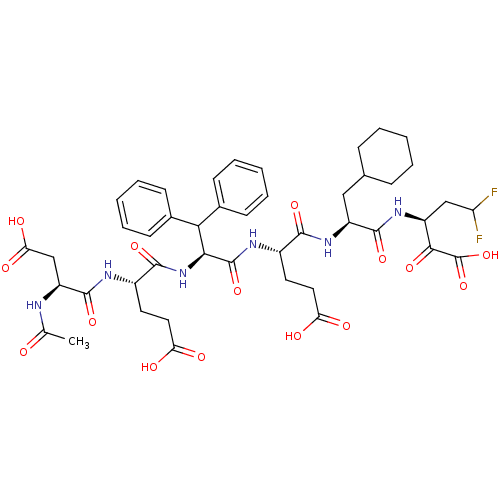
(3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(O)=O Show InChI InChI=1S/C45H56F2N6O15/c1-24(54)48-32(23-36(59)60)43(65)49-29(18-20-35(57)58)41(63)53-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)44(66)50-28(17-19-34(55)56)40(62)52-31(21-25-11-5-2-6-12-25)42(64)51-30(22-33(46)47)39(61)45(67)68/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,48,54)(H,49,65)(H,50,66)(H,51,64)(H,52,62)(H,53,63)(H,55,56)(H,57,58)(H,59,60)(H,67,68)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 705-8 (2002)
BindingDB Entry DOI: 10.7270/Q24X572V |
More data for this
Ligand-Target Pair | |
Genome polyprotein/Non-structural protein 4A
(Hepatitis C virus) | BDBM50110121

(3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...)Show SMILES CC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(F)F)C(=O)C(O)=O Show InChI InChI=1S/C45H56F2N6O15/c1-24(54)48-32(23-36(59)60)43(65)49-29(18-20-35(57)58)41(63)53-38(37(26-13-7-3-8-14-26)27-15-9-4-10-16-27)44(66)50-28(17-19-34(55)56)40(62)52-31(21-25-11-5-2-6-12-25)42(64)51-30(22-33(46)47)39(61)45(67)68/h3-4,7-10,13-16,25,28-33,37-38H,2,5-6,11-12,17-23H2,1H3,(H,48,54)(H,49,65)(H,50,66)(H,51,64)(H,52,62)(H,53,63)(H,55,56)(H,57,58)(H,59,60)(H,67,68)/t28-,29-,30-,31-,32-,38-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome
Curated by ChEMBL
| Assay Description
Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease |
Bioorg Med Chem Lett 12: 701-4 (2002)
BindingDB Entry DOI: 10.7270/Q28P5ZSS |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50066789

(3-((S)-1-Azetidin-2-ylmethoxy)-5-iodo-pyridine | A...)Show InChI InChI=1S/C9H11IN2O/c10-7-3-9(5-11-4-7)13-6-8-1-2-12-8/h3-5,8,12H,1-2,6H2/t8-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50466543

(CHEMBL4276961)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C185H286N52O55S2/c1-20-92(10)144(174(284)226-124(84-137(190)247)163(273)214-115(64-75-294-19)158(268)220-120(78-90(6)7)166(276)231-145(98(16)238)175(285)218-116(33-24-68-202-185(197)198)177(287)235-71-27-36-131(235)169(279)215-110(32-23-67-201-184(195)196)154(264)228-128(181(291)292)82-103-45-53-107(243)54-46-103)230-167(277)122(80-101-41-49-105(241)50-42-101)223-153(263)109(31-22-66-200-183(193)194)210-152(262)108(30-21-65-199-182(191)192)211-161(271)118(76-88(2)3)221-165(275)126(86-142(255)256)219-149(259)95(13)204-147(257)94(12)206-159(269)121(79-100-39-47-104(240)48-40-100)222-157(267)111(55-59-134(187)244)209-148(258)96(14)205-151(261)114(63-74-293-18)213-155(265)112(56-60-135(188)245)212-156(266)113(57-61-139(249)250)216-170(280)132-37-29-73-237(132)180(290)146(99(17)239)232-150(260)97(15)207-160(270)123(83-136(189)246)224-164(274)125(85-141(253)254)208-138(248)87-203-168(278)129-34-25-70-234(129)179(289)127(81-102-43-51-106(242)52-44-102)227-173(283)143(91(8)9)229-172(282)133-38-28-72-236(133)178(288)117(58-62-140(251)252)217-162(272)119(77-89(4)5)225-171(281)130-35-26-69-233(130)176(286)93(11)186/h39-54,88-99,108-133,143-146,238-243H,20-38,55-87,186H2,1-19H3,(H2,187,244)(H2,188,245)(H2,189,246)(H2,190,247)(H,203,278)(H,204,257)(H,205,261)(H,206,269)(H,207,270)(H,208,248)(H,209,258)(H,210,262)(H,211,271)(H,212,266)(H,213,265)(H,214,273)(H,215,279)(H,216,280)(H,217,272)(H,218,285)(H,219,259)(H,220,268)(H,221,275)(H,222,267)(H,223,263)(H,224,274)(H,225,281)(H,226,284)(H,227,283)(H,228,264)(H,229,282)(H,230,277)(H,231,276)(H,232,260)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,291,292)(H4,191,192,199)(H4,193,194,200)(H4,195,196,201)(H4,197,198,202)/t92-,93-,94-,95-,96-,97-,98+,99+,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,143-,144-,145-,146-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay |
J Med Chem 61: 10519-10530 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01046
BindingDB Entry DOI: 10.7270/Q2ZC85JC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50466543

(CHEMBL4276961)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C185H286N52O55S2/c1-20-92(10)144(174(284)226-124(84-137(190)247)163(273)214-115(64-75-294-19)158(268)220-120(78-90(6)7)166(276)231-145(98(16)238)175(285)218-116(33-24-68-202-185(197)198)177(287)235-71-27-36-131(235)169(279)215-110(32-23-67-201-184(195)196)154(264)228-128(181(291)292)82-103-45-53-107(243)54-46-103)230-167(277)122(80-101-41-49-105(241)50-42-101)223-153(263)109(31-22-66-200-183(193)194)210-152(262)108(30-21-65-199-182(191)192)211-161(271)118(76-88(2)3)221-165(275)126(86-142(255)256)219-149(259)95(13)204-147(257)94(12)206-159(269)121(79-100-39-47-104(240)48-40-100)222-157(267)111(55-59-134(187)244)209-148(258)96(14)205-151(261)114(63-74-293-18)213-155(265)112(56-60-135(188)245)212-156(266)113(57-61-139(249)250)216-170(280)132-37-29-73-237(132)180(290)146(99(17)239)232-150(260)97(15)207-160(270)123(83-136(189)246)224-164(274)125(85-141(253)254)208-138(248)87-203-168(278)129-34-25-70-234(129)179(289)127(81-102-43-51-106(242)52-44-102)227-173(283)143(91(8)9)229-172(282)133-38-28-72-236(133)178(288)117(58-62-140(251)252)217-162(272)119(77-89(4)5)225-171(281)130-35-26-69-233(130)176(286)93(11)186/h39-54,88-99,108-133,143-146,238-243H,20-38,55-87,186H2,1-19H3,(H2,187,244)(H2,188,245)(H2,189,246)(H2,190,247)(H,203,278)(H,204,257)(H,205,261)(H,206,269)(H,207,270)(H,208,248)(H,209,258)(H,210,262)(H,211,271)(H,212,266)(H,213,265)(H,214,273)(H,215,279)(H,216,280)(H,217,272)(H,218,285)(H,219,259)(H,220,268)(H,221,275)(H,222,267)(H,223,263)(H,224,274)(H,225,281)(H,226,284)(H,227,283)(H,228,264)(H,229,282)(H,230,277)(H,231,276)(H,232,260)(H,249,250)(H,251,252)(H,253,254)(H,255,256)(H,291,292)(H4,191,192,199)(H4,193,194,200)(H4,195,196,201)(H4,197,198,202)/t92-,93-,94-,95-,96-,97-,98+,99+,108-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,143-,144-,145-,146-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay |
J Med Chem 61: 10519-10530 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01046
BindingDB Entry DOI: 10.7270/Q2ZC85JC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50466554

(CHEMBL4286615)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N)C(C)C)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N(C)[C@@H](CCC(N)=O)CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(O)=O |r| Show InChI InChI=1S/C187H291N53O56S2/c1-21-93(10)147(176(288)229-126(85-139(193)251)166(278)218-117(65-75-298-20)161(273)223-122(78-91(6)7)169(281)234-148(99(16)241)177(289)221-118(33-25-69-205-187(200)201)179(291)236(18)105(46-59-135(189)247)83-140(252)211-110(30-22-66-202-184(194)195)155(267)231-130(183(295)296)82-104-44-53-109(246)54-45-104)233-170(282)124(80-102-40-49-107(244)50-41-102)226-157(269)112(32-24-68-204-186(198)199)214-156(268)111(31-23-67-203-185(196)197)215-164(276)120(76-89(2)3)224-168(280)128(87-145(260)261)222-152(264)96(13)207-150(262)95(12)209-162(274)123(79-101-38-47-106(243)48-39-101)225-160(272)113(55-60-136(190)248)213-151(263)97(14)208-154(266)116(64-74-297-19)217-158(270)114(56-61-137(191)249)216-159(271)115(57-62-142(254)255)219-172(284)133-36-29-73-240(133)182(294)149(100(17)242)235-153(265)98(15)210-163(275)125(84-138(192)250)227-167(279)127(86-144(258)259)212-141(253)88-206-171(283)131-34-26-71-238(131)181(293)129(81-103-42-51-108(245)52-43-103)230-175(287)146(92(8)9)232-174(286)134-37-28-72-239(134)180(292)119(58-63-143(256)257)220-165(277)121(77-90(4)5)228-173(285)132-35-27-70-237(132)178(290)94(11)188/h38-45,47-54,89-100,105,110-134,146-149,241-246H,21-37,46,55-88,188H2,1-20H3,(H2,189,247)(H2,190,248)(H2,191,249)(H2,192,250)(H2,193,251)(H,206,283)(H,207,262)(H,208,266)(H,209,274)(H,210,275)(H,211,252)(H,212,253)(H,213,263)(H,214,268)(H,215,276)(H,216,271)(H,217,270)(H,218,278)(H,219,284)(H,220,277)(H,221,289)(H,222,264)(H,223,273)(H,224,280)(H,225,272)(H,226,269)(H,227,279)(H,228,285)(H,229,288)(H,230,287)(H,231,267)(H,232,286)(H,233,282)(H,234,281)(H,235,265)(H,254,255)(H,256,257)(H,258,259)(H,260,261)(H,295,296)(H4,194,195,202)(H4,196,197,203)(H4,198,199,204)(H4,200,201,205)/t93-,94-,95-,96-,97-,98-,99+,100+,105-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,127-,128-,129-,130-,131-,132-,133-,134-,146-,147-,148-,149-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]-PP from human Y4R expressed in CHO-K1 cell membranes after 2 hrs by scintillation proximity assay |
J Med Chem 61: 10519-10530 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01046
BindingDB Entry DOI: 10.7270/Q2ZC85JC |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(RAT) | BDBM50170654
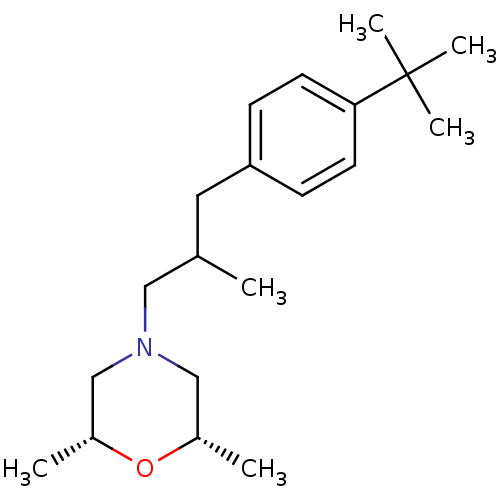
((+-)-cis-4-(3-(4-tert-butylphenyl)-2-methylpropyl)...)Show SMILES CC(CN1C[C@H](C)O[C@H](C)C1)Cc1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C20H33NO/c1-15(12-21-13-16(2)22-17(3)14-21)11-18-7-9-19(10-8-18)20(4,5)6/h7-10,15-17H,11-14H2,1-6H3/t15?,16-,17+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pentazocine from sigma 1 opioid receptor in rat liver homogenate by liquid scintillation counting |
ACS Med Chem Lett 2: 834-839 (2011)
Article DOI: 10.1021/ml2001505
BindingDB Entry DOI: 10.7270/Q2RR208C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537063

(CHEMBL4590517)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(O)=O |r,t:17,19| Show InChI InChI=1S/C83H108ClN13O21S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)95-83)45(2)71-82(5,118-71)66(38-68(101)97(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)96(6)67(100)29-31-119-120-43-61(79(109)110)93-77(107)60-42-122-121-41-59(91-72(102)54(86)33-48-19-11-10-12-20-48)76(106)89-57(34-49-25-27-52(99)28-26-49)74(104)90-58(37-51-40-87-55-22-14-13-21-53(51)55)75(105)88-56(23-15-16-30-85)73(103)94-70(47(4)98)78(108)92-60/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,87,98-99,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H,88,105)(H,89,106)(H,90,104)(H,91,102)(H,92,108)(H,93,107)(H,94,103)(H,95,112)(H,109,110)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50033535
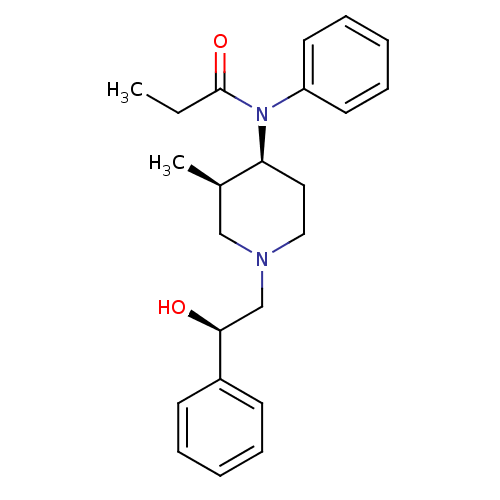
(CHEMBL331883 | N-[(3R,4S)-1-((R)-2-Hydroxy-2-pheny...)Show SMILES CCC(=O)N([C@H]1CCN(C[C@H](O)c2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O2/c1-3-23(27)25(20-12-8-5-9-13-20)21-14-15-24(16-18(21)2)17-22(26)19-10-6-4-7-11-19/h4-13,18,21-22,26H,3,14-17H2,1-2H3/t18-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Vesicular acetylcholine transporter
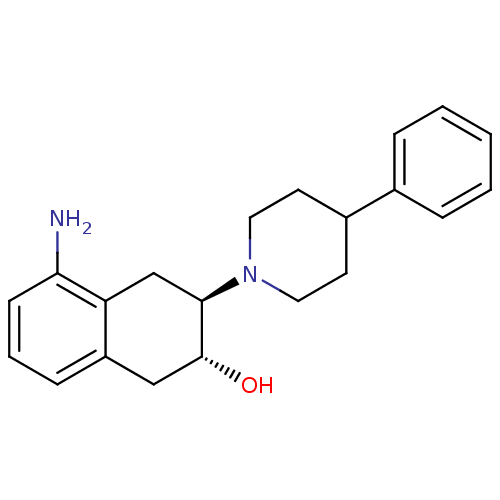
(Torpedo californica) | BDBM50039623
((2R,3R)-5-Amino-3-(4-phenyl-piperidin-1-yl)-1,2,3,...)Show SMILES Nc1cccc2C[C@@H](O)[C@@H](Cc12)N1CCC(CC1)c1ccccc1 Show InChI InChI=1S/C21H26N2O/c22-19-8-4-7-17-13-21(24)20(14-18(17)19)23-11-9-16(10-12-23)15-5-2-1-3-6-15/h1-8,16,20-21,24H,9-14,22H2/t20-,21-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Radiopharmaceutical Cancer Research
Curated by ChEMBL
| Assay Description
Displacement of (-)-[3H]vesamicol from VAChT in Torpedo californica electric organ synaptic vesicles |
Eur J Med Chem 100: 50-67 (2015)
Article DOI: 10.1016/j.ejmech.2015.05.033
BindingDB Entry DOI: 10.7270/Q28C9XZ1 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50471125
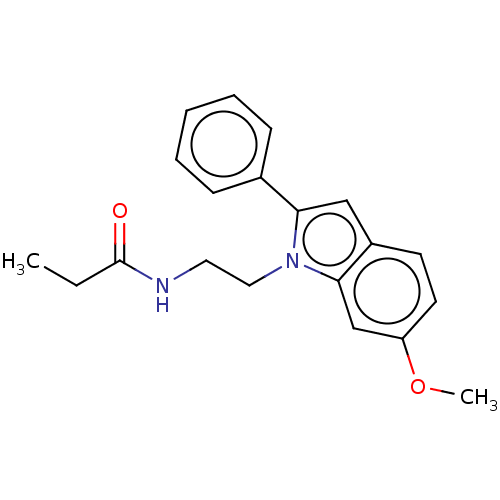
(CHEMBL433237)Show InChI InChI=1S/C20H22N2O2/c1-3-20(23)21-11-12-22-18(15-7-5-4-6-8-15)13-16-9-10-17(24-2)14-19(16)22/h4-10,13-14H,3,11-12H2,1-2H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino
Curated by ChEMBL
| Assay Description
Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin (100 pM) as labelled ligand |
J Med Chem 40: 2003-10 (1997)
Article DOI: 10.1021/jm960653j
BindingDB Entry DOI: 10.7270/Q2CR5X2K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537077

(CHEMBL4550617)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCOc1ccc(C[C@@H](N)C(=O)N[C@H]2CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc3c[nH]c4ccccc34)NC(=O)[C@H](Cc3ccc(O)cc3)NC2=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O)cc1 |r,t:17,19| Show InChI InChI=1S/C86H114ClN13O23S4/c1-45-16-15-20-67(119-10)86(117)41-66(121-84(116)98-86)46(2)74-85(6,123-74)68(40-70(105)100(8)64-37-52(34-45)38-65(118-9)71(64)87)122-83(115)47(3)99(7)69(104)29-32-124-125-33-31-120-55-27-23-50(24-28-55)35-57(89)75(106)94-62-43-126-127-44-63(80(111)97-73(49(5)102)82(113)114)95-81(112)72(48(4)101)96-76(107)59(19-13-14-30-88)91-78(109)61(39-53-42-90-58-18-12-11-17-56(53)58)93-77(108)60(92-79(62)110)36-51-21-25-54(103)26-22-51/h11-12,15-18,20-28,37-38,42,46-49,57,59-63,66-68,72-74,90,101-103,117H,13-14,19,29-36,39-41,43-44,88-89H2,1-10H3,(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,116)(H,113,114)/b20-15+,45-16+/t46-,47+,48-,49-,57-,59+,60+,61-,62+,63+,66+,67-,68+,72+,73+,74+,85-,86+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537066

(CHEMBL4541310)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSCCCN[C@H](Cc1ccccc1)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@]([H])(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC1=O)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O |r,t:17,19| Show InChI InChI=1S/C87H116ClN13O22S4/c1-47-20-18-26-68(120-10)87(118)43-67(121-85(117)99-87)48(2)75-86(6,123-75)69(42-71(106)101(8)65-39-54(36-47)40-66(119-9)72(65)88)122-84(116)49(3)100(7)70(105)31-35-125-124-34-19-33-90-60(37-52-21-12-11-13-22-52)77(108)95-63-45-126-127-46-64(81(112)98-74(51(5)103)83(114)115)96-82(113)73(50(4)102)97-76(107)59(25-16-17-32-89)92-79(110)62(41-55-44-91-58-24-15-14-23-57(55)58)94-78(109)61(93-80(63)111)38-53-27-29-56(104)30-28-53/h11-15,18,20-24,26-30,39-40,44,48-51,59-64,67-69,73-75,90-91,102-104,118H,16-17,19,25,31-38,41-43,45-46,89H2,1-10H3,(H,92,110)(H,93,111)(H,94,109)(H,95,108)(H,96,113)(H,97,107)(H,98,112)(H,99,117)(H,114,115)/b26-18+,47-20+/t48-,49+,50-,51-,59+,60-,61+,62-,63+,64+,67+,68-,69+,73+,74+,75+,86-,87+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537069
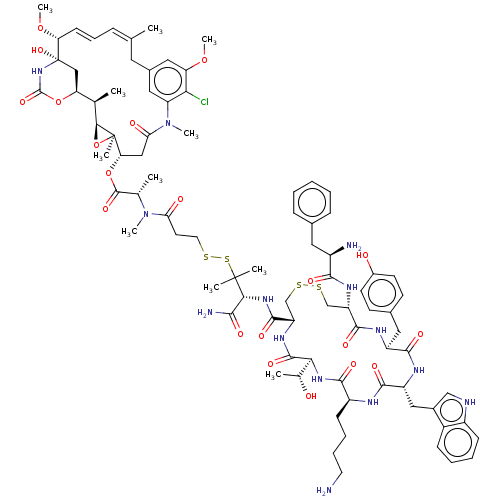
(CHEMBL4584764)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC(C)(C)[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C85H113ClN14O20S4/c1-45-20-19-26-65(117-11)85(115)41-64(118-82(114)98-85)46(2)72-84(7,120-72)66(40-68(104)100(9)62-37-51(34-45)38-63(116-10)69(62)86)119-81(113)47(3)99(8)67(103)31-33-121-124-83(5,6)71(73(89)105)97-79(111)61-44-123-122-43-60(94-74(106)55(88)35-49-21-13-12-14-22-49)78(110)92-58(36-50-27-29-53(102)30-28-50)76(108)93-59(39-52-42-90-56-24-16-15-23-54(52)56)77(109)91-57(25-17-18-32-87)75(107)96-70(48(4)101)80(112)95-61/h12-16,19-24,26-30,37-38,42,46-48,55,57-61,64-66,70-72,90,101-102,115H,17-18,25,31-36,39-41,43-44,87-88H2,1-11H3,(H2,89,105)(H,91,109)(H,92,110)(H,93,108)(H,94,106)(H,95,112)(H,96,107)(H,97,111)(H,98,114)/b26-19+,45-20+/t46-,47+,48-,55-,57+,58+,59-,60+,61+,64+,65-,66+,70+,71-,72+,84-,85+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50034110
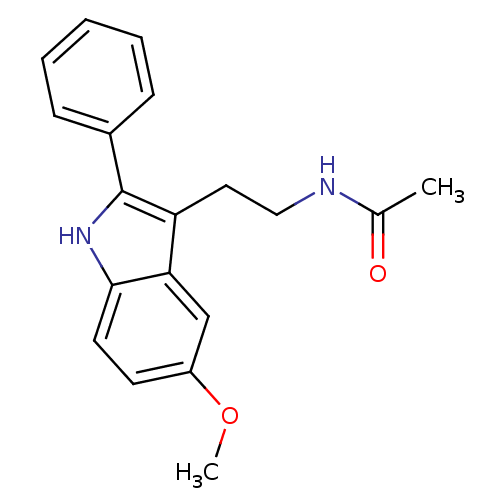
(CHEMBL15060 | Melatonin,2-Phenyl | N-[2-(5-Methoxy...)Show InChI InChI=1S/C19H20N2O2/c1-13(22)20-11-10-16-17-12-15(23-2)8-9-18(17)21-19(16)14-6-4-3-5-7-14/h3-9,12,21H,10-11H2,1-2H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino
Curated by ChEMBL
| Assay Description
Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as labelled ligand |
J Med Chem 40: 1990-2002 (1997)
Article DOI: 10.1021/jm960651z
BindingDB Entry DOI: 10.7270/Q2HD7ZCK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50160893
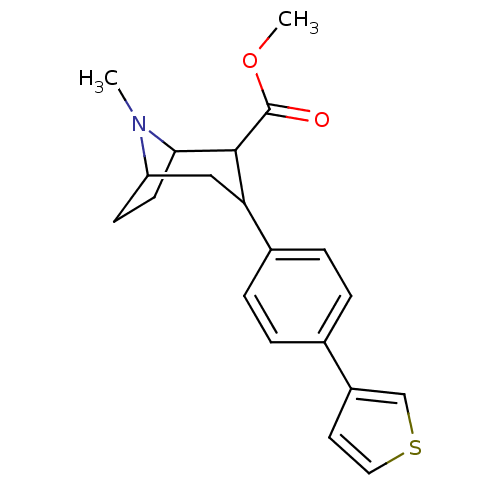
(8-Methyl-3-(4-thiophen-3-yl-phenyl)-8-aza-bicyclo[...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(cc1)-c1ccsc1)N2C |TLB:23:22:4.10.9:6.7,THB:2:4:22:6.7,11:10:22:6.7| Show InChI InChI=1S/C20H23NO2S/c1-21-16-7-8-18(21)19(20(22)23-2)17(11-16)14-5-3-13(4-6-14)15-9-10-24-12-15/h3-6,9-10,12,16-19H,7-8,11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Displacement of [3H]paroxetine from serotonin transporter of rat cerebral cortex |
Bioorg Med Chem Lett 15: 1131-3 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.014
BindingDB Entry DOI: 10.7270/Q2PK0GXK |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50034110

(CHEMBL15060 | Melatonin,2-Phenyl | N-[2-(5-Methoxy...)Show InChI InChI=1S/C19H20N2O2/c1-13(22)20-11-10-16-17-12-15(23-2)8-9-18(17)21-19(16)14-6-4-3-5-7-14/h3-9,12,21H,10-11H2,1-2H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino
Curated by ChEMBL
| Assay Description
Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin (100 pM) as labelled ligand |
J Med Chem 40: 2003-10 (1997)
Article DOI: 10.1021/jm960653j
BindingDB Entry DOI: 10.7270/Q2CR5X2K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537076

(CHEMBL4564727)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1 |r,t:17,19| Show InChI InChI=1S/C83H110ClN13O20S4/c1-45-18-17-24-66(114-9)83(112)39-65(115-81(111)95-83)46(2)72-82(5,117-72)67(38-69(102)97(7)63-35-51(32-45)36-64(113-8)70(63)84)116-80(110)47(3)96(6)68(101)29-31-118-119-42-53(41-98)88-77(107)61-43-120-121-44-62(92-73(103)56(86)33-49-19-11-10-12-20-49)78(108)90-59(34-50-25-27-54(100)28-26-50)75(105)91-60(37-52-40-87-57-22-14-13-21-55(52)57)76(106)89-58(23-15-16-30-85)74(104)94-71(48(4)99)79(109)93-61/h10-14,17-22,24-28,35-36,40,46-48,53,56,58-62,65-67,71-72,87,98-100,112H,15-16,23,29-34,37-39,41-44,85-86H2,1-9H3,(H,88,107)(H,89,106)(H,90,108)(H,91,105)(H,92,103)(H,93,109)(H,94,104)(H,95,111)/b24-17+,45-18+/t46-,47+,48-,53-,56-,58+,59+,60-,61+,62+,65+,66-,67+,71+,72+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM94503
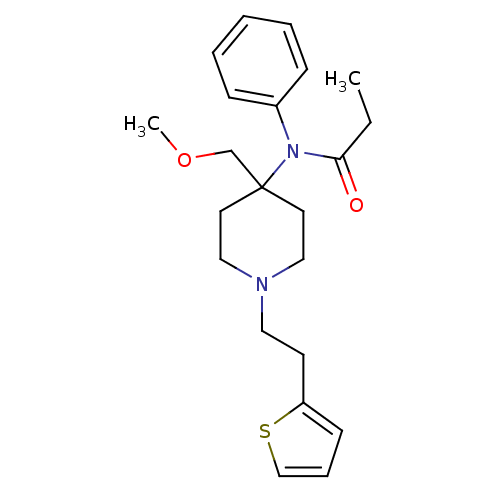
(2-hydroxypropane-1,2,3-tricarboxylic acid;N-[4-(me...)Show InChI InChI=1S/C22H30N2O2S/c1-3-21(25)24(19-8-5-4-6-9-19)22(18-26-2)12-15-23(16-13-22)14-11-20-10-7-17-27-20/h4-10,17H,3,11-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anaquest Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro affinity to displace [3H]naloxone from opiate receptor in freshly prepared rat brain homogenates |
J Med Chem 32: 968-74 (1989)
BindingDB Entry DOI: 10.7270/Q2XG9TBN |
More data for this
Ligand-Target Pair | |
Prolyl endopeptidase
(Rattus norvegicus) | BDBM50155838
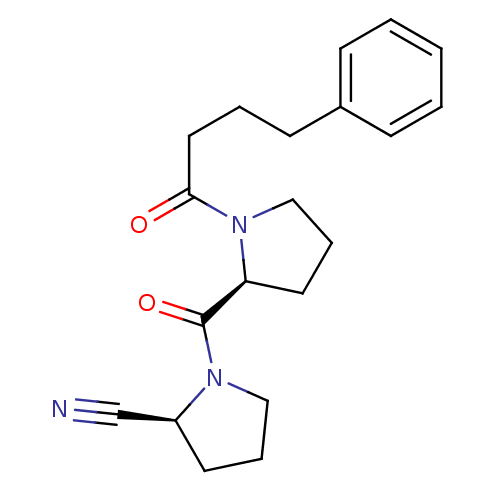
((S)-1-((S)-1-(4-phenylbutanoyl)pyrrolidine-2-carbo...)Show SMILES O=C(CCCc1ccccc1)N1CCC[C@H]1C(=O)N1CCC[C@H]1C#N Show InChI InChI=1S/C20H25N3O2/c21-15-17-10-5-13-22(17)20(25)18-11-6-14-23(18)19(24)12-4-9-16-7-2-1-3-8-16/h1-3,7-8,17-18H,4-6,9-14H2/t17-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of POP in Han/Wistar rat brain using Suc-Gly-Pro-AMC substrate incubated for 60 mins |
J Med Chem 59: 4221-34 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01296
BindingDB Entry DOI: 10.7270/Q2Z89FBB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50100712
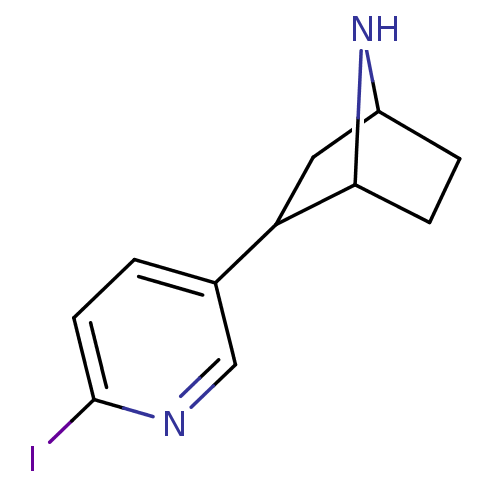
(2-(6-Iodo-pyridin-3-yl)-7-aza-bicyclo[2.2.1]heptan...)Show InChI InChI=1S/C11H13IN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse
Curated by PDSP Ki Database
| |
Mol Pharmacol 57: 642-9 (2000)
Article DOI: 10.1124/mol.57.3.642
BindingDB Entry DOI: 10.7270/Q21C1VF8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50021347
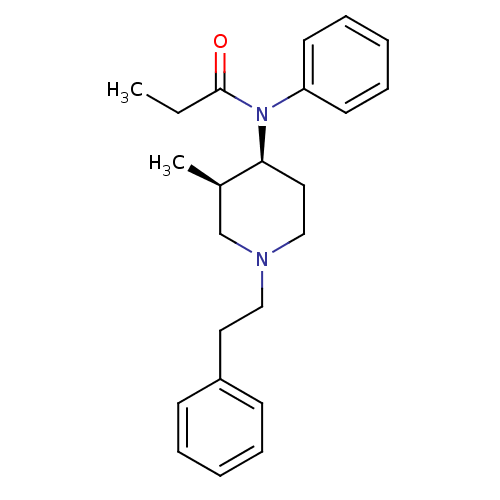
(CHEMBL12391 | N-(3-Methyl-1-phenethyl-piperidin-4-...)Show SMILES CCC(=O)N([C@H]1CCN(CCc2ccccc2)C[C@H]1C)c1ccccc1 Show InChI InChI=1S/C23H30N2O/c1-3-23(26)25(21-12-8-5-9-13-21)22-15-17-24(18-19(22)2)16-14-20-10-6-4-7-11-20/h4-13,19,22H,3,14-18H2,1-2H3/t19-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86660

(OFQ/N UFP-102)Show SMILES [#6]-[#6@@H](-[#8])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(F)cc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C73H123FN28O17/c1-41(92-57(107)39-91-70(119)59(43(3)104)102-68(117)53(34-44-24-26-46(74)27-25-44)94-58(108)38-90-56(106)37-89-55(105)36-85-35-45-16-5-4-6-17-45)61(110)96-50(21-13-31-86-71(79)80)65(114)99-49(20-9-12-30-77)67(116)101-54(40-103)69(118)93-42(2)62(111)97-51(22-14-32-87-72(81)82)66(115)98-48(19-8-11-29-76)64(113)100-52(23-15-33-88-73(83)84)63(112)95-47(60(78)109)18-7-10-28-75/h4-6,16-17,24-27,41-43,47-54,59,85,103-104H,7-15,18-23,28-40,75-77H2,1-3H3,(H2,78,109)(H,89,105)(H,90,106)(H,91,119)(H,92,107)(H,93,118)(H,94,108)(H,95,112)(H,96,110)(H,97,111)(H,98,115)(H,99,114)(H,100,113)(H,101,116)(H,102,117)(H4,79,80,86)(H4,81,82,87)(H4,83,84,88)/t41-,42-,43+,47-,48-,49-,50-,51-,52-,53-,54-,59-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Section of Pharmacology
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 312: 1114-23 (2005)
Article DOI: 10.1124/jpet.104.077339
BindingDB Entry DOI: 10.7270/Q2222SC4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50537061
(CHEMBL4527856)Show SMILES [H][C@@]12O[C@]1(C)[C@H](CC(=O)N(C)c1cc(C\C(C)=C\C=C\[C@@H](OC)[C@@]3(O)C[C@]([H])(OC(=O)N3)[C@H]2C)cc(OC)c1Cl)OC(=O)[C@H](C)N(C)C(=O)CCSSC[C@H](NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@]([H])([C@@H](C)O)C(=O)N1)C(N)=O |r,t:17,19| Show InChI InChI=1S/C83H109ClN14O20S4/c1-44-18-17-24-65(115-9)83(113)39-64(116-81(112)96-83)45(2)71-82(5,118-71)66(38-68(102)98(7)62-35-50(32-44)36-63(114-8)69(62)84)117-80(111)46(3)97(6)67(101)29-31-119-120-41-59(72(87)103)92-78(109)61-43-122-121-42-60(93-73(104)54(86)33-48-19-11-10-12-20-48)77(108)90-57(34-49-25-27-52(100)28-26-49)75(106)91-58(37-51-40-88-55-22-14-13-21-53(51)55)76(107)89-56(23-15-16-30-85)74(105)95-70(47(4)99)79(110)94-61/h10-14,17-22,24-28,35-36,40,45-47,54,56-61,64-66,70-71,88,99-100,113H,15-16,23,29-34,37-39,41-43,85-86H2,1-9H3,(H2,87,103)(H,89,107)(H,90,108)(H,91,106)(H,92,109)(H,93,104)(H,94,110)(H,95,105)(H,96,112)/b24-17+,44-18+/t45-,46+,47-,54-,56+,57+,58-,59+,60+,61+,64+,65-,66+,70+,71+,82-,83+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tarveda Therapeutics
Curated by ChEMBL
| Assay Description
Displacement of [125I]somatostatin from human SSTR2 expressed in CHO-K1 cell membranes after 240 mins |
J Med Chem 62: 2708-2719 (2019)
Article DOI: 10.1021/acs.jmedchem.8b02036
BindingDB Entry DOI: 10.7270/Q2NK3JJ9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50012477
(1-Phenethyl-4-(phenyl-propionyl-amino)-piperidine-...)Show SMILES CCC(=O)N(c1ccccc1)C1(CCN(CCc2ccccc2)CC1)C(=O)OC Show InChI InChI=1S/C24H30N2O3/c1-3-22(27)26(21-12-8-5-9-13-21)24(23(28)29-2)15-18-25(19-16-24)17-14-20-10-6-4-7-11-20/h4-13H,3,14-19H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Binding affinity against Opioid receptor mu 1 |
J Med Chem 43: 381-91 (2000)
BindingDB Entry DOI: 10.7270/Q2DN45RB |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50341578
(3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...)Show SMILES Cc1cc(C)cc(c1)-c1cnc2cc(Cl)c(cc2c1OCCCN)-c1cccc(O)c1 Show InChI InChI=1S/C26H25ClN2O2/c1-16-9-17(2)11-19(10-16)23-15-29-25-14-24(27)21(18-5-3-6-20(30)12-18)13-22(25)26(23)31-8-4-7-28/h3,5-6,9-15,30H,4,7-8,28H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting |
J Med Chem 54: 2351-8 (2011)
Article DOI: 10.1021/jm101501b
BindingDB Entry DOI: 10.7270/Q2668DH8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50341575
(7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...)Show SMILES Cc1cc(C)cc(c1)-c1cnc2cc(Cl)c(cc2c1OCC[C@H]1CCCCN1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C28H31ClN4O/c1-18-10-19(2)12-20(11-18)25-16-31-27-14-26(29)23(21-15-32-33(3)17-21)13-24(27)28(25)34-9-7-22-6-4-5-8-30-22/h10-17,22,30H,4-9H2,1-3H3/t22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting |
J Med Chem 54: 2351-8 (2011)
Article DOI: 10.1021/jm101501b
BindingDB Entry DOI: 10.7270/Q2668DH8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50146585
(CHEMBL3763396)Show SMILES CC1CCN(CCCCc2cccc3cc(OCCCCCCN4C(=O)c5ccc(cc5C4=O)N(C)C)ccc23)CC1 Show InChI InChI=1S/C36H47N3O3/c1-27-18-22-38(23-19-27)20-8-6-11-28-12-10-13-29-25-31(15-17-32(28)29)42-24-9-5-4-7-21-39-35(40)33-16-14-30(37(2)3)26-34(33)36(39)41/h10,12-17,25-27H,4-9,11,18-24H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50043287
(CHEMBL33415 | Melatonin,2-Bromo | N-[2-(2-Bromo-5-...)Show InChI InChI=1S/C13H15BrN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino
Curated by ChEMBL
| Assay Description
Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as labelled ligand |
J Med Chem 40: 1990-2002 (1997)
Article DOI: 10.1021/jm960651z
BindingDB Entry DOI: 10.7270/Q2HD7ZCK |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A/1B
(Homo sapiens (Human)) | BDBM50043287

(CHEMBL33415 | Melatonin,2-Bromo | N-[2-(2-Bromo-5-...)Show InChI InChI=1S/C13H15BrN2O2/c1-8(17)15-6-5-10-11-7-9(18-2)3-4-12(11)16-13(10)14/h3-4,7,16H,5-6H2,1-2H3,(H,15,17) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino
Curated by ChEMBL
| Assay Description
Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin (100 pM) as labelled ligand |
J Med Chem 40: 2003-10 (1997)
Article DOI: 10.1021/jm960653j
BindingDB Entry DOI: 10.7270/Q2CR5X2K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50341572
(3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...)Show SMILES Cc1cc(C)cc(c1)-c1cnc2cc(Cl)c(cc2c1OCC[C@H]1CCCCN1)-c1cccc(O)c1 |r| Show InChI InChI=1S/C30H31ClN2O2/c1-19-12-20(2)14-22(13-19)27-18-33-29-17-28(31)25(21-6-5-8-24(34)15-21)16-26(29)30(27)35-11-9-23-7-3-4-10-32-23/h5-6,8,12-18,23,32,34H,3-4,7,9-11H2,1-2H3/t23-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting |
J Med Chem 54: 2351-8 (2011)
Article DOI: 10.1021/jm101501b
BindingDB Entry DOI: 10.7270/Q2668DH8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50341574
(3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...)Show SMILES Cc1cc(C)cc(c1)-c1cnc2cc(Cl)c(cc2c1OCC[C@H]1CCCCN1)-c1ccc(cc1)C(N)=O |r| Show InChI InChI=1S/C31H32ClN3O2/c1-19-13-20(2)15-23(14-19)27-18-35-29-17-28(32)25(21-6-8-22(9-7-21)31(33)36)16-26(29)30(27)37-12-10-24-5-3-4-11-34-24/h6-9,13-18,24,34H,3-5,10-12H2,1-2H3,(H2,33,36)/t24-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting |
J Med Chem 54: 2351-8 (2011)
Article DOI: 10.1021/jm101501b
BindingDB Entry DOI: 10.7270/Q2668DH8 |
More data for this
Ligand-Target Pair | |
Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50274054
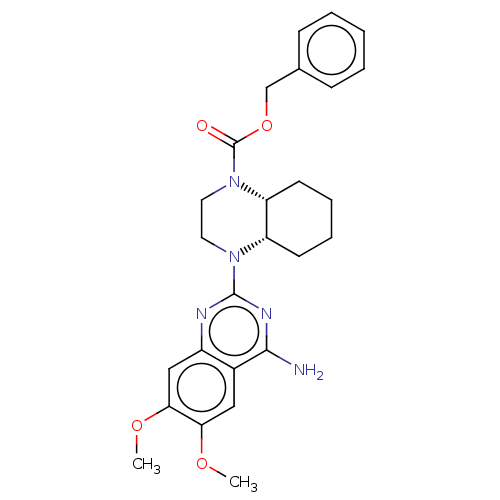
(CHEMBL4128084)Show SMILES [H][C@@]12CCCC[C@]1([H])N(CCN2C(=O)OCc1ccccc1)c1nc(N)c2cc(OC)c(OC)cc2n1 |r| Show InChI InChI=1S/C26H31N5O4/c1-33-22-14-18-19(15-23(22)34-2)28-25(29-24(18)27)30-12-13-31(21-11-7-6-10-20(21)30)26(32)35-16-17-8-4-3-5-9-17/h3-5,8-9,14-15,20-21H,6-7,10-13,16H2,1-2H3,(H2,27,28,29)/t20-,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino
Curated by ChEMBL
| Assay Description
Displacement of [3H]prazosin from human alpha1D adrenergic receptor expressed in CHO cell membranes after 30 mins by rapid filtration method |
Bioorg Med Chem 26: 3502-3513 (2018)
Article DOI: 10.1016/j.bmc.2018.05.023
BindingDB Entry DOI: 10.7270/Q2X92DS4 |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17355
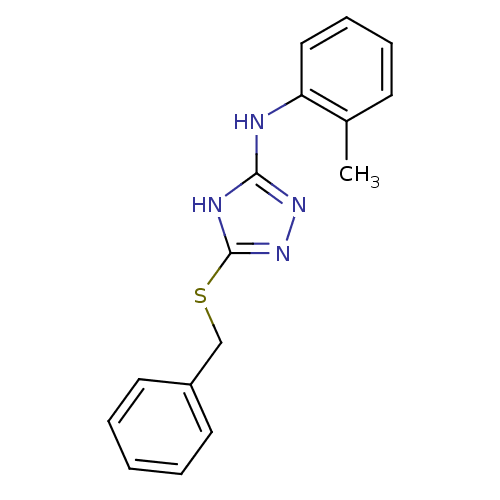
(1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...)Show InChI InChI=1S/C16H16N4S/c1-12-7-5-6-10-14(12)17-15-18-16(20-19-15)21-11-13-8-3-2-4-9-13/h2-10H,11H2,1H3,(H2,17,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Methionine aminopeptidase 2
(Homo sapiens (Human)) | BDBM17428
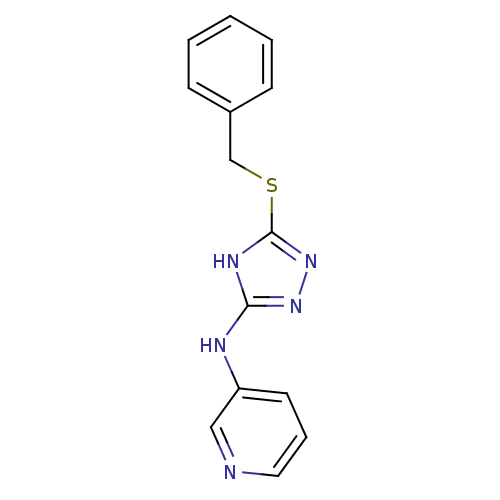
(1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...)Show InChI InChI=1S/C14H13N5S/c1-2-5-11(6-3-1)10-20-14-17-13(18-19-14)16-12-7-4-8-15-9-12/h1-9H,10H2,(H2,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... |
J Med Chem 50: 3777-85 (2007)
Article DOI: 10.1021/jm061182w
BindingDB Entry DOI: 10.7270/Q2B856D7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data