 Found 21373 hits with Last Name = 'mac' and Initial = 'm'
Found 21373 hits with Last Name = 'mac' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM82547
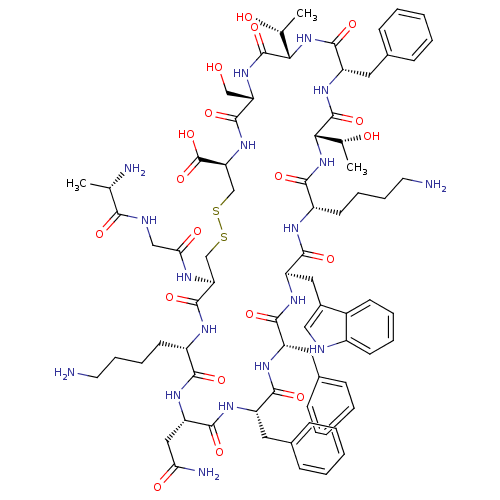
(SRIF-D-Trp8)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55+,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163573

((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccccn1 Show InChI InChI=1S/C28H21N5O2/c34-27-20-5-1-2-7-23(20)32-25-21(27)16-33(26(25)18-8-9-24-17(13-18)10-12-35-24)28-30-14-19(15-31-28)22-6-3-4-11-29-22/h1-9,11,13-15,26H,10,12,16H2,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50133069
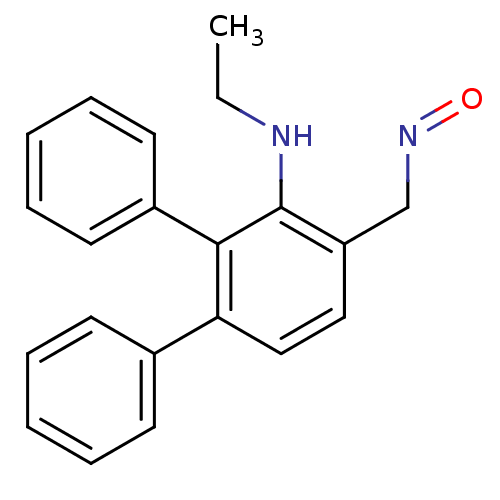
(3'-Ethylamino-[1,1';2',1'']terphenyl-4'-carbaldehy...)Show InChI InChI=1S/C21H20N2O/c1-2-22-21-18(15-23-24)13-14-19(16-9-5-3-6-10-16)20(21)17-11-7-4-8-12-17/h3-14,22H,2,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Relative binding affinity for human estrogen receptor betacompared to [3H]-estradiol |
J Med Chem 46: 4032-42 (2003)
Article DOI: 10.1021/jm0308390
BindingDB Entry DOI: 10.7270/Q27W6BKS |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50192018

(CHEMBL3350037)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H](CO)[C@@H](C)O)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39+,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR2 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099273
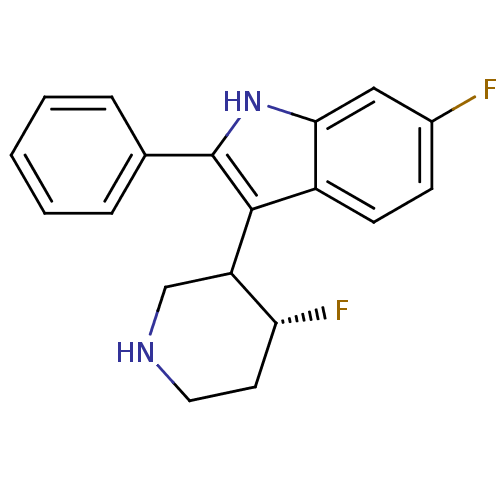
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099273

(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccccc1 Show InChI InChI=1S/C19H18F2N2/c20-13-6-7-14-17(10-13)23-19(12-4-2-1-3-5-12)18(14)15-11-22-9-8-16(15)21/h1-7,10,15-16,22-23H,8-9,11H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320375
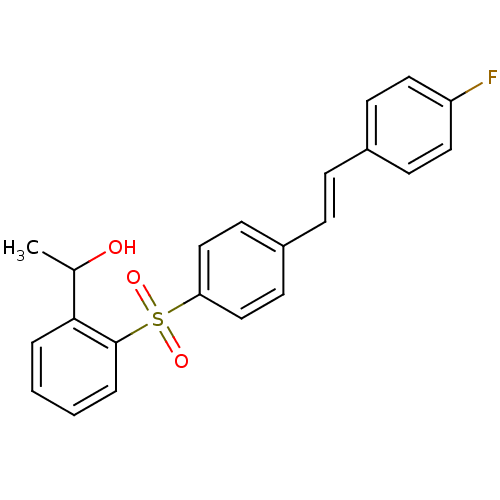
((E)-1-(2-(4-(4-fluorostyryl)phenylsulfonyl)phenyl)...)Show SMILES CC(O)c1ccccc1S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2)cc1 Show InChI InChI=1S/C22H19FO3S/c1-16(24)21-4-2-3-5-22(21)27(25,26)20-14-10-18(11-15-20)7-6-17-8-12-19(23)13-9-17/h2-16,24H,1H3/b7-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50017931

(CHEMBL3289302)Show SMILES NCCC1CCN(CC1)C(=O)[C@@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)C1CCC(N)=NC1 |r,c:39| Show InChI InChI=1S/C28H39N7O3S/c29-12-9-19-10-13-35(14-11-19)28(36)25(16-20-3-1-5-22(15-20)27(31)32)34-39(37,38)24-6-2-4-21(17-24)23-7-8-26(30)33-18-23/h1-6,15,17,19,23,25,34H,7-14,16,18,29H2,(H2,30,33)(H3,31,32)/t23?,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aurigene Discovery Technologies Limited
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matriptase (unknown origin) using Boc-Gln-Ala-Arg-7-amido-4-methyl coumarin hydrobromide |
Bioorg Med Chem 22: 3187-203 (2014)
Article DOI: 10.1016/j.bmc.2014.04.013
BindingDB Entry DOI: 10.7270/Q2J967ZD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099255
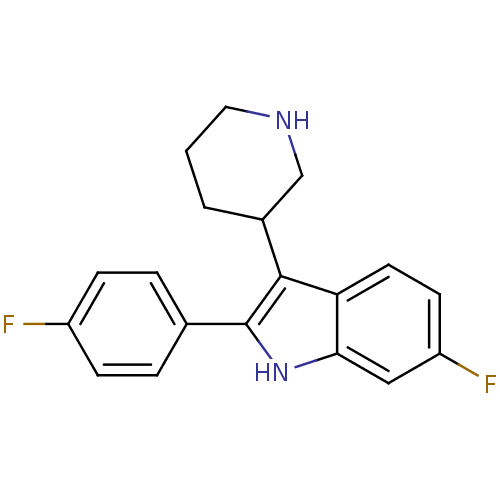
(6-Fluoro-2-(4-fluoro-phenyl)-3-piperidin-3-yl-1H-i...)Show InChI InChI=1S/C19H18F2N2/c20-14-5-3-12(4-6-14)19-18(13-2-1-9-22-11-13)16-8-7-15(21)10-17(16)23-19/h3-8,10,13,22-23H,1-2,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099275
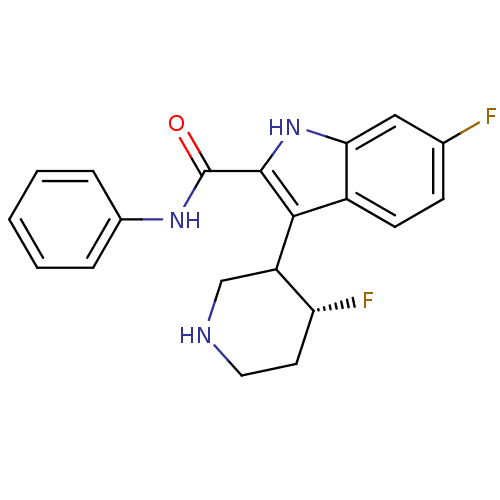
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-1H-indole-2-c...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C20H19F2N3O/c21-12-6-7-14-17(10-12)25-19(18(14)15-11-23-9-8-16(15)22)20(26)24-13-4-2-1-3-5-13/h1-7,10,15-16,23,25H,8-9,11H2,(H,24,26)/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50095027
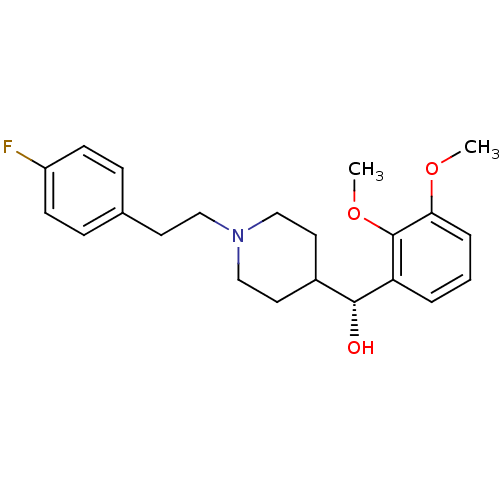
((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...)Show SMILES COc1cccc([C@H](O)C2CCN(CCc3ccc(F)cc3)CC2)c1OC |r| Show InChI InChI=1S/C22H28FNO3/c1-26-20-5-3-4-19(22(20)27-2)21(25)17-11-14-24(15-12-17)13-10-16-6-8-18(23)9-7-16/h3-9,17,21,25H,10-15H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099259
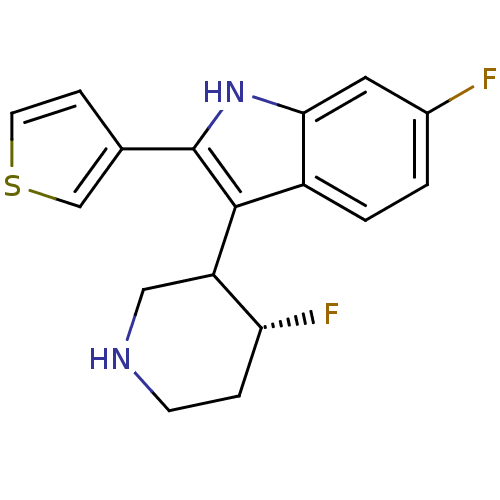
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-thiophen-3-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccsc1 Show InChI InChI=1S/C17H16F2N2S/c18-11-1-2-12-15(7-11)21-17(10-4-6-22-9-10)16(12)13-8-20-5-3-14(13)19/h1-2,4,6-7,9,13-14,20-21H,3,5,8H2/t13?,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598740
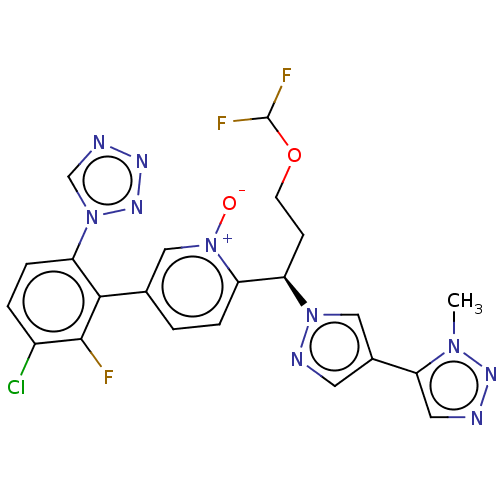
(CHEMBL5175227)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163576
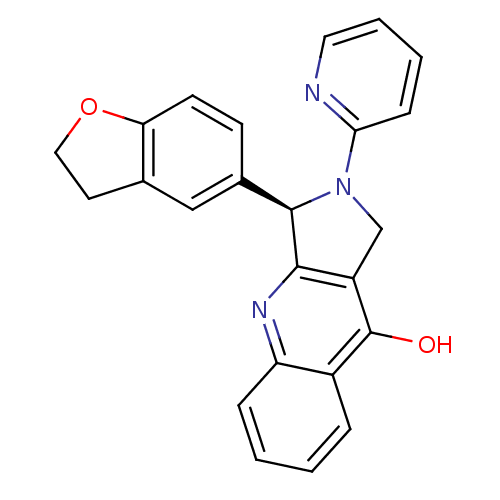
((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-pyridin-2-yl...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCCc2c1)c1ccccn1 Show InChI InChI=1S/C24H19N3O2/c28-24-17-5-1-2-6-19(17)26-22-18(24)14-27(21-7-3-4-11-25-21)23(22)16-8-9-20-15(13-16)10-12-29-20/h1-9,11,13,23H,10,12,14H2,(H,26,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099269
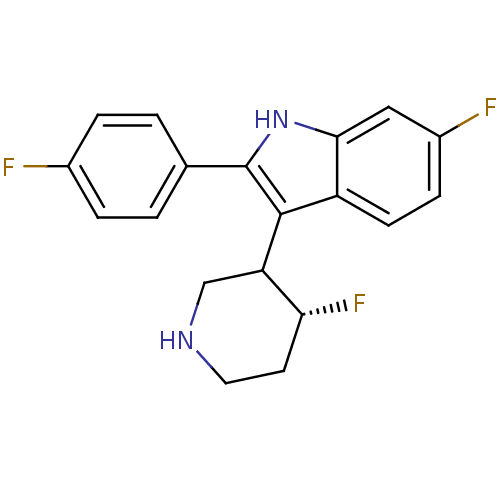
(6-Fluoro-2-(4-fluoro-phenyl)-3-(4-fluoro-piperidin...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccc(F)cc1 Show InChI InChI=1S/C19H17F3N2/c20-12-3-1-11(2-4-12)19-18(15-10-23-8-7-16(15)22)14-6-5-13(21)9-17(14)24-19/h1-6,9,15-16,23-24H,7-8,10H2/t15?,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029978
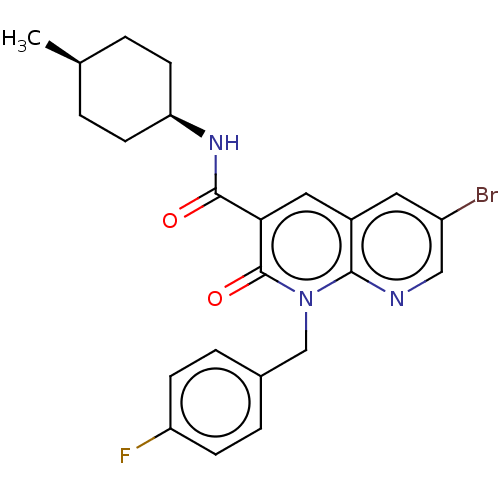
(CHEMBL3353441)Show SMILES C[C@H]1CC[C@H](CC1)NC(=O)c1cc2cc(Br)cnc2n(Cc2ccc(F)cc2)c1=O |r,wU:4.7,1.0,(16.02,-5.47,;14.69,-6.25,;14.69,-7.79,;13.37,-8.56,;12.03,-7.79,;12.02,-6.26,;13.35,-5.48,;10.7,-8.57,;9.36,-7.8,;9.35,-6.26,;8.03,-8.58,;6.69,-7.82,;5.37,-8.6,;4.03,-7.83,;2.7,-8.6,;1.37,-7.83,;2.7,-10.15,;4.04,-10.92,;5.37,-10.14,;6.7,-10.91,;6.71,-12.45,;8.04,-13.21,;8.04,-14.75,;9.37,-15.52,;10.71,-14.74,;12.04,-15.51,;10.7,-13.19,;9.36,-12.43,;8.04,-10.13,;9.38,-10.9,)| Show InChI InChI=1S/C23H23BrFN3O2/c1-14-2-8-19(9-3-14)27-22(29)20-11-16-10-17(24)12-26-21(16)28(23(20)30)13-15-4-6-18(25)7-5-15/h4-7,10-12,14,19H,2-3,8-9,13H2,1H3,(H,27,29)/t14-,19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598738
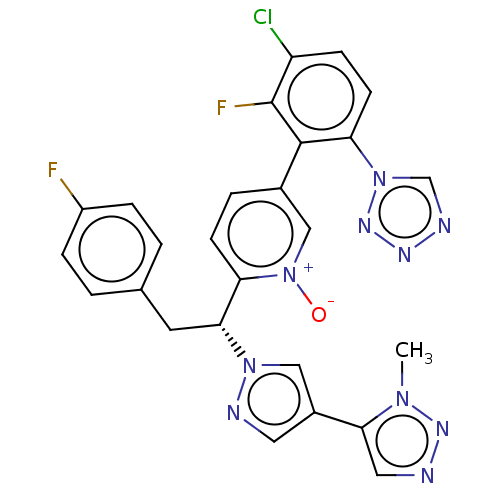
(CHEMBL5204065)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598739

(CHEMBL5188215)Show SMILES COCC[C@H](c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1)n1cc(cn1)-c1cnnn1C |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122970

(3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)c1ccc(o1)-c1cccnc1 Show InChI InChI=1S/C28H19N3O5/c32-27-18-5-1-2-6-20(18)30-25-19(27)14-31(26(25)16-7-8-22-24(12-16)35-15-34-22)28(33)23-10-9-21(36-23)17-4-3-11-29-13-17/h1-13,26H,14-15H2,(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50133070
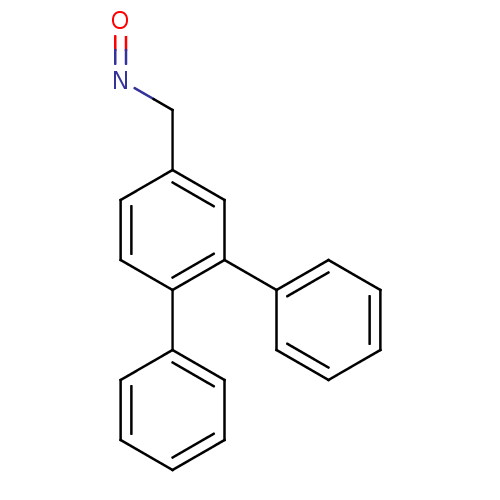
(CHEMBL335465 | [1,1';2',1'']Terphenyl-4'-carbaldeh...)Show InChI InChI=1S/C19H15NO/c21-20-14-15-11-12-18(16-7-3-1-4-8-16)19(13-15)17-9-5-2-6-10-17/h1-13H,14H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Pisa
Curated by ChEMBL
| Assay Description
Relative binding affinity for human estrogen receptor alpha compared to [3H]-estradiol |
J Med Chem 46: 4032-42 (2003)
Article DOI: 10.1021/jm0308390
BindingDB Entry DOI: 10.7270/Q27W6BKS |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163581

(2-[2,3'']Bipyridinyl-6''-yl-3-(2,3-dihydro-benzofu...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ccc(cn1)-c1ccccn1 Show InChI InChI=1S/C29H22N4O2/c34-29-21-5-1-2-7-24(21)32-27-22(29)17-33(28(27)19-8-10-25-18(15-19)12-14-35-25)26-11-9-20(16-31-26)23-6-3-4-13-30-23/h1-11,13,15-16,28H,12,14,17H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM50395423
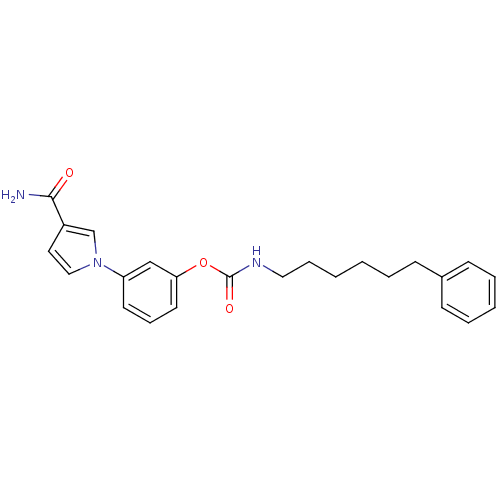
(CHEMBL2165084)Show SMILES NC(=O)c1ccn(c1)-c1cccc(OC(=O)NCCCCCCc2ccccc2)c1 Show InChI InChI=1S/C24H27N3O3/c25-23(28)20-14-16-27(18-20)21-12-8-13-22(17-21)30-24(29)26-15-7-2-1-4-9-19-10-5-3-6-11-19/h3,5-6,8,10-14,16-18H,1-2,4,7,9,15H2,(H2,25,28)(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... |
J Med Chem 55: 6898-915 (2012)
Article DOI: 10.1021/jm300689c
BindingDB Entry DOI: 10.7270/Q2ZK5HSK |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598724

(CHEMBL5170592)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CCOC(F)F)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029963
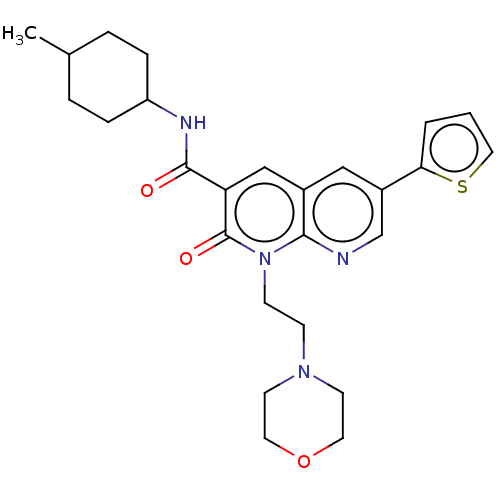
(CHEMBL3353452)Show SMILES CC1CCC(CC1)NC(=O)c1cc2cc(cnc2n(CCN2CCOCC2)c1=O)-c1cccs1 |(26.44,-2.21,;25.11,-2.98,;25.11,-4.52,;23.79,-5.29,;22.45,-4.52,;22.44,-2.99,;23.77,-2.21,;21.12,-5.3,;19.78,-4.54,;19.77,-3,;18.45,-5.31,;17.12,-4.55,;15.79,-5.33,;14.46,-4.56,;13.13,-5.33,;13.13,-6.88,;14.46,-7.65,;15.79,-6.87,;17.13,-7.64,;17.13,-9.17,;18.46,-9.94,;18.47,-11.48,;17.14,-12.25,;17.14,-13.78,;18.47,-14.56,;19.81,-13.79,;19.81,-12.24,;18.46,-6.86,;19.8,-7.63,;11.79,-4.56,;10.38,-5.19,;9.35,-4.05,;10.12,-2.71,;11.63,-3.03,)| Show InChI InChI=1S/C26H32N4O3S/c1-18-4-6-21(7-5-18)28-25(31)22-16-19-15-20(23-3-2-14-34-23)17-27-24(19)30(26(22)32)9-8-29-10-12-33-13-11-29/h2-3,14-18,21H,4-13H2,1H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163570

(2-[5-(3-Benzyl-3H-imidazol-4-yl)-pyridin-2-yl]-3-(...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ccc(cn1)-c1cncn1Cc1ccccc1 Show InChI InChI=1S/C34H27N5O2/c40-34-26-8-4-5-9-28(26)37-32-27(34)20-39(33(32)24-10-12-30-23(16-24)14-15-41-30)31-13-11-25(17-36-31)29-18-35-21-38(29)19-22-6-2-1-3-7-22/h1-13,16-18,21,33H,14-15,19-20H2,(H,37,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50131925
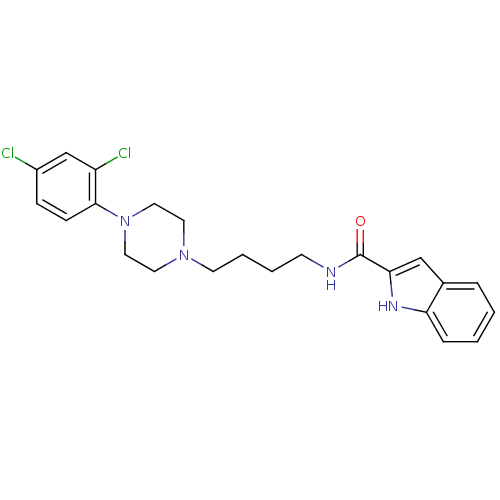
(1H-Indole-2-carboxylic acid {4-[4-(2,4-dichloro-ph...)Show SMILES Clc1ccc(N2CCN(CCCCNC(=O)c3cc4ccccc4[nH]3)CC2)c(Cl)c1 Show InChI InChI=1S/C23H26Cl2N4O/c24-18-7-8-22(19(25)16-18)29-13-11-28(12-14-29)10-4-3-9-26-23(30)21-15-17-5-1-2-6-20(17)27-21/h1-2,5-8,15-16,27H,3-4,9-14H2,(H,26,30) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Displacement of [3H]-7-OH-DPAT from rat brain membrane D3 receptor expressed in Sf9 cells incubated for 60 mins by liquid scintillation counting meth... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111674
BindingDB Entry DOI: 10.7270/Q2FR012J |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50029958
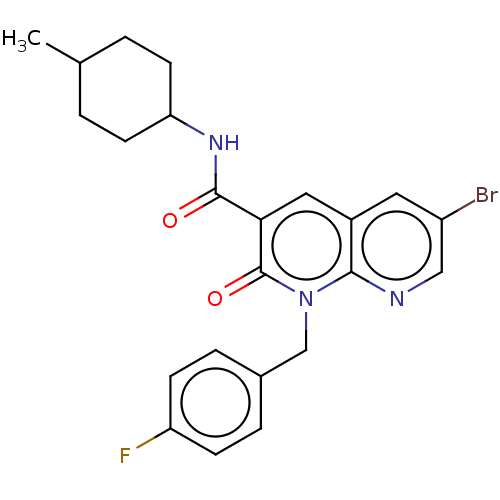
(CHEMBL3353439)Show SMILES CC1CCC(CC1)NC(=O)c1cc2cc(Br)cnc2n(Cc2ccc(F)cc2)c1=O |(16.02,-5.47,;14.69,-6.25,;14.69,-7.79,;13.37,-8.56,;12.03,-7.79,;12.02,-6.26,;13.35,-5.48,;10.7,-8.57,;9.36,-7.8,;9.35,-6.26,;8.03,-8.58,;6.69,-7.82,;5.37,-8.6,;4.03,-7.83,;2.7,-8.6,;1.37,-7.83,;2.7,-10.15,;4.04,-10.92,;5.37,-10.14,;6.7,-10.91,;6.71,-12.45,;8.04,-13.21,;8.04,-14.75,;9.37,-15.52,;10.71,-14.74,;12.04,-15.51,;10.7,-13.19,;9.36,-12.43,;8.04,-10.13,;9.38,-10.9,)| Show InChI InChI=1S/C23H23BrFN3O2/c1-14-2-8-19(9-3-14)27-22(29)20-11-16-10-17(24)12-26-21(16)28(23(20)30)13-15-4-6-18(25)7-5-15/h4-7,10-12,14,19H,2-3,8-9,13H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa
Curated by ChEMBL
| Assay Description
HEK293DHEK293iHEK293sHEK293pHEK293lHEK293aHEK293cHEK293eHEK293mHEK293eHEK293nHEK293tHEK293 HEK293oHEK293fHEK293 HEK293[HEK2933HEK293HHEK293]HEK293CHE... |
J Med Chem 57: 8777-91 (2014)
Article DOI: 10.1021/jm500807e
BindingDB Entry DOI: 10.7270/Q2QC054S |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM50395424
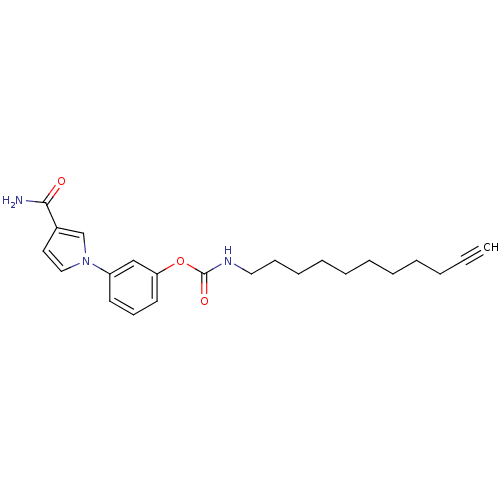
(CHEMBL2165083)Show SMILES NC(=O)c1ccn(c1)-c1cccc(OC(=O)NCCCCCCCCCC#C)c1 Show InChI InChI=1S/C23H29N3O3/c1-2-3-4-5-6-7-8-9-10-15-25-23(28)29-21-13-11-12-20(17-21)26-16-14-19(18-26)22(24)27/h1,11-14,16-18H,3-10,15H2,(H2,24,27)(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena
Curated by ChEMBL
| Assay Description
Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... |
J Med Chem 55: 6898-915 (2012)
Article DOI: 10.1021/jm300689c
BindingDB Entry DOI: 10.7270/Q2ZK5HSK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690
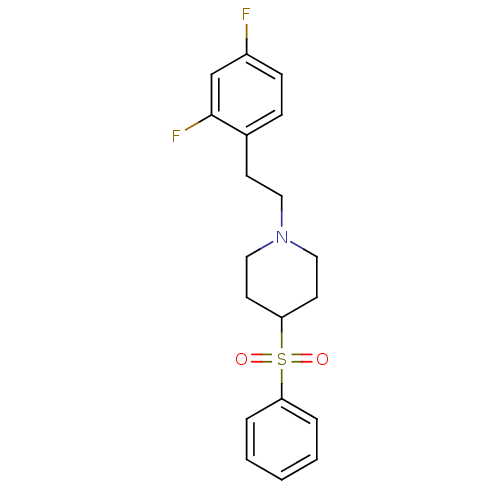
(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 45: 492-503 (2002)
BindingDB Entry DOI: 10.7270/Q2KH0P27 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50108690

(1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...)Show SMILES Fc1ccc(CCN2CCC(CC2)S(=O)(=O)c2ccccc2)c(F)c1 Show InChI InChI=1S/C19H21F2NO2S/c20-16-7-6-15(19(21)14-16)8-11-22-12-9-18(10-13-22)25(23,24)17-4-2-1-3-5-17/h1-7,14,18H,8-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Binding affinity against rat 5-hydroxytryptamine 2A receptor |
Bioorg Med Chem Lett 15: 3665-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.104
BindingDB Entry DOI: 10.7270/Q2HM580P |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50205787

((E)-5-fluoro-2-(2-(5-(2-fluorophenylsulfonyl)pyrid...)Show SMILES Oc1cc(F)ccc1\C=C\c1ccc(cn1)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C19H13F2NO3S/c20-14-7-5-13(18(23)11-14)6-8-15-9-10-16(12-22-15)26(24,25)19-4-2-1-3-17(19)21/h1-12,23H/b8-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human 5HT2A receptor expressed in CHO cells |
Bioorg Med Chem Lett 17: 2643-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.098
BindingDB Entry DOI: 10.7270/Q2F47PZH |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163577

(3-Benzofuran-5-yl-2-(5-pyridin-2-yl-pyrimidin-2-yl...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2occc2c1)c1ncc(cn1)-c1ccccn1 Show InChI InChI=1S/C28H19N5O2/c34-27-20-5-1-2-7-23(20)32-25-21(27)16-33(26(25)18-8-9-24-17(13-18)10-12-35-24)28-30-14-19(15-31-28)22-6-3-4-11-29-22/h1-15,26H,16H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320373

((E)-3-(4-(2,4-difluorostyryl)phenylsulfonyl)benzam...)Show SMILES NC(=O)c1cccc(c1)S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2F)cc1 Show InChI InChI=1S/C21H15F2NO3S/c22-17-9-8-15(20(23)13-17)7-4-14-5-10-18(11-6-14)28(26,27)19-3-1-2-16(12-19)21(24)25/h1-13H,(H2,24,25)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM17659

((R,S)-2-phosphonomethylpentanedioic acid | 2-(phos...)Show InChI InChI=1S/C6H11O7P/c7-5(8)2-1-4(6(9)10)3-14(11,12)13/h4H,1-3H2,(H,7,8)(H,9,10)(H2,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against glutamate carboxypeptidase II (GCP II) using N-acetyl-L-aspartyl-[3H]-L-glutamate as a substrate |
J Med Chem 46: 1989-96 (2003)
Article DOI: 10.1021/jm020515w
BindingDB Entry DOI: 10.7270/Q2SQ8ZRG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598737
(CHEMBL5205631)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](CC1CC1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50495074
(CHEMBL3098601)Show SMILES [H][C@]1(NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C\C=C/C[C@H](NC(=O)[C@H](CO)NC(=O)[C@@]([H])(NC(=O)[C@H](Cc2ccccc2)NC1=O)[C@@H](C)O)C(N)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r,c:73| Show InChI InChI=1S/C78H107N19O18/c1-43(81)67(104)85-41-63(102)86-53-30-16-15-29-52(66(83)103)87-76(113)61(42-98)95-78(115)65(45(3)100)97-75(112)58(37-48-25-11-6-12-26-48)94-77(114)64(44(2)99)96-70(107)55(32-18-20-34-80)89-73(110)59(38-49-40-84-51-28-14-13-27-50(49)51)92-72(109)57(36-47-23-9-5-10-24-47)90-71(108)56(35-46-21-7-4-8-22-46)91-74(111)60(39-62(82)101)93-69(106)54(88-68(53)105)31-17-19-33-79/h4-16,21-28,40,43-45,52-61,64-65,84,98-100H,17-20,29-39,41-42,79-81H2,1-3H3,(H2,82,101)(H2,83,103)(H,85,104)(H,86,102)(H,87,113)(H,88,105)(H,89,110)(H,90,108)(H,91,111)(H,92,109)(H,93,106)(H,94,114)(H,95,115)(H,96,107)(H,97,112)/b16-15-/t43-,44+,45+,52-,53-,54-,55-,56-,57-,58-,59-,60-,61-,64-,65-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR5 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122969
(3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...)Show SMILES CN1CCN(CC1)C(=O)c1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C35H30N4O6/c1-37-14-16-38(17-15-37)34(41)22-8-6-21(7-9-22)27-12-13-29(45-27)35(42)39-19-25-31(36-26-5-3-2-4-24(26)33(25)40)32(39)23-10-11-28-30(18-23)44-20-43-28/h2-13,18,32H,14-17,19-20H2,1H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50370143
(CHEMBL1744059)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)[C@H]1c1ccc2OCOc2c1 |r| Show InChI InChI=1S/C30H24N4O5/c1-36-23-9-7-17(11-25(23)37-2)19-13-31-30(32-14-19)34-15-21-27(33-22-6-4-3-5-20(22)29(21)35)28(34)18-8-10-24-26(12-18)39-16-38-24/h3-14,28H,15-16H2,1-2H3,(H,33,35)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118249
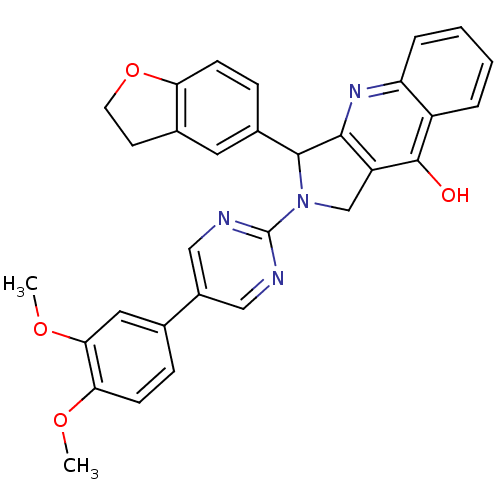
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3,4-dimethox...)Show SMILES COc1ccc(cc1OC)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCCc2c1 Show InChI InChI=1S/C31H26N4O4/c1-37-26-10-7-18(14-27(26)38-2)21-15-32-31(33-16-21)35-17-23-28(34-24-6-4-3-5-22(24)30(23)36)29(35)20-8-9-25-19(13-20)11-12-39-25/h3-10,13-16,29H,11-12,17H2,1-2H3,(H,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138930
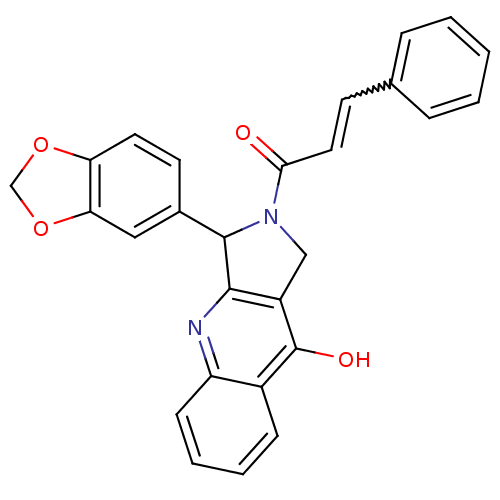
(3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-acryloyl)-1,2,...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)C=Cc1ccccc1 |w:26.31| Show InChI InChI=1S/C27H20N2O4/c30-24(13-10-17-6-2-1-3-7-17)29-15-20-25(28-21-9-5-4-8-19(21)27(20)31)26(29)18-11-12-22-23(14-18)33-16-32-22/h1-14,26H,15-16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50320376
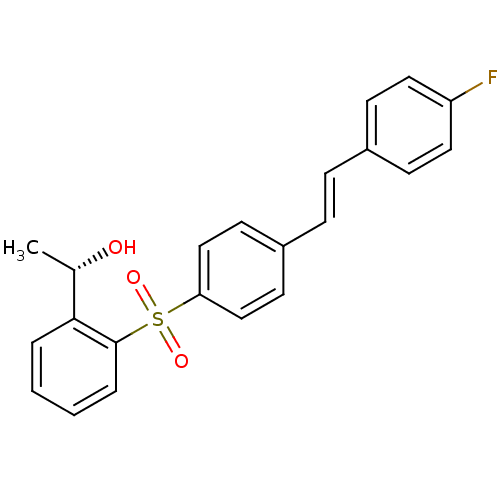
((S,E)-1-(2-(4-(4-fluorostyryl)phenylsulfonyl)pheny...)Show SMILES C[C@H](O)c1ccccc1S(=O)(=O)c1ccc(\C=C\c2ccc(F)cc2)cc1 |r| Show InChI InChI=1S/C22H19FO3S/c1-16(24)21-4-2-3-5-22(21)27(25,26)20-14-10-18(11-15-20)7-6-17-8-12-19(23)13-9-17/h2-16,24H,1H3/b7-6+/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp& Dohme
Curated by ChEMBL
| Assay Description
Displacement of [3H]- ketanserin from human 5HT2A receptor expressed CHO cells |
Bioorg Med Chem Lett 20: 3708-12 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.090
BindingDB Entry DOI: 10.7270/Q2RX9C89 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50598741

(CHEMBL5204894)Show SMILES Cn1nncc1-c1cnn(c1)[C@H](Cc1ccc(F)cc1)c1ccc(c[n+]1[O-])-c1c(F)c(Cl)ccc1-n1cc(Cl)nn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00442
BindingDB Entry DOI: 10.7270/Q2C82FBD |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163578

(3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-4-yl-...)Show SMILES Oc1c2CN(C(c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccncc1 Show InChI InChI=1S/C28H21N5O2/c34-27-21-3-1-2-4-23(21)32-25-22(27)16-33(26(25)19-5-6-24-18(13-19)9-12-35-24)28-30-14-20(15-31-28)17-7-10-29-11-8-17/h1-8,10-11,13-15,26H,9,12,16H2,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50099258
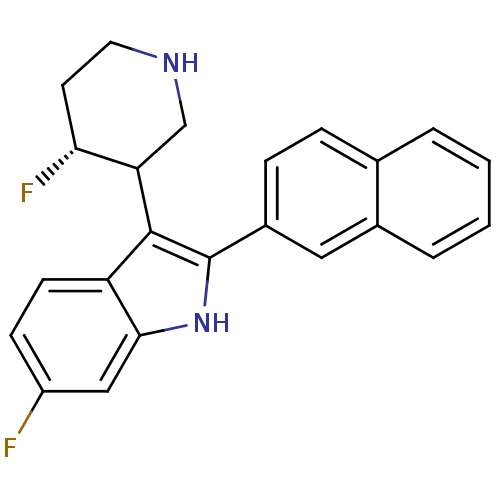
(6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-naphthalen-...)Show SMILES F[C@@H]1CCNCC1c1c([nH]c2cc(F)ccc12)-c1ccc2ccccc2c1 Show InChI InChI=1S/C23H20F2N2/c24-17-7-8-18-21(12-17)27-23(22(18)19-13-26-10-9-20(19)25)16-6-5-14-3-1-2-4-15(14)11-16/h1-8,11-12,19-20,26-27H,9-10,13H2/t19?,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme
Curated by ChEMBL
| Assay Description
Ability to displace [3H]-ketanserin binding to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells |
J Med Chem 44: 1603-14 (2001)
BindingDB Entry DOI: 10.7270/Q27M076K |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50118248
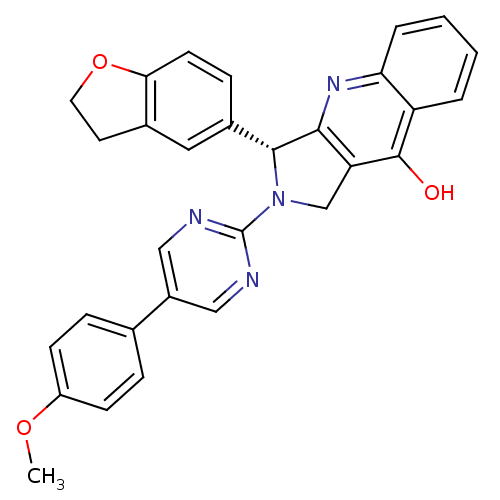
(3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...)Show SMILES COc1ccc(cc1)-c1cnc(nc1)N1Cc2c(nc3ccccc3c2O)[C@H]1c1ccc2OCCc2c1 Show InChI InChI=1S/C30H24N4O3/c1-36-22-9-6-18(7-10-22)21-15-31-30(32-16-21)34-17-24-27(33-25-5-3-2-4-23(25)29(24)35)28(34)20-8-11-26-19(14-20)12-13-37-26/h2-11,14-16,28H,12-13,17H2,1H3,(H,33,35)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Phosphodiesterase 5 activity of human corpus cavernosum |
J Med Chem 45: 4094-6 (2002)
BindingDB Entry DOI: 10.7270/Q2R49RGM |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50138939
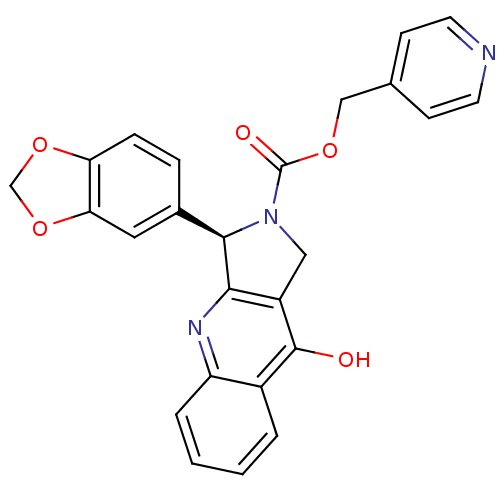
((R)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCOc2c1)C(=O)OCc1ccncc1 Show InChI InChI=1S/C25H19N3O5/c29-24-17-3-1-2-4-19(17)27-22-18(24)12-28(25(30)31-13-15-7-9-26-10-8-15)23(22)16-5-6-20-21(11-16)33-14-32-20/h1-11,23H,12-14H2,(H,27,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Phosphodiesterase 5 |
J Med Chem 47: 656-62 (2004)
Article DOI: 10.1021/jm020521s
BindingDB Entry DOI: 10.7270/Q228070W |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Mus musculus (mouse)) | BDBM50528930
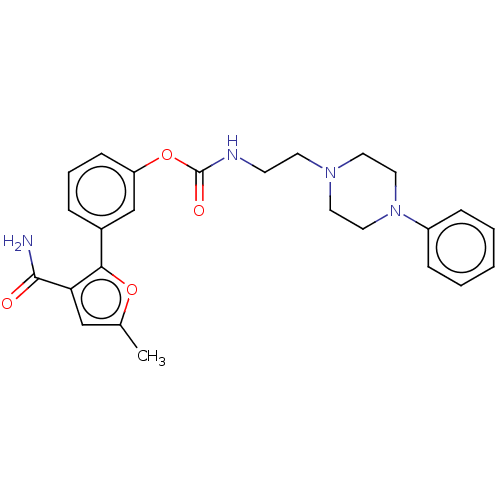
(CHEMBL4471658)Show SMILES Cc1cc(C(N)=O)c(o1)-c1cccc(OC(=O)NCCN2CCN(CC2)c2ccccc2)c1 Show InChI InChI=1S/C25H28N4O4/c1-18-16-22(24(26)30)23(32-18)19-6-5-9-21(17-19)33-25(31)27-10-11-28-12-14-29(15-13-28)20-7-3-2-4-8-20/h2-9,16-17H,10-15H2,1H3,(H2,26,30)(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Siena
Curated by ChEMBL
| Assay Description
Inhibition of FAAH in mouse brain membranes assessed as inhibitory constant using [14C]-AEA as substrate incubated for 15 mins by scintillation count... |
Eur J Med Chem 183: (2019)
Article DOI: 10.1016/j.ejmech.2019.111674
BindingDB Entry DOI: 10.7270/Q2FR012J |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM81767

(15-28-Somatostatin-28 | CAS_38916-34-6 | CB6417646...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@H](NC(=O)[C@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)[C@@H](C)O |r| Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42+,43+,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,62-,63-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Research in Biomedicine (IRB Barcelona)
Curated by ChEMBL
| Assay Description
Displacement of [125I]-somatostatin from human SSTR5 expressed in CHO-K1 cells |
Bioorg Med Chem Lett 24: 103-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.11.065
BindingDB Entry DOI: 10.7270/Q24X5BR2 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50122990
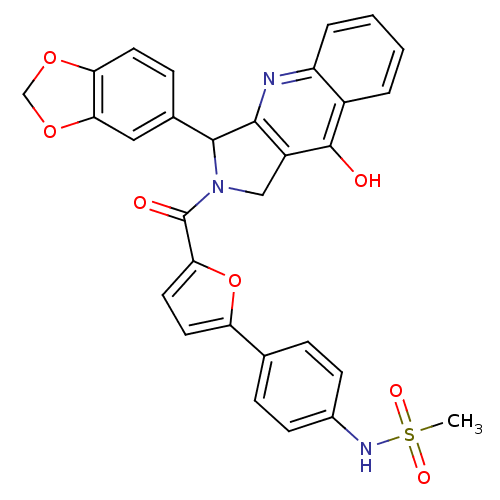
(CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...)Show SMILES CS(=O)(=O)Nc1ccc(cc1)-c1ccc(o1)C(=O)N1Cc2c(nc3ccccc3c2O)C1c1ccc2OCOc2c1 Show InChI InChI=1S/C30H23N3O7S/c1-41(36,37)32-19-9-6-17(7-10-19)23-12-13-25(40-23)30(35)33-15-21-27(31-22-5-3-2-4-20(22)29(21)34)28(33)18-8-11-24-26(14-18)39-16-38-24/h2-14,28,32H,15-16H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated |
J Med Chem 46: 441-4 (2003)
Article DOI: 10.1021/jm0202573
BindingDB Entry DOI: 10.7270/Q2QR4WHQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data