

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210938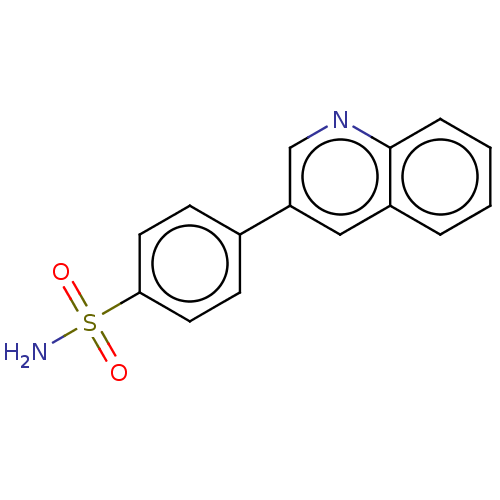 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 [A65S,N67Q,E69T,I91L,F131V,K170E,L204A] (Homo sapiens (Human)) | BDBM210935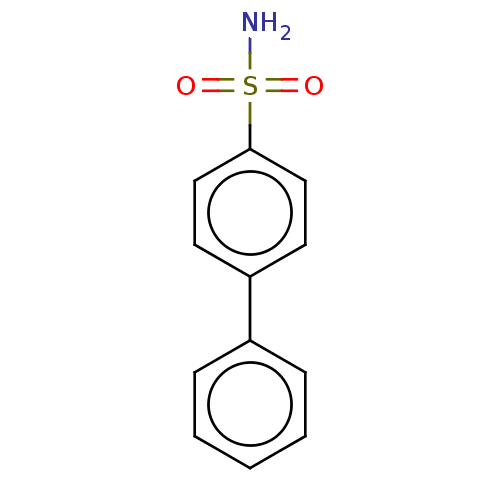 (4-(phenyl)-bezenesulfonamide (4a)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50334361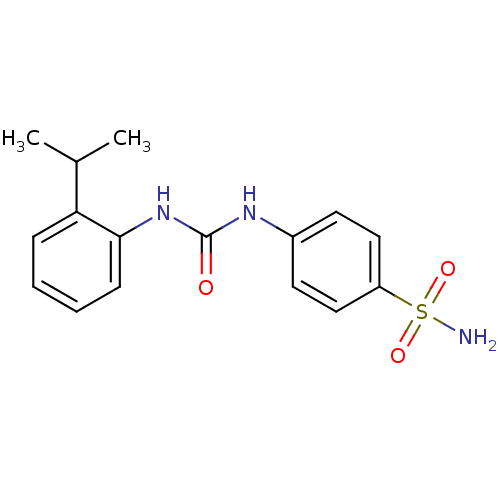 (4-(3-(2-isopropylphenyl)ureido)benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM210937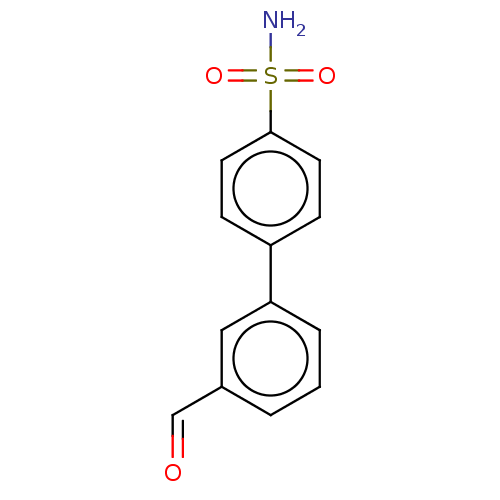 (4-(3-formylphenyl)-benzenesulfonamide (4e)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210935 (4-(phenyl)-bezenesulfonamide (4a)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50334349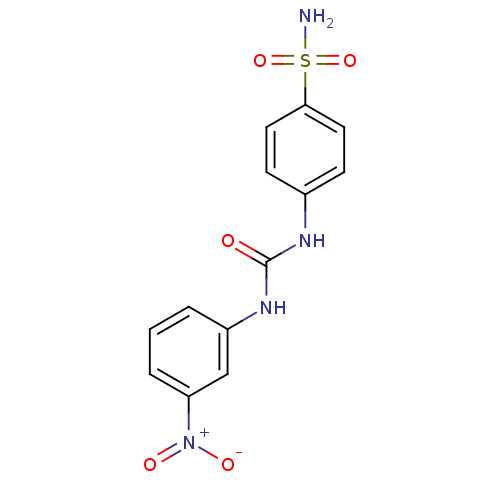 (4-(3-(3-nitrophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50028265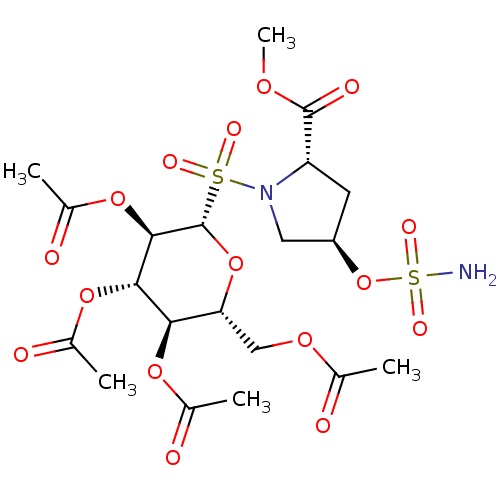 (CHEMBL3342252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50028265 (CHEMBL3342252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 expressed in Escherichia coli by stopped flow CO2 hydration assay | J Med Chem 58: 6630-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00845 BindingDB Entry DOI: 10.7270/Q24F1SHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50028308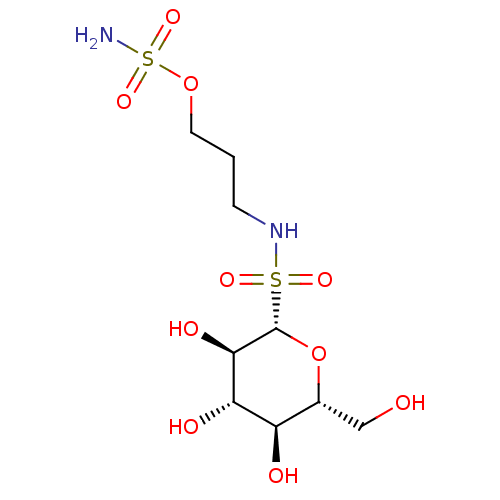 (CHEMBL3342254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210936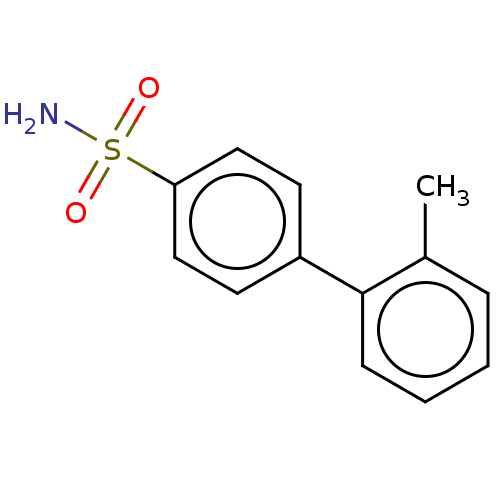 (4-(2-methylphenyl)-bezenesulfonamide (4b)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210937 (4-(3-formylphenyl)-benzenesulfonamide (4e)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-2 by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028268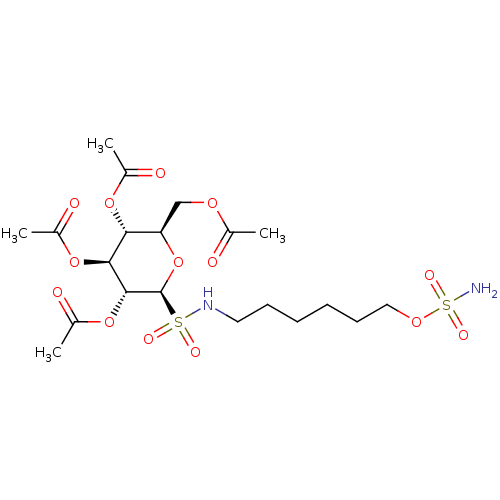 (CHEMBL3342249) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028267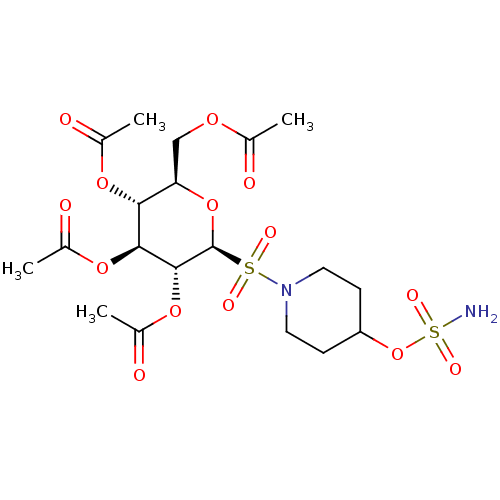 (CHEMBL3342250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028266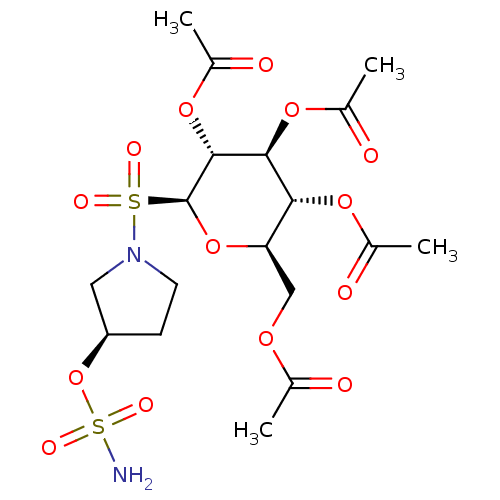 (CHEMBL3342251) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50108106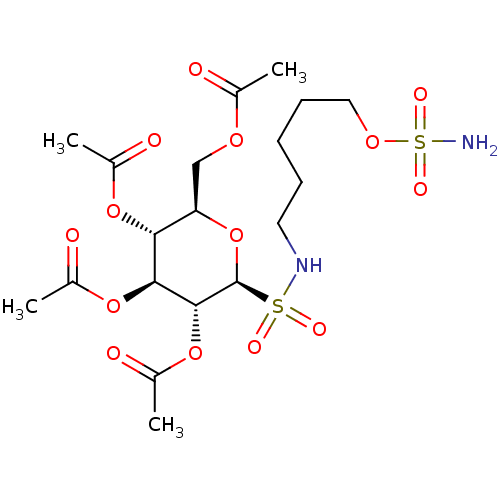 (CHEMBL3601602) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 expressed in Escherichia coli by stopped flow CO2 hydration assay | J Med Chem 58: 6630-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00845 BindingDB Entry DOI: 10.7270/Q24F1SHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028279 (CHEMBL3342255) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028271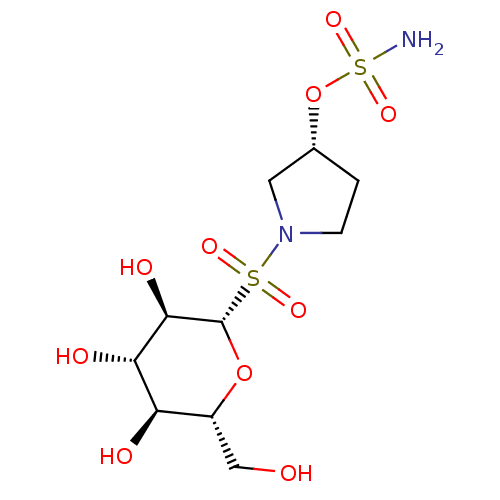 (CHEMBL3342257) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028265 (CHEMBL3342252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028265 (CHEMBL3342252) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 expressed in Escherichia coli by stopped flow CO2 hydration assay | J Med Chem 58: 6630-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00845 BindingDB Entry DOI: 10.7270/Q24F1SHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028267 (CHEMBL3342250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant CA9 expressed in Escherichia coli by stopped flow CO2 hydration assay | J Med Chem 58: 6630-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00845 BindingDB Entry DOI: 10.7270/Q24F1SHZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50028308 (CHEMBL3342254) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10875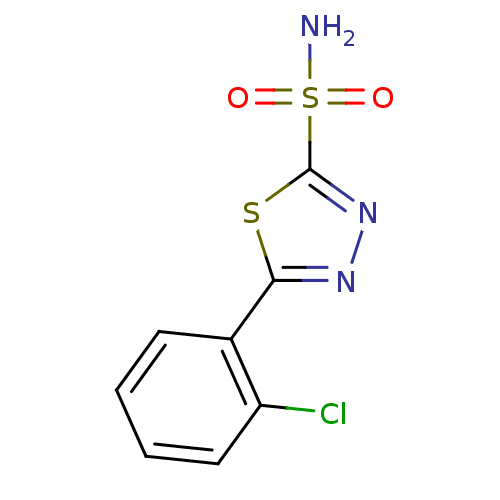 (5-(2-chlorophenyl)-1,3,4-thiadiazole-2-sulfonamide...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM210938 (4-(3-quinolinyl)-benzenesulfonamide (4p)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10886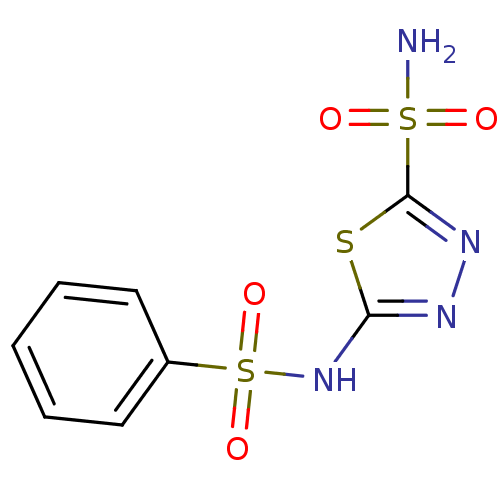 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-2 by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50334361 (4-(3-(2-isopropylphenyl)ureido)benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50143994 (CHEMBL3759678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10885 ((4R)-4-(ethylamino)-2-(3-methoxypropyl)-1,1-dioxo-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50334361 (4-(3-(2-isopropylphenyl)ureido)benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50334354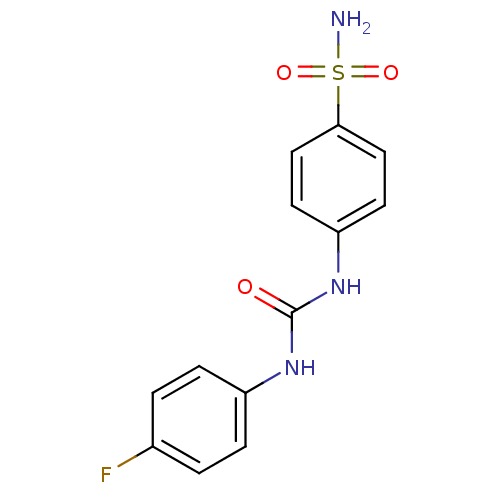 (4-(3-(4-fluorophenyl)ureido)benzenesulfonamide | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10869 (5-imino-4-methyl-4,5-dihydro-1,3,4-thiadiazole-2-s...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50028308 (CHEMBL3342254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 by stopped-flow CO2 hydration assay | J Med Chem 57: 8635-45 (2014) Article DOI: 10.1021/jm5012935 BindingDB Entry DOI: 10.7270/Q2QF8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50146756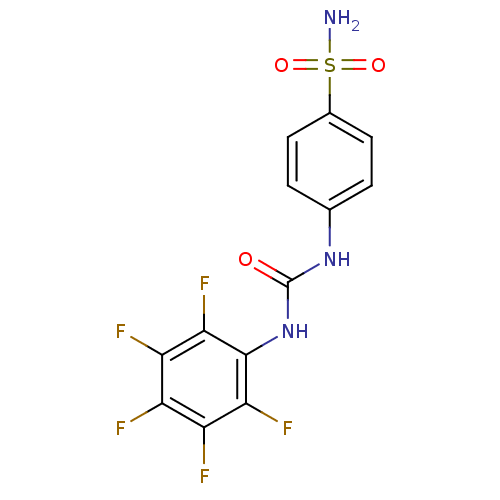 (4-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM13063 (4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50146756 (4-(3-Pentafluorophenyl-ureido)-benzenesulfonamide ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 9 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase, alpha family (Thiomicrospira crunogena (strain XCL-2)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50334349 (4-(3-(3-nitrophenyl)ureido)benzenesulfonamide | 4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 12 for 15 mins by stopped flow CO2 hydration assay | Bioorg Med Chem 24: 976-81 (2016) Article DOI: 10.1016/j.bmc.2016.01.019 BindingDB Entry DOI: 10.7270/Q2445P9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida | Assay Description CA-catalyzed CO2 hydration activity methods were published previously by Cornelio et al. as part of a larger series of benzenesulfonamide-based inhib... | Chembiochem 18: 213-222 (2017) Article DOI: 10.1002/cbic.201600513 BindingDB Entry DOI: 10.7270/Q27H1HFC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sassari Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 12 catalytic domain preincubated for 15 mins prior to testing measured for 10 to 100 secs by pheno... | ACS Med Chem Lett 8: 941-946 (2017) Article DOI: 10.1021/acsmedchemlett.7b00229 BindingDB Entry DOI: 10.7270/Q2ZK5K8P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human recombinant CA12 expressed in Escherichia coli by stopped flow CO2 hydration assay | J Med Chem 58: 6630-8 (2015) Article DOI: 10.1021/acs.jmedchem.5b00845 BindingDB Entry DOI: 10.7270/Q24F1SHZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10870 (2-N-(4-aminobenzene)-1,3,4-thiadiazole-2,5-disulfo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase-1 by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10873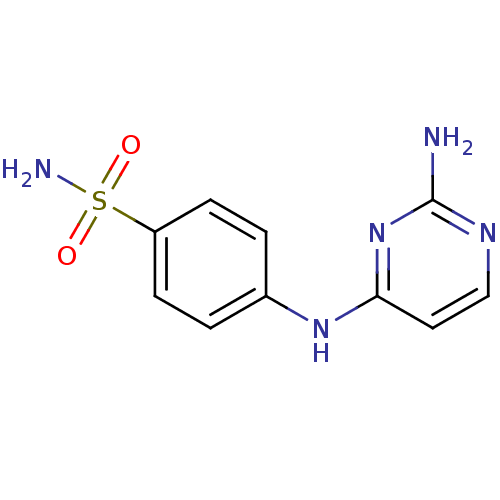 (4-[(2-aminopyrimidin-4-yl)amino]benzene-1-sulfonam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase (Sulfurihydrogenibium sp. (strain YO3AOP1)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assay | Bioorg Med Chem Lett 26: 401-5 (2016) Article DOI: 10.1016/j.bmcl.2015.11.104 BindingDB Entry DOI: 10.7270/Q2DB83QG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 326 total ) | Next | Last >> |