

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH2B adapter protein 2 (Human) | BDBM484440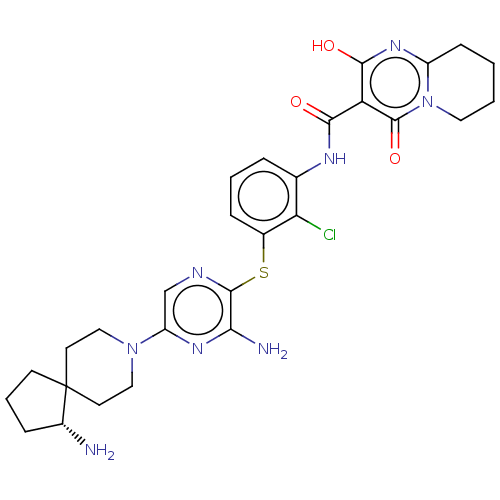 (US10934285, Example 24) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350832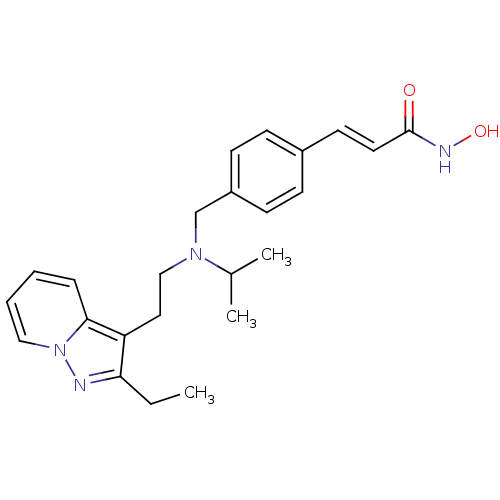 (CHEMBL1819274) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350831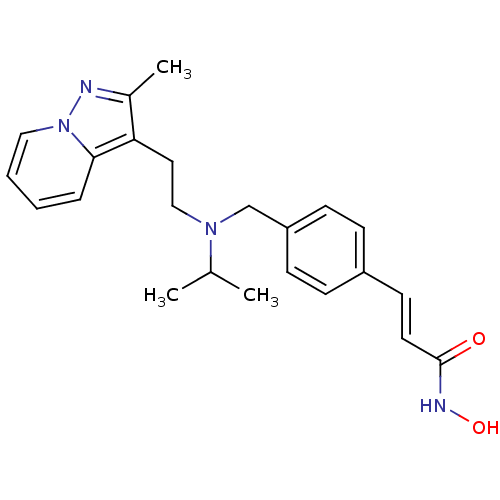 (CHEMBL1819273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50531540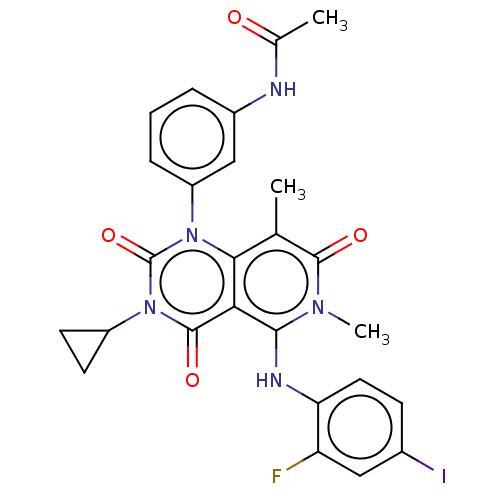 (CHEBI:75998 | GSK-1120212 | GSK1120212 | JTP 74057...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MEK in human KYSE-520 cells assessed as reduction in p-ERK levels | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350818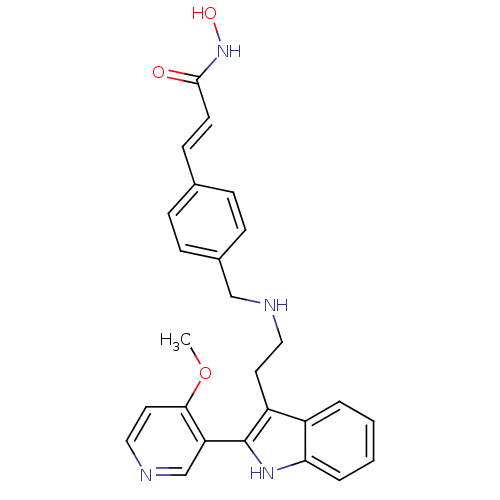 (CHEMBL1819257) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484457 (US10934285, Example 43) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350827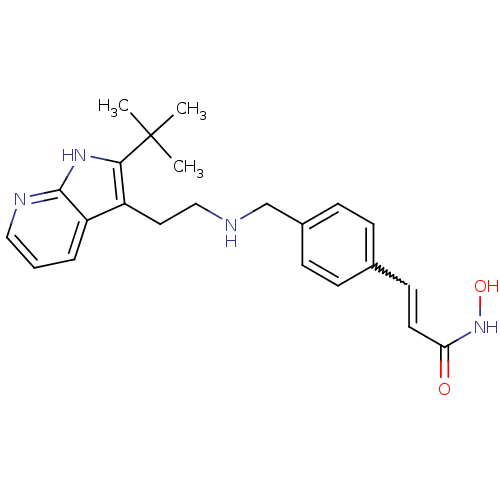 (CHEMBL1819267) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350835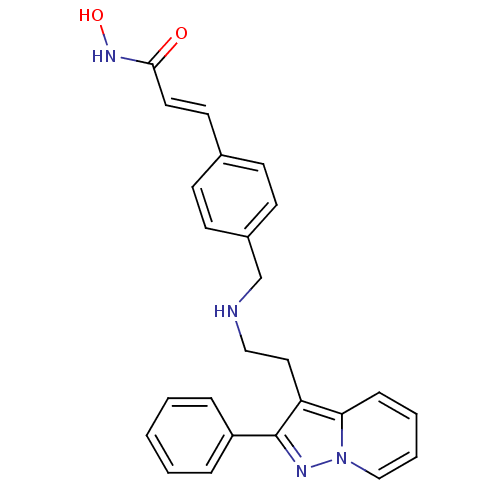 (CHEMBL1819272) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350820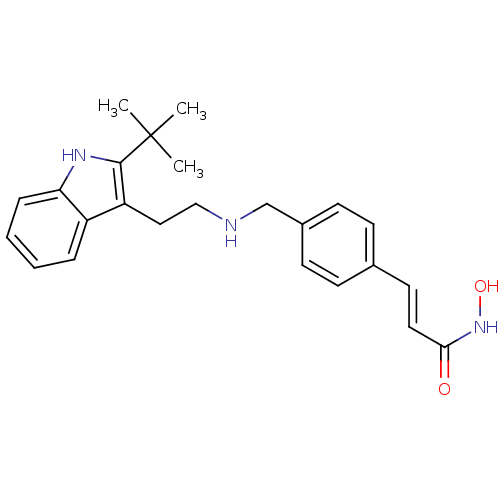 (CHEMBL1819260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484469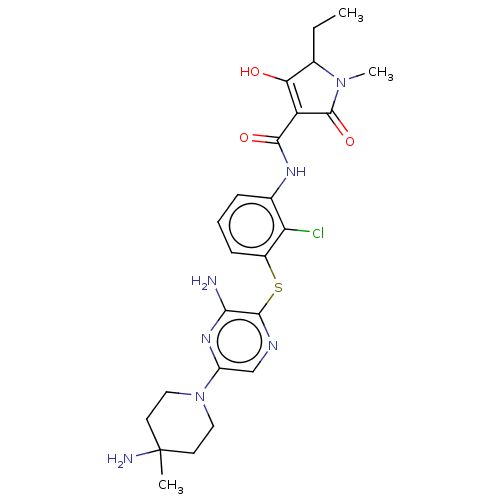 (US10934285, Example 55) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484472 (US10934285, Example 59) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484461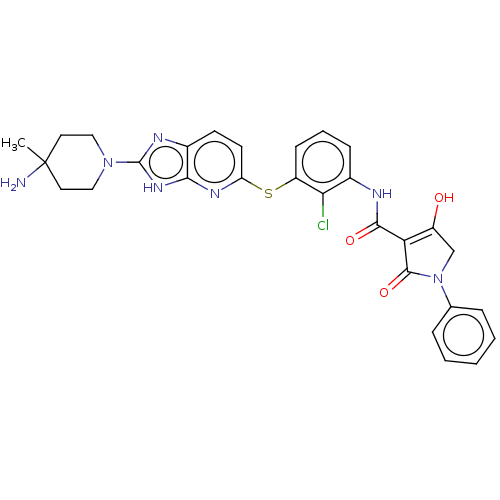 (US10934285, Example 47) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484466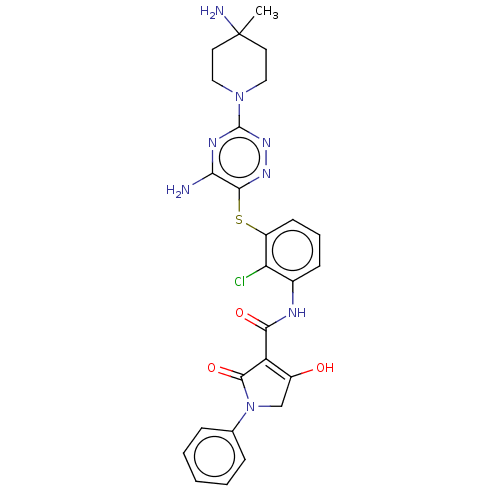 (US10934285, Example 52) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350833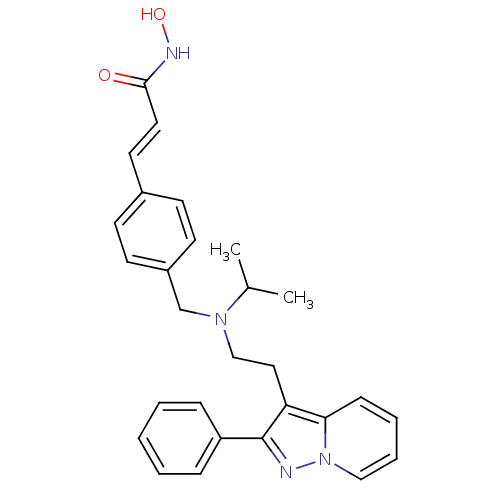 (CHEMBL1819275) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350830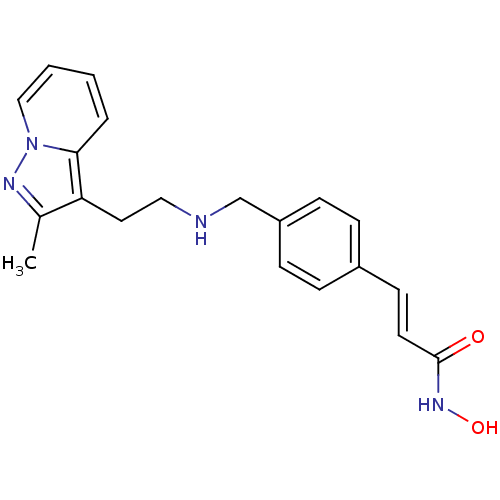 (CHEMBL1819270) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484421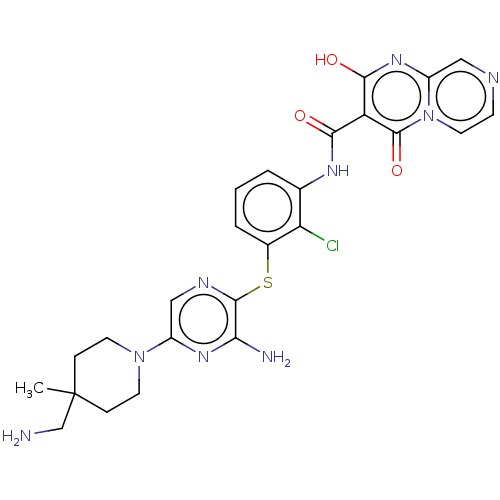 (US10934285, Example 5) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Wnt-3a (Homo sapiens (Human)) | BDBM50439791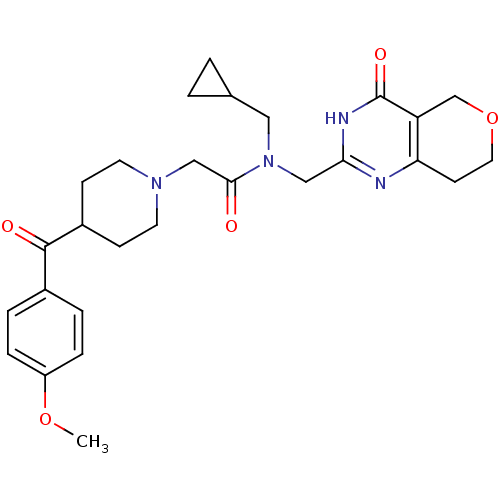 (CHEMBL2419706 | US9181266, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolin | J Med Chem 56: 6495-511 (2013) Article DOI: 10.1021/jm400807n BindingDB Entry DOI: 10.7270/Q24Q7WFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein Wnt-3a (Homo sapiens (Human)) | BDBM50439799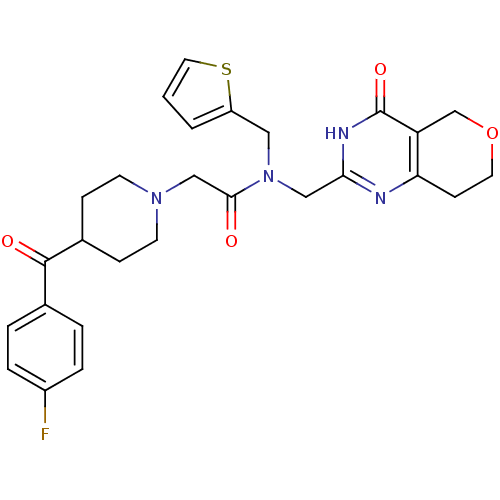 (CHEMBL2419698 | US9181266, 13) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of WNT3A signaling in HEK293 cells by luciferase reporter gene assay in presence of forskolin | J Med Chem 56: 6495-511 (2013) Article DOI: 10.1021/jm400807n BindingDB Entry DOI: 10.7270/Q24Q7WFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350821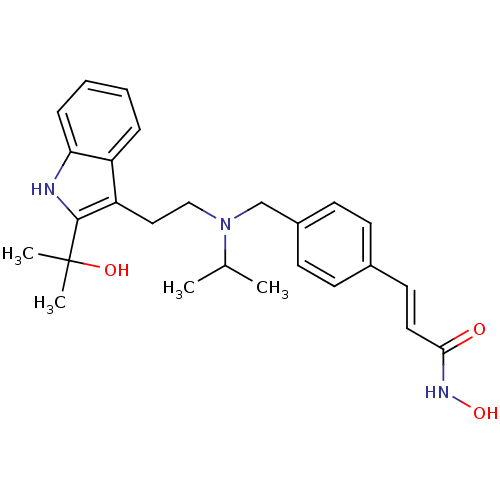 (CHEMBL1819261) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484467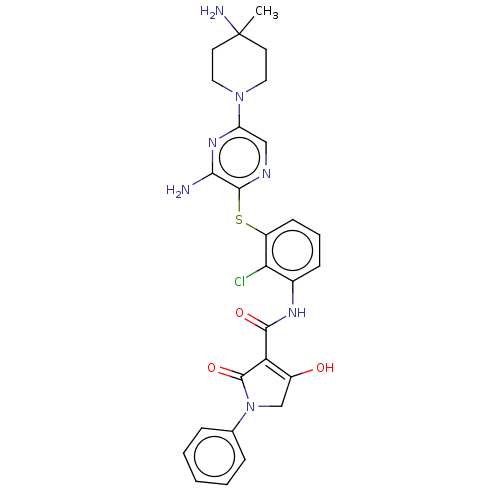 (US10934285, Example 53) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484429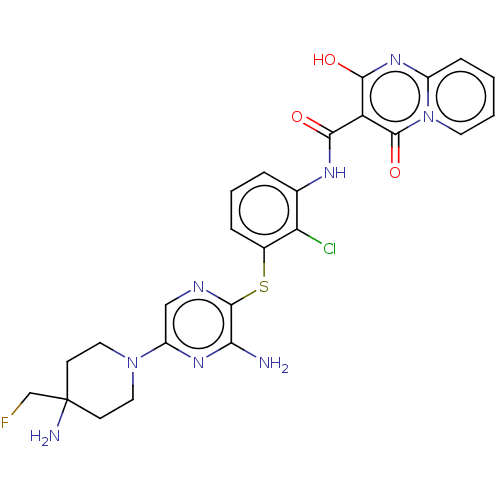 (US10934285, Example 13) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350826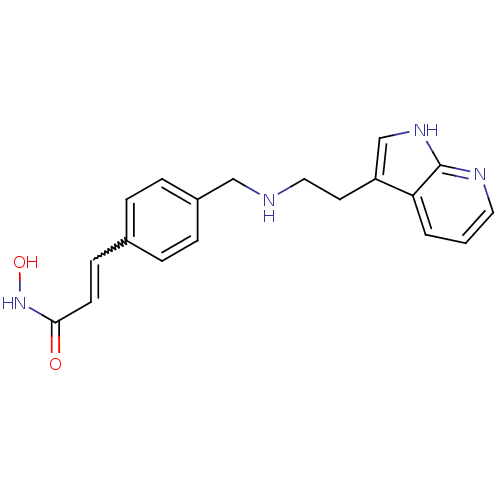 (CHEMBL1819266) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484422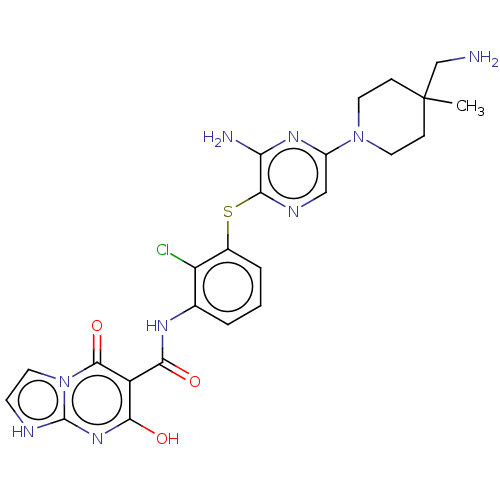 (US10934285, Example 6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484439 (US10934285, Example 23) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484430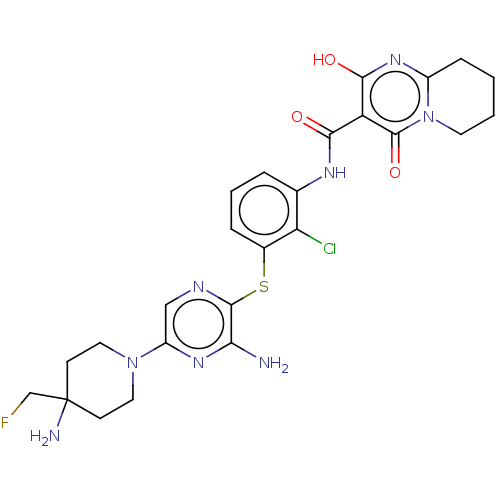 (US10934285, Example 14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484419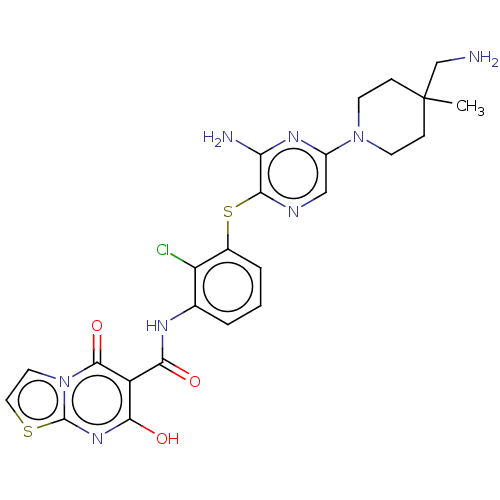 (US10934285, Example 3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350829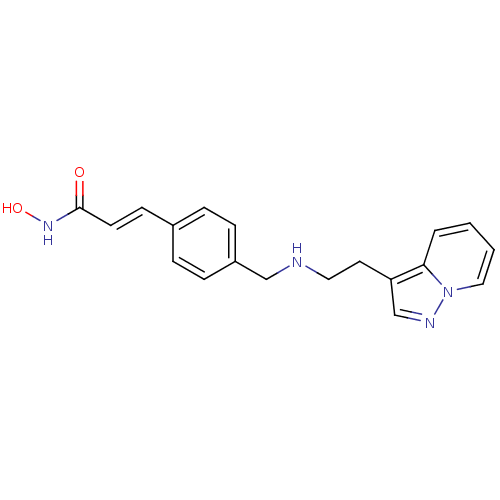 (CHEMBL1819269) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484451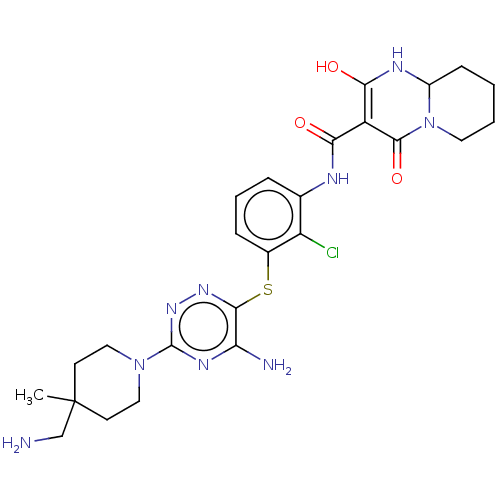 (US10934285, Example 37) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484426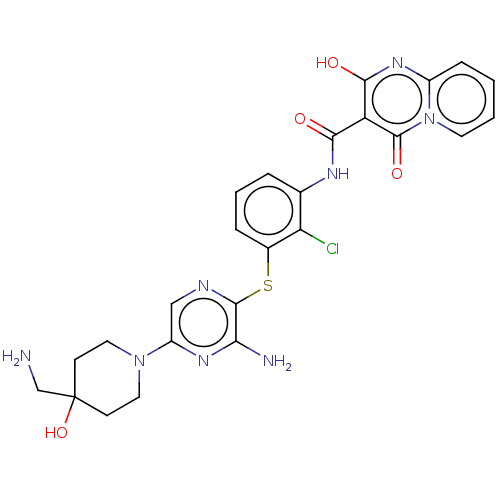 (US10934285, Example 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484423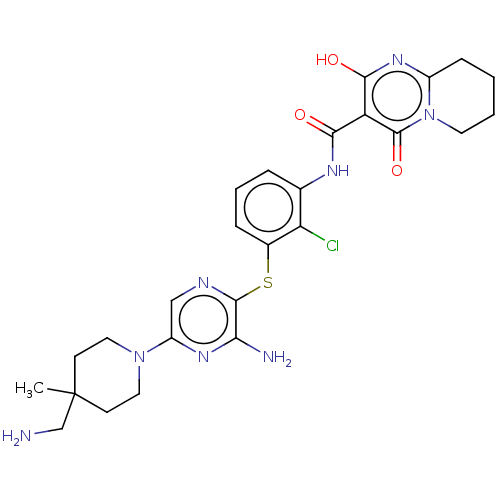 (US10934285, Example 7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350838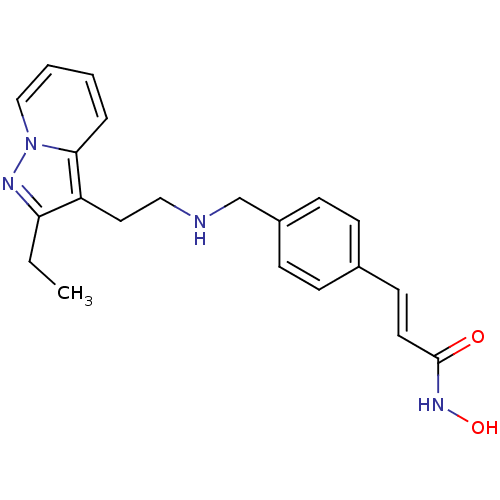 (CHEMBL1819271) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484470 (US10934285, Example 56) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484418 (US10934285, Example 2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484425 (US10934285, Example 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408067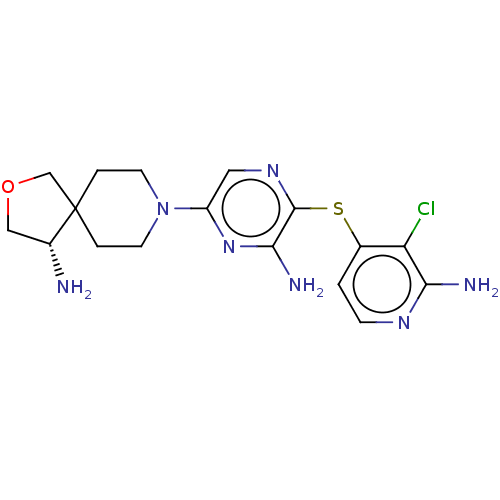 (US10336774, Example 52) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484459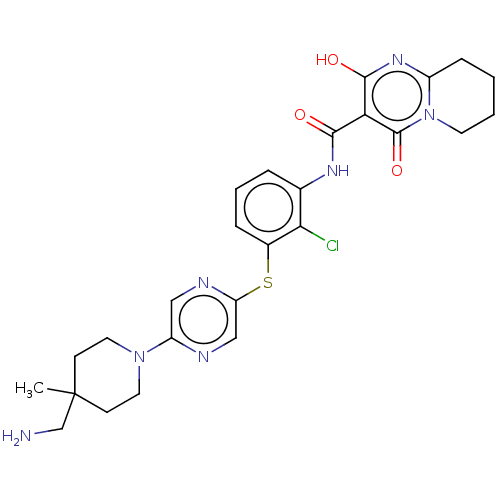 (US10934285, Example 45) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484468 (US10934285, Example 54) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350824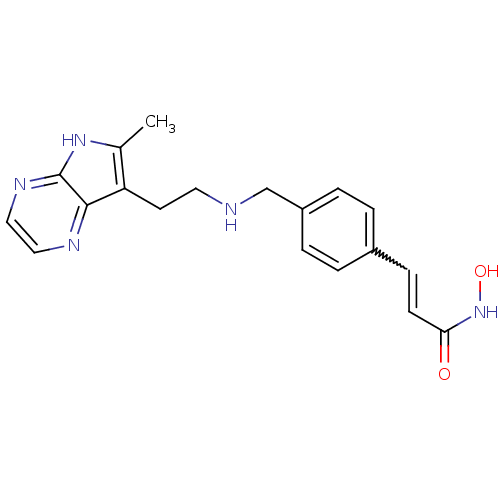 (CHEMBL1819264) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484446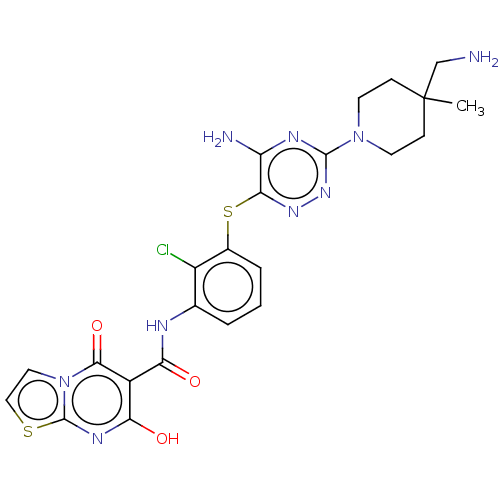 (US10934285, Example 32) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484432 (US10934285, Example 16) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50539763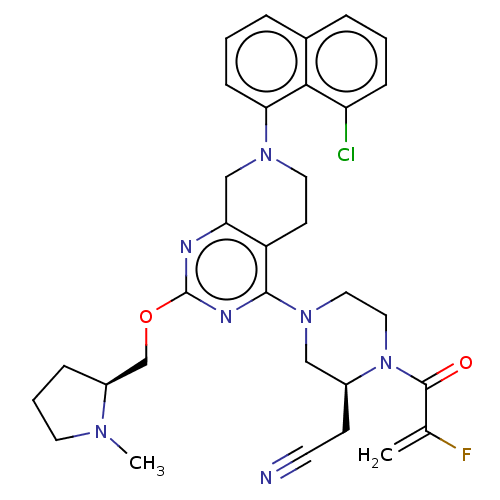 (Adagrasib | Mrtx-849 | Mrtx849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of KRAS G12C mutant in human MIA PaCa-2 cells assessed as reduction in p-ERK levels | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484438 (US10934285, Example 22) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350828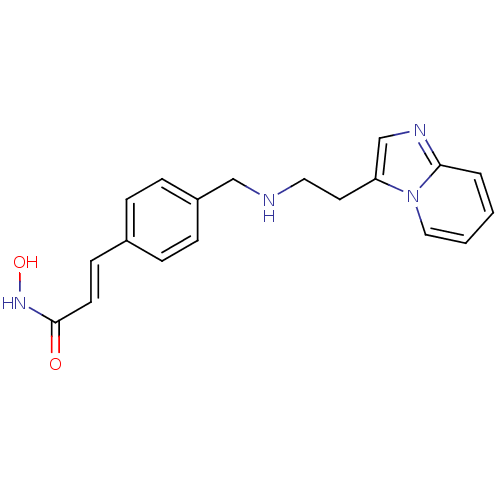 (CHEMBL1819268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484447 (US10934285, Example 33) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484431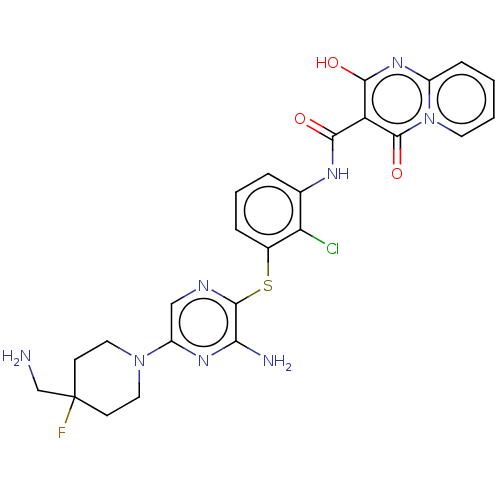 (US10934285, Example 15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553790 (CHEMBL4763213) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM50553783 (Ptpn11 inhibitor tno155 | Shp2 inhibitor tno155 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Allosteric inhibition of 6x-histidine tagged human SHP2 (Met1-L525 residues) expressed in Escherichia coli BL21 Star (DE3) using IRS1_pY1172(dPEG8)pY... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50350822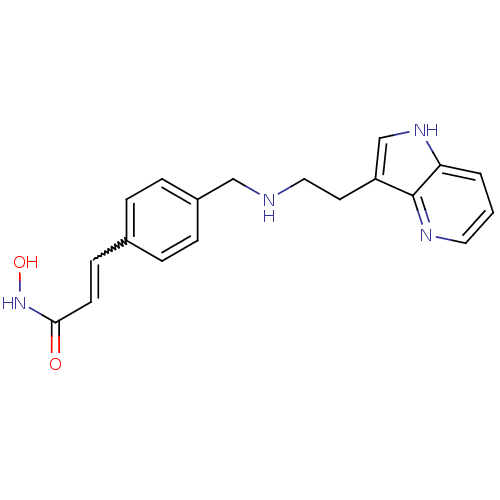 (CHEMBL1819262) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of HDAC1 | J Med Chem 54: 4752-72 (2011) Article DOI: 10.1021/jm200388e BindingDB Entry DOI: 10.7270/Q23N24DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484455 (US10934285, Example 41) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| SH2B adapter protein 2 (Human) | BDBM484471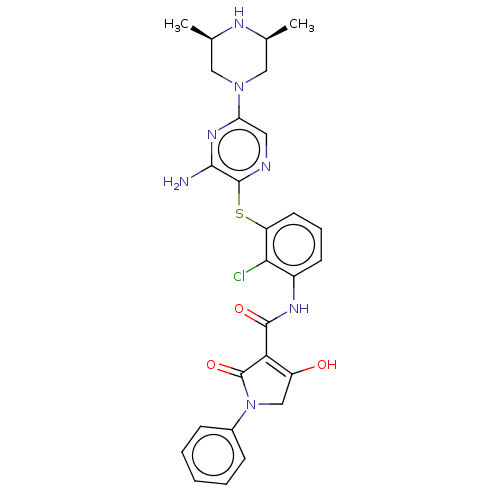 (US10934285, Example 57) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The inhibition of SHP2 by compounds of the invention (concentrations varying from 0.003-100 μM) was monitored using an assay in which 0.5 nM of ... | US Patent US10934285 (2021) BindingDB Entry DOI: 10.7270/Q2MK6H10 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 548 total ) | Next | Last >> |