 Found 1055 hits with Last Name = 'makishima' and Initial = 'm'
Found 1055 hits with Last Name = 'makishima' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50428854
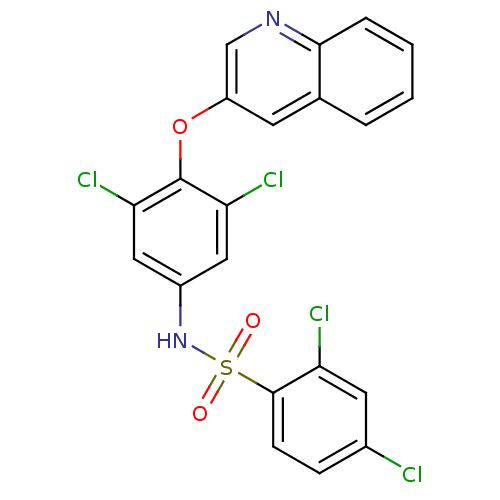
(CHEMBL1236924)Show SMILES Clc1ccc(c(Cl)c1)S(=O)(=O)Nc1cc(Cl)c(Oc2cnc3ccccc3c2)c(Cl)c1 Show InChI InChI=1S/C21H12Cl4N2O3S/c22-13-5-6-20(16(23)8-13)31(28,29)27-14-9-17(24)21(18(25)10-14)30-15-7-12-3-1-2-4-19(12)26-11-15/h1-11,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Displacement of [3H]rosiglitazone from PPARgamma (unknown origin) |
Bioorg Med Chem 24: 5455-5461 (2016)
Article DOI: 10.1016/j.bmc.2016.08.067
BindingDB Entry DOI: 10.7270/Q21V5JGB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339081
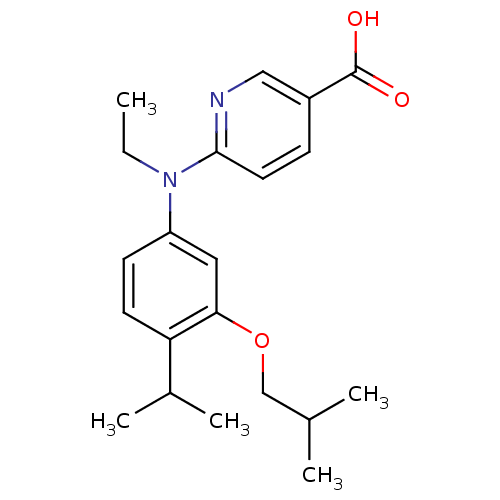
(6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(C)C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H28N2O3/c1-6-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(15(4)5)19(11-17)26-13-14(2)3/h7-12,14-15H,6,13H2,1-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50339081

(6-[Ethyl-(3-isobutoxy-4-isopropylphenyl)amino]nico...)Show SMILES CCN(c1ccc(C(C)C)c(OCC(C)C)c1)c1ccc(cn1)C(O)=O Show InChI InChI=1S/C21H28N2O3/c1-6-23(20-10-7-16(12-22-20)21(24)25)17-8-9-18(15(4)5)19(11-17)26-13-14(2)3/h7-12,14-15H,6,13H2,1-5H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50530499

(CHEMBL4449685)Show SMILES Cc1cc2c(cc1-c1cc3cc(C(O)=O)c(=O)oc3cc1O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H26O5/c1-13-8-18-19(25(4,5)7-6-24(18,2)3)11-15(13)16-9-14-10-17(22(27)28)23(29)30-21(14)12-20(16)26/h8-12,26H,6-7H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50530499

(CHEMBL4449685)Show SMILES Cc1cc2c(cc1-c1cc3cc(C(O)=O)c(=O)oc3cc1O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C25H26O5/c1-13-8-18-19(25(4,5)7-6-24(18,2)3)11-15(13)16-9-14-10-17(22(27)28)23(29)30-21(14)12-20(16)26/h8-12,26H,6-7H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of 9-cis-[11,12-3H]-retinoic acid from human RXRalpha LBD incubated for overnight by scintillation counting method |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50566090
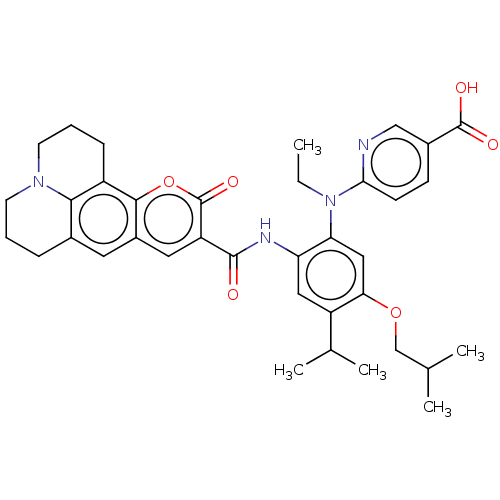
(CHEMBL4788526)Show SMILES CCN(c1ccc(cn1)C(O)=O)c1cc(OCC(C)C)c(cc1NC(=O)c1cc2cc3CCCN4CCCc(c34)c2oc1=O)C(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]9cis-RA from human RXRalpha-LBD by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01883
BindingDB Entry DOI: 10.7270/Q2959N9F |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 379 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50566089

(CHEMBL4778155)Show SMILES CCN(c1ccc(cn1)C(O)=O)c1cc(OCC(C)C)c(cc1NC(=O)c1ccccc1)C(C)C | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]9cis-RA from human RXRalpha-LBD by competitive binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01883
BindingDB Entry DOI: 10.7270/Q2959N9F |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 478 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of 6-(Ethyl-{5-isobutoxy-4-isopropyl-2-[(10-oxo-2,3,5,6-tetrahydro-1H,4H,10H-11-oxa-3a-aza-benzo[de]anthracene-9-carbonyl)-amino]-phenyl... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01883
BindingDB Entry DOI: 10.7270/Q2959N9F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 583 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50382930
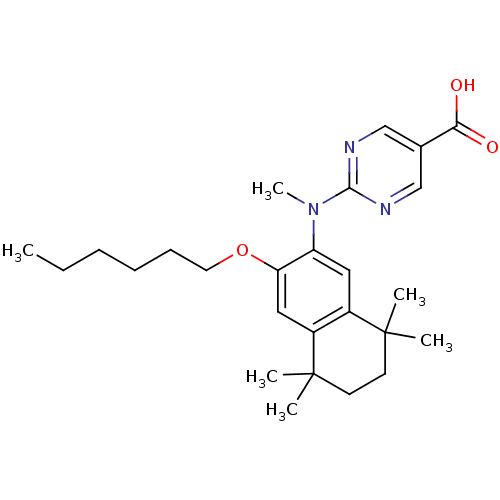
(CHEMBL115097)Show SMILES CCCCCCOc1cc2c(cc1N(C)c1ncc(cn1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H37N3O3/c1-7-8-9-10-13-32-22-15-20-19(25(2,3)11-12-26(20,4)5)14-21(22)29(6)24-27-16-18(17-28-24)23(30)31/h14-17H,7-13H2,1-6H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-9-cis retinoic acid from recombinant human RXR-alpha LBD by liquid scintillation counting based competitive ligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01354
BindingDB Entry DOI: 10.7270/Q2KP85WJ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50565932

(CHEMBL4777069)Show SMILES CCOCCOc1cc2c(cc1-n1c(nc3cc(ccc13)C(O)=O)C(F)(F)F)C(C)(C)CCC2(C)C |(58.28,-21.07,;58.28,-22.61,;56.95,-23.39,;56.96,-24.93,;55.62,-25.71,;55.63,-27.25,;54.3,-28.03,;52.96,-27.26,;51.63,-28.04,;51.63,-29.58,;52.96,-30.36,;54.3,-29.58,;55.64,-30.35,;57.05,-29.72,;58.08,-30.86,;57.31,-32.2,;57.79,-33.65,;56.77,-34.79,;55.26,-34.48,;54.79,-33.02,;55.81,-31.88,;57.26,-36.26,;56.23,-37.41,;58.77,-36.57,;57.37,-28.21,;58.83,-27.73,;56.22,-27.18,;57.75,-26.7,;50.29,-30.34,;51.05,-31.67,;49.52,-31.67,;48.97,-29.57,;48.97,-28.03,;50.31,-27.27,;49.53,-25.93,;51.08,-25.93,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-9-cis retinoic acid from recombinant human RXR-alpha LBD by liquid scintillation counting based competitive ligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01354
BindingDB Entry DOI: 10.7270/Q2KP85WJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50253371
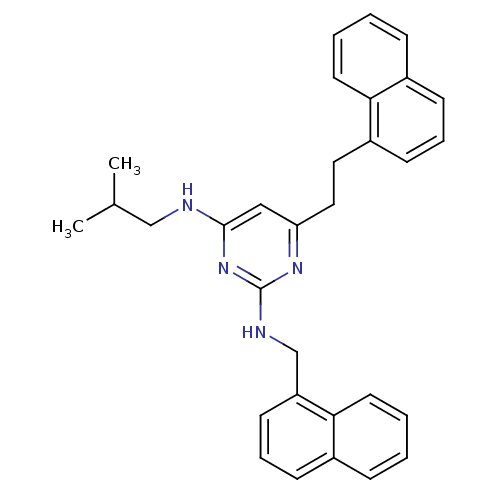
(CHEMBL522172 | N4-Isobutyl-6-(2-naphthalen-1-yl-et...)Show SMILES CC(C)CNc1cc(CCc2cccc3ccccc23)nc(NCc2cccc3ccccc23)n1 Show InChI InChI=1S/C31H32N4/c1-22(2)20-32-30-19-27(18-17-25-13-7-11-23-9-3-5-15-28(23)25)34-31(35-30)33-21-26-14-8-12-24-10-4-6-16-29(24)26/h3-16,19,22H,17-18,20-21H2,1-2H3,(H2,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of AR |
Bioorg Med Chem Lett 20: 1712-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.079
BindingDB Entry DOI: 10.7270/Q2TM7B76 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50313149

(6-(2-benzylpyridin-4-yl)-3-hydroxy-5-isobutyl-1-(n...)Show SMILES CC(C)Cc1cc(O)c(=O)n(Cc2ccc3ccccc3c2)c1-c1ccnc(Cc2ccccc2)c1 Show InChI InChI=1S/C32H30N2O2/c1-22(2)16-28-20-30(35)32(36)34(21-24-12-13-25-10-6-7-11-26(25)17-24)31(28)27-14-15-33-29(19-27)18-23-8-4-3-5-9-23/h3-15,17,19-20,22,35H,16,18,21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of ER |
Bioorg Med Chem Lett 20: 1712-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.079
BindingDB Entry DOI: 10.7270/Q2TM7B76 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50210259
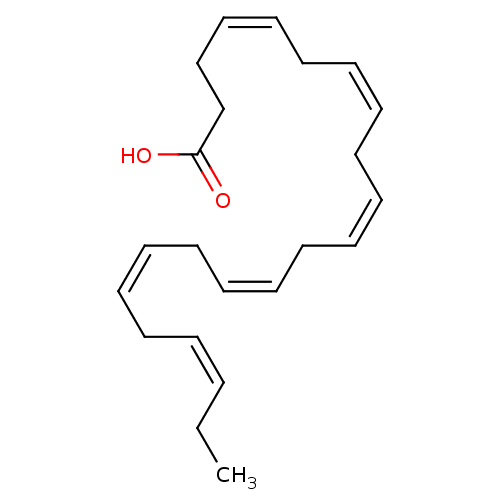
((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O Show InChI InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50210259

((4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hex...)Show SMILES CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCC(O)=O Show InChI InChI=1S/C22H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22(23)24/h3-4,6-7,9-10,12-13,15-16,18-19H,2,5,8,11,14,17,20-21H2,1H3,(H,23,24)/b4-3-,7-6-,10-9-,13-12-,16-15-,19-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50242349

((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...)Show InChI InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50242349

((5Z,8Z,11Z,14Z,17Z)-5,8,11,14,17-eicosapentaenoic ...)Show InChI InChI=1S/C20H30O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20(21)22/h3-4,6-7,9-10,12-13,15-16H,2,5,8,11,14,17-19H2,1H3,(H,21,22)/b4-3-,7-6-,10-9-,13-12-,16-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Okayama University Graduate School of Medicine
Curated by ChEMBL
| Assay Description
Displacement of CU-6PMN from human RXRalpha LBD incubated for 2 hrs by fluorescence based assay |
J Med Chem 62: 8809-8818 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00995
BindingDB Entry DOI: 10.7270/Q2G44TRN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50313148
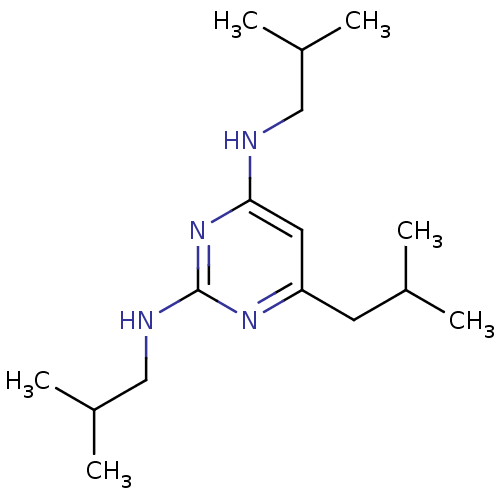
(CHEMBL1087884 | N2,N4,6-triisobutylpyrimidine-2,4-...)Show InChI InChI=1S/C16H30N4/c1-11(2)7-14-8-15(17-9-12(3)4)20-16(19-14)18-10-13(5)6/h8,11-13H,7,9-10H2,1-6H3,(H2,17,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Inhibition of ER |
Bioorg Med Chem Lett 20: 1712-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.079
BindingDB Entry DOI: 10.7270/Q2TM7B76 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Transrepression of VP16-tagged VDR (unknown origin) expressed in HEK293 cells harboring pCMX-GAL4-NCoR and MH100(UAS) X 4tk-LUC reporter plasmid asse... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50121317
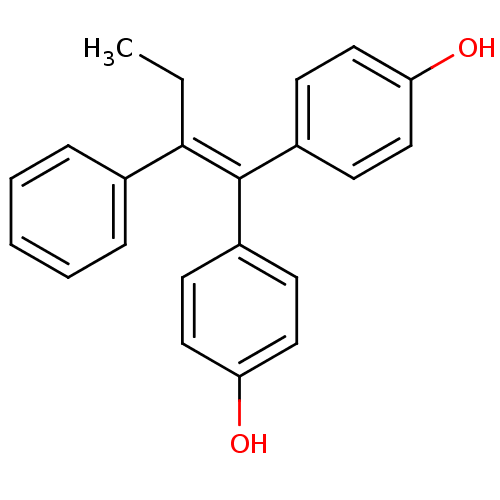
(1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...)Show SMILES [#6]-[#6]\[#6](=[#6](\c1ccc(-[#8])cc1)-c1ccc(-[#8])cc1)-c1ccccc1 Show InChI InChI=1S/C22H20O2/c1-2-21(16-6-4-3-5-7-16)22(17-8-12-19(23)13-9-17)18-10-14-20(24)15-11-18/h3-15,23-24H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human ER alpha ligand-binding domain by TR-FRET assay |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity measured after 24 hrs |
ACS Med Chem Lett 9: 641-645 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00058
BindingDB Entry DOI: 10.7270/Q2PN986S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50135039
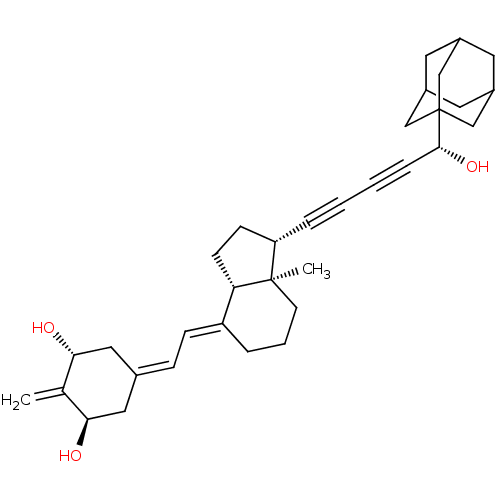
(CHEMBL3745849)Show SMILES [H][C@@]12[#6]-[#6]-[#6@H](C#CC#C[#6@@H](-[#8])C34[#6]-[#6]-5-[#6]-[#6](-[#6]-[#6](-[#6]-5)-[#6]3)-[#6]4)[C@@]1([#6])[#6]-[#6]-[#6]\[#6]2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C34H44O3/c1-22-30(35)17-23(18-31(22)36)9-10-27-6-5-13-33(2)28(11-12-29(27)33)7-3-4-8-32(37)34-19-24-14-25(20-34)16-26(15-24)21-34/h9-10,24-26,28-32,35-37H,1,5-6,11-21H2,2H3/b27-10+/t24?,25?,26?,28-,29-,30+,31+,32+,33+,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Agonist activity at VDR (unknown origin) expressed in HEK293 cells cotransfected with NCoR assessed as decrease in NCoR recruitment by two-hybrid ass... |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50281390
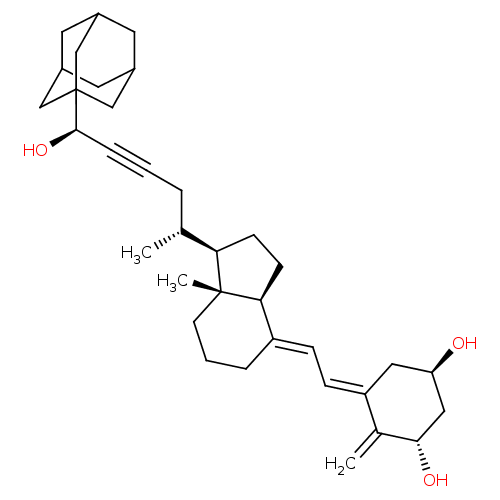
(CHEMBL4159525)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)CC#C[C@@H](O)C12CC3CC(CC(C3)C1)C2 |r,TLB:33:34:32.31.37:38,THB:35:34:31:37.36.38,35:36:33.34.39:31,33:32:34.39.35:38| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-24-14-25(20-35)16-26(15-24)21-35)30-11-12-31-27(7-5-13-34(30,31)3)9-10-28-17-29(36)18-32(37)23(28)2/h9-10,22,24-26,29-33,36-38H,2,5-7,11-21H2,1,3H3/b27-9+,28-10-/t22-,24?,25?,26?,29-,30-,31+,32+,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Transrepression of VP16-tagged VDR (unknown origin) expressed in HEK293 cells harboring pCMX-GAL4-NCoR and MH100(UAS) X 4tk-LUC reporter plasmid asse... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Agonist activity at VDR (unknown origin) expressed in HEK293 cells cotransfected with NCoR assessed as decrease in NCoR recruitment by two-hybrid ass... |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as reduction in E2-induced ER-alpha-mediated transcrip... |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50281390

(CHEMBL4159525)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)CC#C[C@@H](O)C12CC3CC(CC(C3)C1)C2 |r,TLB:33:34:32.31.37:38,THB:35:34:31:37.36.38,35:36:33.34.39:31,33:32:34.39.35:38| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-24-14-25(20-35)16-26(15-24)21-35)30-11-12-31-27(7-5-13-34(30,31)3)9-10-28-17-29(36)18-32(37)23(28)2/h9-10,22,24-26,29-33,36-38H,2,5-7,11-21H2,1,3H3/b27-9+,28-10-/t22-,24?,25?,26?,29-,30-,31+,32+,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [26,27-Methyl-3H]-1,25(OH)2D3 from recombinant human GST-tagged VDR LBD (140 to 427 residues) expressed in Escherichia coli BL21 prei... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at human PRB expressed in African green monkey CV1 cells |
ACS Med Chem Lett 9: 641-645 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00058
BindingDB Entry DOI: 10.7270/Q2PN986S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50200182

((1S,3R,5Z,7E)-9,10-secocholesta-5,7,10(19)-triene-...)Show SMILES C[C@H](CCCC(C)(C)O)[C@H]1CC[C@H]2\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C |r| Show InChI InChI=1S/C27H44O3/c1-18(8-6-14-26(3,4)30)23-12-13-24-20(9-7-15-27(23,24)5)10-11-21-16-22(28)17-25(29)19(21)2/h10-11,18,22-25,28-30H,2,6-9,12-17H2,1,3-5H3/b20-10+,21-11-/t18-,22-,23-,24+,25+,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from recombinant human VDR ligand binding domain |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50015318
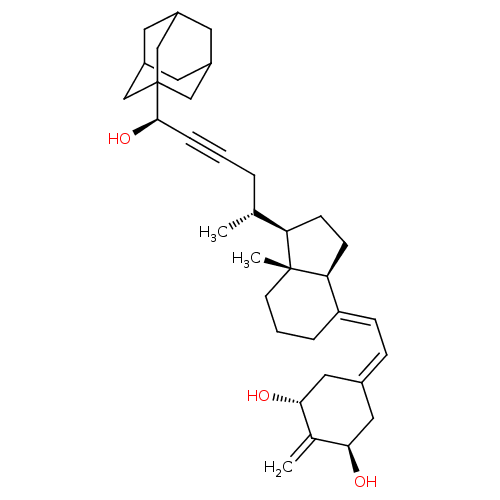
(CHEMBL3263871)Show SMILES [H][C@@]1([#6]-[#6][C@@]2([H])\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1\[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1)[#6@H](-[#6])-[#6]C#C[#6@@H](-[#8])C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2 |r,THB:35:34:31:37.36.38,35:36:33.34.39:31,38:36:33:39.30.31,38:30:33:37.35.36| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-25-14-26(20-35)16-27(15-25)21-35)29-11-12-30-28(7-5-13-34(29,30)3)10-9-24-17-31(36)23(2)32(37)18-24/h9-10,22,25-27,29-33,36-38H,2,5-7,11-21H2,1,3H3/b28-10+/t22-,25?,26?,27?,29-,30+,31-,32-,33-,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from GST-fused human VDR-LBD expressed in Escherichia coli BL21 |
J Med Chem 57: 4073-87 (2014)
Article DOI: 10.1021/jm401989c
BindingDB Entry DOI: 10.7270/Q2ZS2Z2G |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50238741
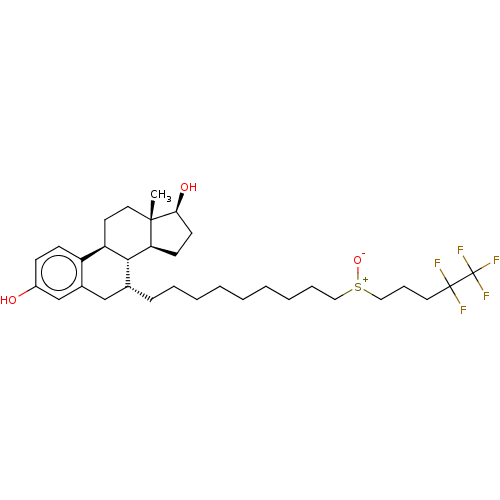
(CHEBI:31638 | Faslodex | Fulvestrant | ICI-182780 ...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@]1([H])c3ccc(O)cc3C[C@@H](CCCCCCCCC[S+]([O-])CCCC(F)(F)C(F)(F)F)[C@@]21[H] Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as reduction in E2-induced ER-alpha-mediated transcrip... |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
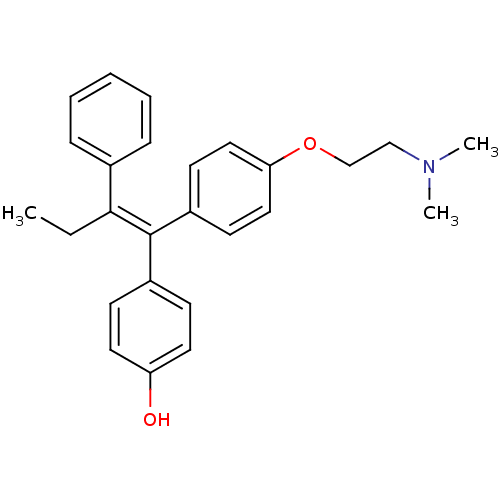
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as reduction in E2-induced ER-alpha-mediated transcrip... |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at human GR assessed as reduction in dexamethasone-induced transactivation |
ACS Med Chem Lett 9: 641-645 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00058
BindingDB Entry DOI: 10.7270/Q2PN986S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437046

(CHEMBL2403356)Show InChI InChI=1S/C21H28O2/c1-5-11-21(12-6-2,17-7-9-19(22)15(3)13-17)18-8-10-20(23)16(4)14-18/h7-10,13-14,22-23H,5-6,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human ER alpha ligand-binding domain by TR-FRET assay |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437049

(CHEMBL2403355)Show InChI InChI=1S/C19H24O2/c1-5-19(6-2,15-7-9-17(20)13(3)11-15)16-8-10-18(21)14(4)12-16/h7-12,20-21H,5-6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human ER alpha ligand-binding domain by TR-FRET assay |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50523937

(CHEMBL4473971)Show InChI InChI=1S/C21H29NO/c1-5-11-21(12-6-2,17-7-9-19(22)15(3)13-17)18-8-10-20(23)16(4)14-18/h7-10,13-14,23H,5-6,11-12,22H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human ER alpha ligand-binding domain by TR-FRET assay |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50281389

(CHEMBL4173602)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)CC#C[C@H](O)C12CC3CC(CC(C3)C1)C2 |r,TLB:33:34:32.31.37:38,THB:35:34:31:37.36.38,35:36:33.34.39:31,33:32:34.39.35:38| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-24-14-25(20-35)16-26(15-24)21-35)30-11-12-31-27(7-5-13-34(30,31)3)9-10-28-17-29(36)18-32(37)23(28)2/h9-10,22,24-26,29-33,36-38H,2,5-7,11-21H2,1,3H3/b27-9+,28-10-/t22-,24?,25?,26?,29-,30-,31+,32+,33+,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Transrepression of VP16-tagged VDR (unknown origin) expressed in HEK293 cells harboring pCMX-GAL4-NCoR and MH100(UAS) X 4tk-LUC reporter plasmid asse... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608

(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged human ER alpha ligand-binding domain by TR-FRET assay |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50292705

((25R)-25-Adamantyl-1alpha,25-dihydroxy-2-methylene...)Show SMILES [#6]-[#6@@H](\[#6]=[#6]\[#6]-[#6@@H](-[#8])C12[#6]-[#6]-3-[#6]-[#6](-[#6]-[#6](-[#6]-3)-[#6]1)-[#6]2)-[#6@H]1-[#6]-[#6]-[#6@H]2\[#6](-[#6]-[#6]-[#6][C@]12[#6])=[#6]\[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:10:11:9.8.14:15,THB:12:11:8:14.13.15,12:13:10.11.16:8,10:9:11.16.12:15| Show InChI InChI=1S/C35H52O3/c1-22(6-4-8-33(38)35-19-25-14-26(20-35)16-27(15-25)21-35)29-11-12-30-28(7-5-13-34(29,30)3)10-9-24-17-31(36)23(2)32(37)18-24/h4,6,9-10,22,25-27,29-33,36-38H,2,5,7-8,11-21H2,1,3H3/b6-4+,28-10+/t22-,25?,26?,27?,29+,30-,31+,32+,33+,34+,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University
Curated by ChEMBL
| Assay Description
Antagonist activity at VDR expressed in COS7 cells assessed as inhibition of 1,25-Dihydroxyvitamin D3-induced response by transient transcription ass... |
J Med Chem 51: 5320-9 (2008)
Article DOI: 10.1021/jm8004477
BindingDB Entry DOI: 10.7270/Q28C9W2Z |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50281389

(CHEMBL4173602)Show SMILES [H][C@@]1(CC[C@@]2([H])\C(CCC[C@]12C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C)[C@H](C)CC#C[C@H](O)C12CC3CC(CC(C3)C1)C2 |r,TLB:33:34:32.31.37:38,THB:35:34:31:37.36.38,35:36:33.34.39:31,33:32:34.39.35:38| Show InChI InChI=1S/C35H50O3/c1-22(6-4-8-33(38)35-19-24-14-25(20-35)16-26(15-24)21-35)30-11-12-31-27(7-5-13-34(30,31)3)9-10-28-17-29(36)18-32(37)23(28)2/h9-10,22,24-26,29-33,36-38H,2,5-7,11-21H2,1,3H3/b27-9+,28-10-/t22-,24?,25?,26?,29-,30-,31+,32+,33+,34-,35?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Santiago de Compostela
Curated by ChEMBL
| Assay Description
Displacement of [26,27-Methyl-3H]-1,25(OH)2D3 from recombinant human GST-tagged VDR LBD (140 to 427 residues) expressed in Escherichia coli BL21 prei... |
J Med Chem 61: 6658-6673 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00427
BindingDB Entry DOI: 10.7270/Q2M04801 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50121317

(1,1-bis(4,4'-hydroxyphenyl)-2-phenylbut-1-ene | 4,...)Show SMILES [#6]-[#6]\[#6](=[#6](\c1ccc(-[#8])cc1)-c1ccc(-[#8])cc1)-c1ccccc1 Show InChI InChI=1S/C22H20O2/c1-2-21(16-6-4-3-5-7-16)22(17-8-12-19(23)13-9-17)18-10-14-20(24)15-11-18/h3-15,23-24H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as reduction in E2-induced ER-alpha-mediated transcrip... |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437049

(CHEMBL2403355)Show InChI InChI=1S/C19H24O2/c1-5-19(6-2,15-7-9-17(20)13(3)11-15)16-8-10-18(21)14(4)12-16/h7-12,20-21H,5-6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at human Gal4-fused ER-alpha expressed in HEK293 cells assessed as inhibition of 17beta-estradiol-induced effect by luciferase re... |
Bioorg Med Chem 22: 2244-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.025
BindingDB Entry DOI: 10.7270/Q2H996QP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437046

(CHEMBL2403356)Show InChI InChI=1S/C21H28O2/c1-5-11-21(12-6-2,17-7-9-19(22)15(3)13-17)18-8-10-20(23)16(4)14-18/h7-10,13-14,22-23H,5-6,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at human Gal4-fused ER-alpha expressed in HEK293 cells assessed as inhibition of 17beta-estradiol-induced effect by luciferase re... |
Bioorg Med Chem 22: 2244-52 (2014)
Article DOI: 10.1016/j.bmc.2014.02.025
BindingDB Entry DOI: 10.7270/Q2H996QP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50437046

(CHEMBL2403356)Show InChI InChI=1S/C21H28O2/c1-5-11-21(12-6-2,17-7-9-19(22)15(3)13-17)18-8-10-20(23)16(4)14-18/h7-10,13-14,22-23H,5-6,11-12H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
the University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at FLAG-tagged ERalpha (unknown origin) expressed in HEK293 cells assessed as reduction in E2-induced ER-alpha-mediated transcrip... |
Bioorg Med Chem 27: 1952-1961 (2019)
Article DOI: 10.1016/j.bmc.2019.03.042
BindingDB Entry DOI: 10.7270/Q24171GJ |
More data for this
Ligand-Target Pair | |
Vitamin D3 receptor
(Homo sapiens (Human)) | BDBM50135039

(CHEMBL3745849)Show SMILES [H][C@@]12[#6]-[#6]-[#6@H](C#CC#C[#6@@H](-[#8])C34[#6]-[#6]-5-[#6]-[#6](-[#6]-[#6](-[#6]-5)-[#6]3)-[#6]4)[C@@]1([#6])[#6]-[#6]-[#6]\[#6]2=[#6]/[#6]=[#6]-1/[#6]-[#6@@H](-[#8])-[#6](=[#6])-[#6@H](-[#8])-[#6]-1 |r,TLB:18:13:20:17.19.16,18:17:20:12.13.14,THB:16:15:12:18.17.19,16:17:12:20.14.15| Show InChI InChI=1S/C34H44O3/c1-22-30(35)17-23(18-31(22)36)9-10-27-6-5-13-33(2)28(11-12-29(27)33)7-3-4-8-32(37)34-19-24-14-25(20-34)16-26(15-24)21-34/h9-10,24-26,28-32,35-37H,1,5-6,11-21H2,2H3/b27-10+/t24?,25?,26?,28-,29-,30+,31+,32+,33+,34?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rikkyo University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-1,25(OH)2D3 from recombinant human VDR ligand binding domain |
J Med Chem 58: 9510-21 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00792
BindingDB Entry DOI: 10.7270/Q22V2HZC |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo
Curated by ChEMBL
| Assay Description
Antagonist activity at human AR assessed as reduction in DHT-induced transactivation |
ACS Med Chem Lett 9: 641-645 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00058
BindingDB Entry DOI: 10.7270/Q2PN986S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data