

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185909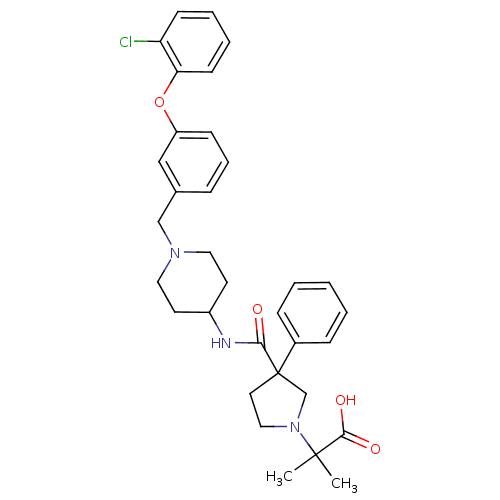 (2-(3-((1-(3-(2-chlorophenoxy)benzyl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185912 (2-(4-{1-[3-(2-chloro-phenoxy)-benzyl]-piperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185908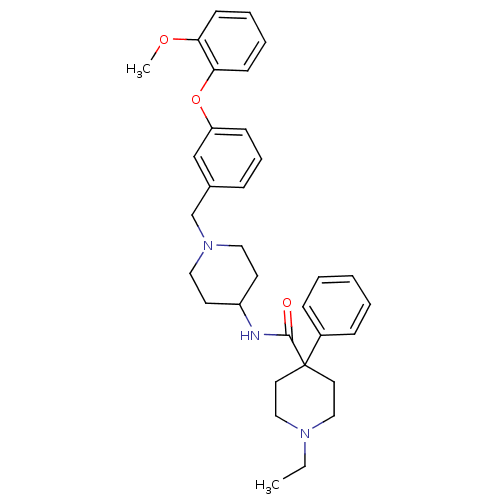 (1-ethyl-4-phenyl-piperidine-4-carboxylic acid {1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185910 (1-ethyl-4-phenyl-piperidine-4-carboxylic acid {1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185906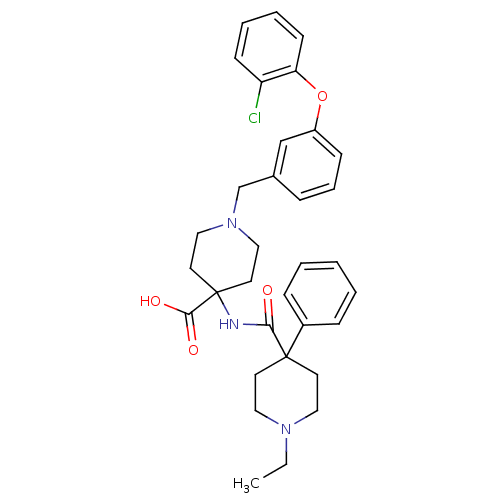 (1-[3-(2-chlorophenoxy)benzyl]-4-{[(1-ethyl-4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185904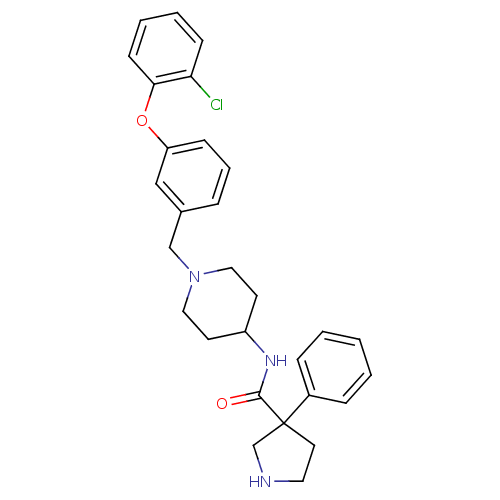 ((+/-) 3-phenyl-pyrrolidine-3-carboxylic acid {1-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185902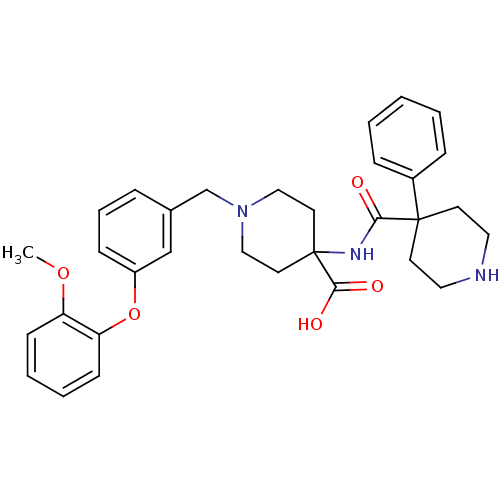 (1-[3-(2-methoxy-phenoxy)-benzyl]-4-[(4-phenyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185905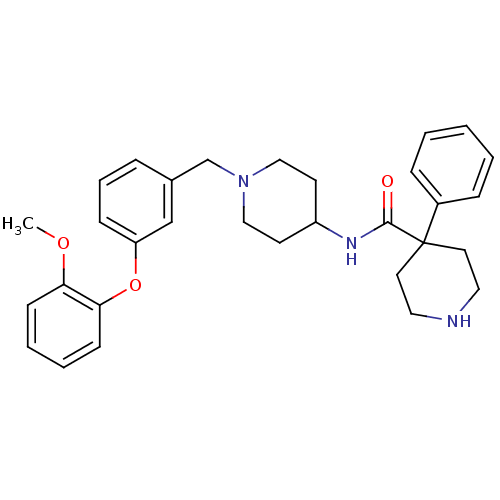 (4-phenyl-piperidine-4-carboxylic acid {1-[3-(2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185915 (1-(4-chloro-phenyl)-cyclohexanecarboxylic acid {1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185907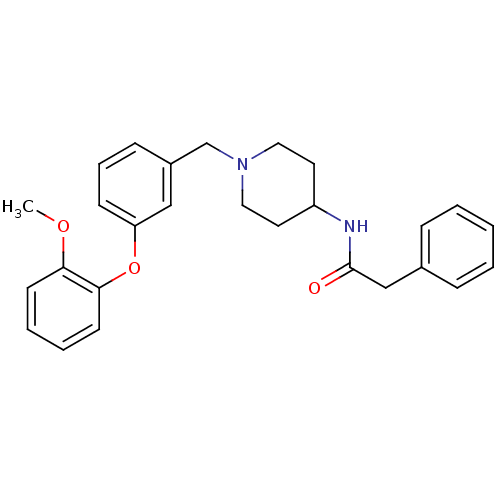 (CHEMBL210322 | N-{1-[3-(2-methoxy-phenoxy)-benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50185905 (4-phenyl-piperidine-4-carboxylic acid {1-[3-(2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from hERG expressed in HEK293 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185911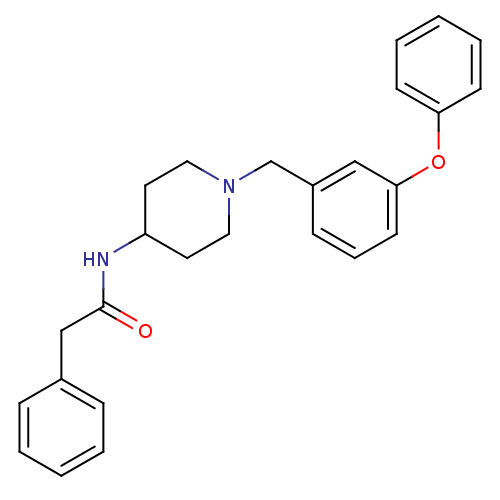 (CHEMBL205692 | N-[1-(3-phenoxy-benzyl)-piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185903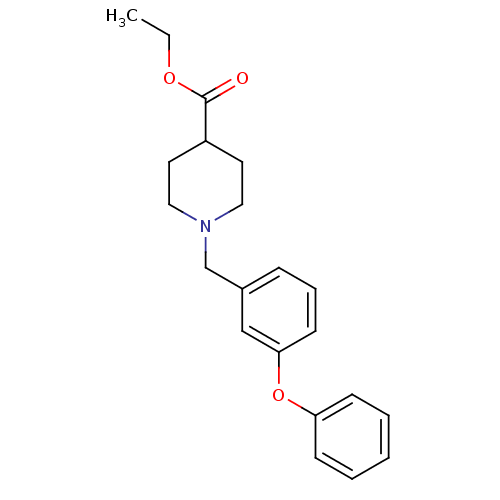 (1-(3-phenoxy-benzyl)-piperidine-4-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185916 (1-(3-phenoxy-benzyl)-piperidine-4-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185913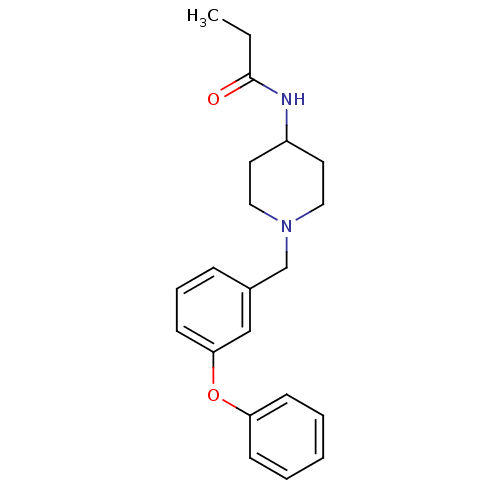 (CHEMBL381354 | N-[1-(3-phenoxy-benzyl)-piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50185902 (1-[3-(2-methoxy-phenoxy)-benzyl]-4-[(4-phenyl-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from hERG expressed in HEK293 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50185909 (2-(3-((1-(3-(2-chlorophenoxy)benzyl)piperidin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from hERG expressed in HEK293 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50185906 (1-[3-(2-chlorophenoxy)benzyl]-4-{[(1-ethyl-4-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from hERG expressed in HEK293 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185914 (1-(3-phenoxybenzyl)piperidine-4-carboxylic acid | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125I]I309 from human CCR8 expressed in L1.2 cells | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185909 (2-(3-((1-(3-(2-chlorophenoxy)benzyl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132322 (({2-[(2-{4-[4-Amino-5-(3-hydroxy-phenyl)-pyrrolo[2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185912 (2-(4-{1-[3-(2-chloro-phenoxy)-benzyl]-piperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM27216 ((2R)-2-({6-[(3-chlorophenyl)amino]-9-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Cyclin-dependent kinase 2 (CDK2) | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97708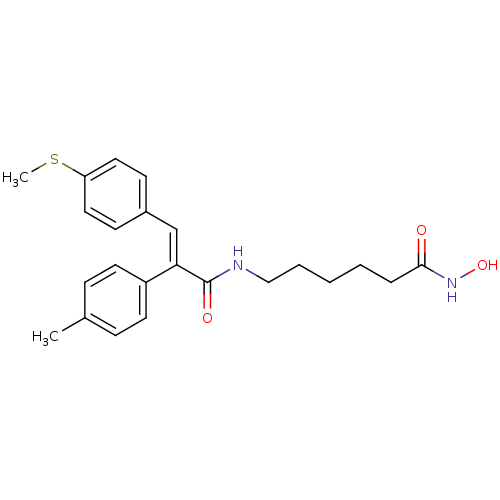 (US8476255, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97707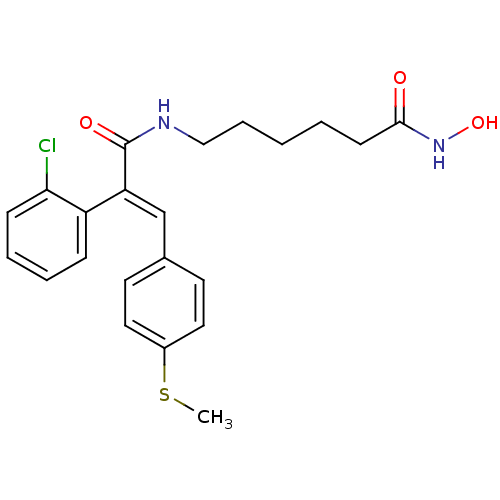 (US8476255, 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185905 (4-phenyl-piperidine-4-carboxylic acid {1-[3-(2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97695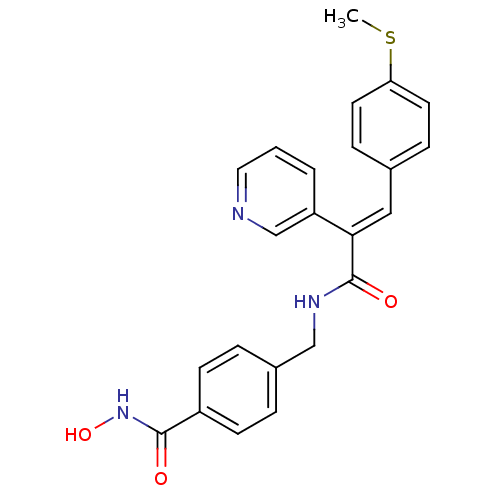 (US8476255, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50221656 (CHEMBL3706663) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185904 ((+/-) 3-phenyl-pyrrolidine-3-carboxylic acid {1-[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97703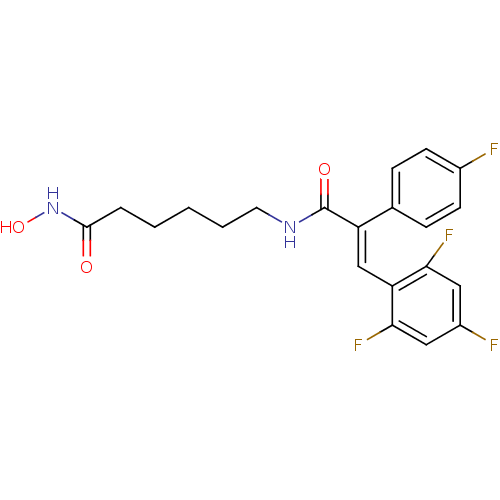 (US8476255, 89) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97698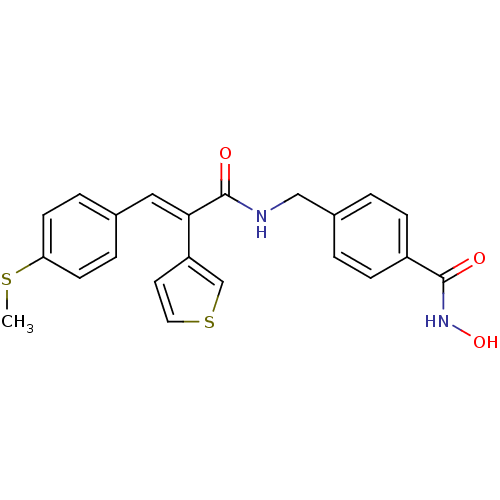 (US8476255, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97685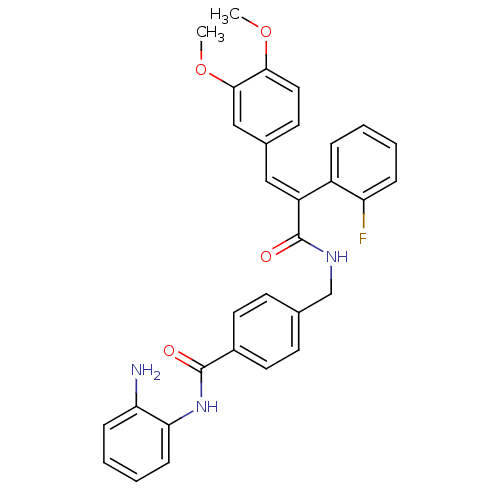 (US8476255, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132339 (3-[4-Amino-7-(4-{2-[(2-hydroxy-ethyl)-methyl-amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97697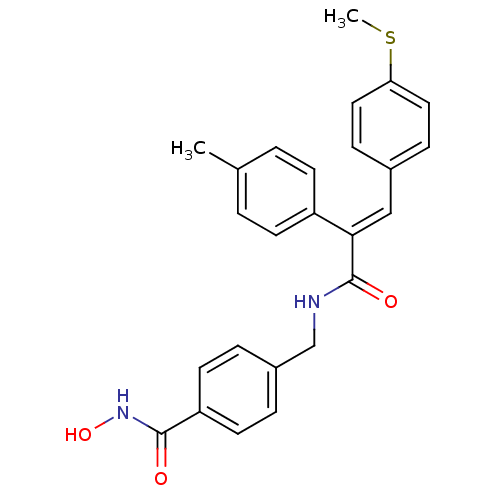 (US8476255, 58) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97706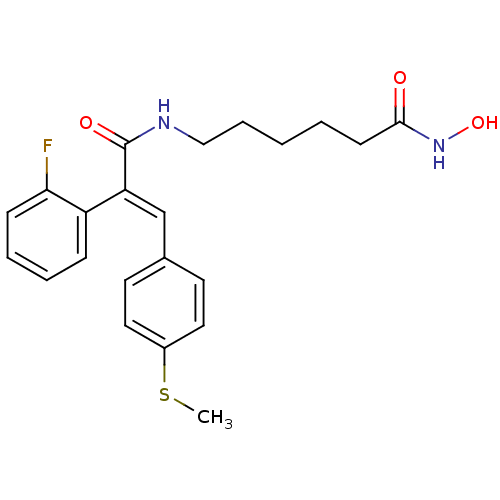 (US8476255, 94) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97704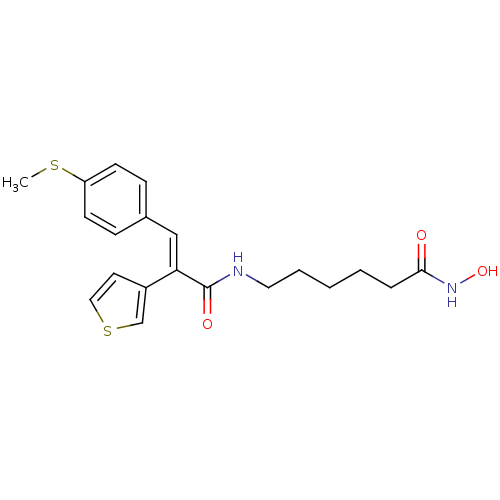 (US8476255, 90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97694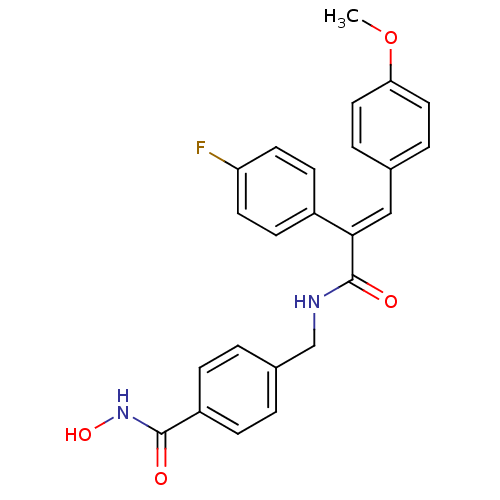 (US8476255, 53) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132324 (3-{4-Amino-7-[4-(2-hydroxy-ethyl)-phenyl]-7H-pyrro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50451556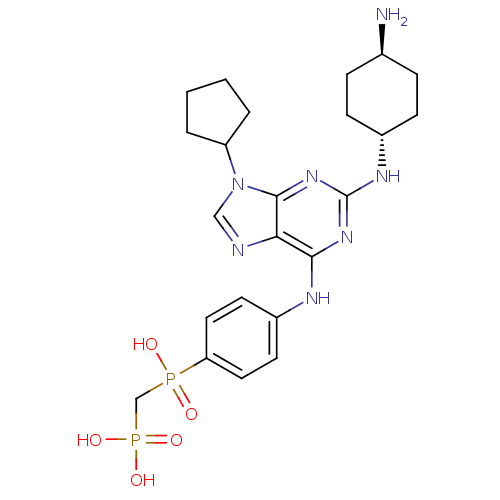 (CHEMBL3084838) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of Src-mediated dentine resorption in rabbit-osteoclast assay | Bioorg Med Chem Lett 13: 3067-70 (2003) BindingDB Entry DOI: 10.7270/Q25B01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97680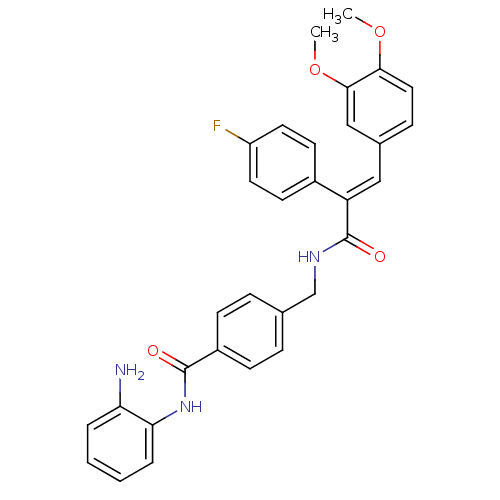 (US8476255, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185910 (1-ethyl-4-phenyl-piperidine-4-carboxylic acid {1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97700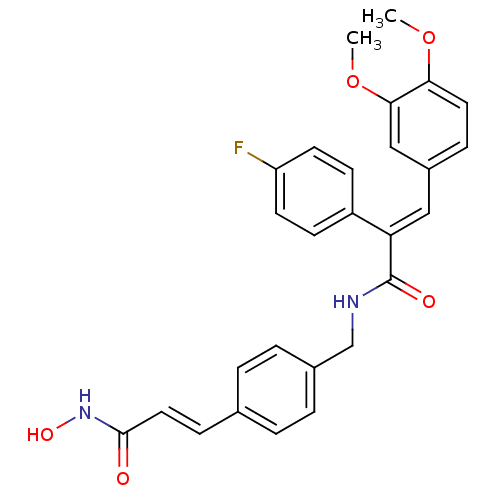 (US8476255, 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97684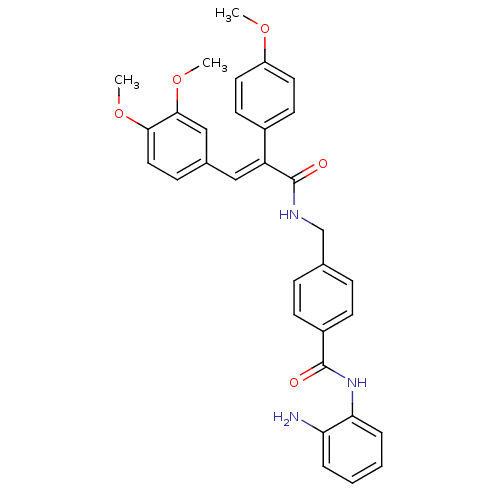 (US8476255, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97688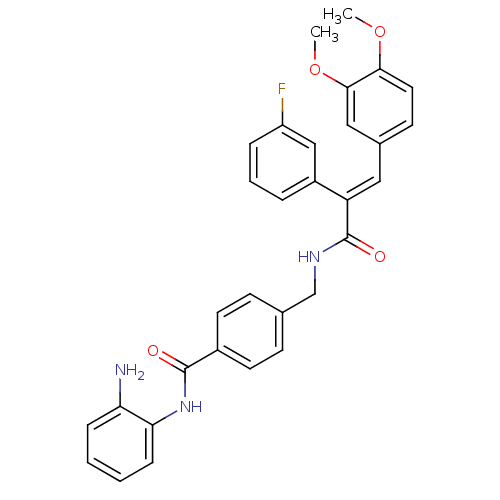 (US8476255, 32) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97687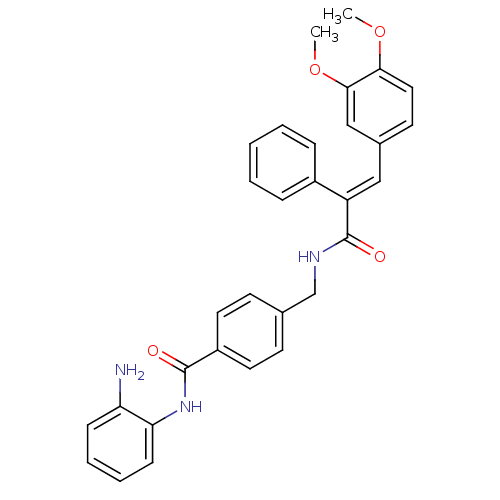 (US8476255, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185907 (CHEMBL210322 | N-{1-[3-(2-methoxy-phenoxy)-benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 8 (Homo sapiens (Human)) | BDBM50185908 (1-ethyl-4-phenyl-piperidine-4-carboxylic acid {1-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of I309-induced chemotaxis in L1.2 cells expressing CCR8 | J Med Chem 49: 2669-72 (2006) Article DOI: 10.1021/jm050965z BindingDB Entry DOI: 10.7270/Q2GH9HJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97686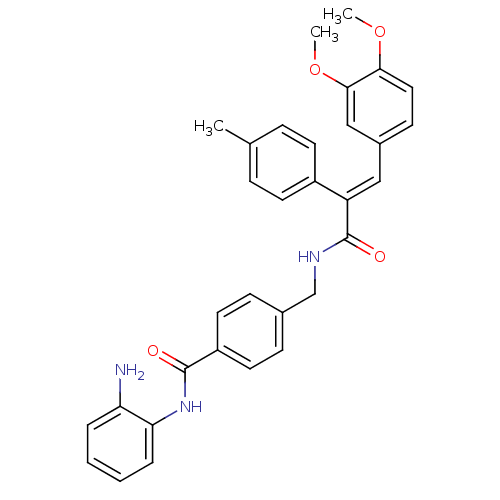 (US8476255, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50132334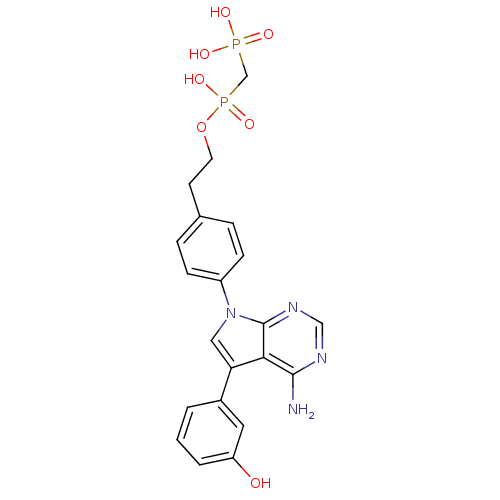 (CHEMBL104215 | [(2-{4-[4-Amino-5-(3-hydroxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of Src protein tryrosine kinase using the Scintillation proximity kinase assay. | Bioorg Med Chem Lett 13: 3063-6 (2003) BindingDB Entry DOI: 10.7270/Q290235N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Mus musculus (Mouse)) | BDBM97699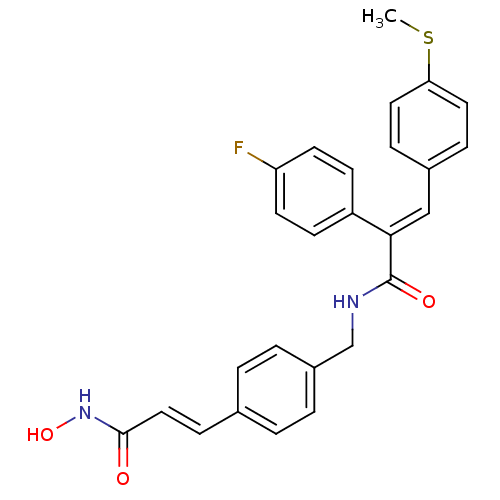 (BOC-LYS(AC)-AMC | US8476255, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | 37 |
Orchid Chemicals & Pharmaceuticals Limited US Patent | Assay Description Histone Deacetylase (HDAC) inhibition assay using Boc-Lys (Ac)-AMC substrate. The fluorometric assay provides a fast and fluorescence based method t... | US Patent US8476255 (2013) BindingDB Entry DOI: 10.7270/Q27S7MCR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |