

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584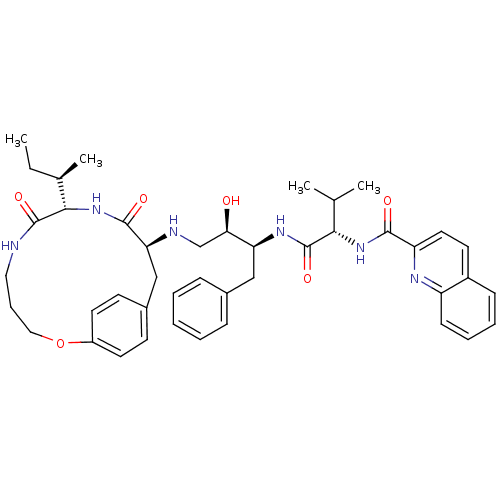 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152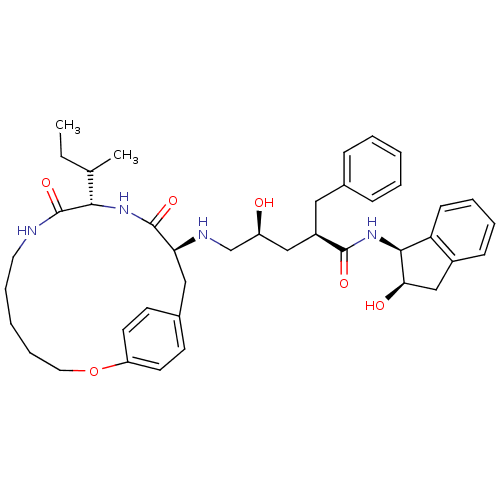 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13928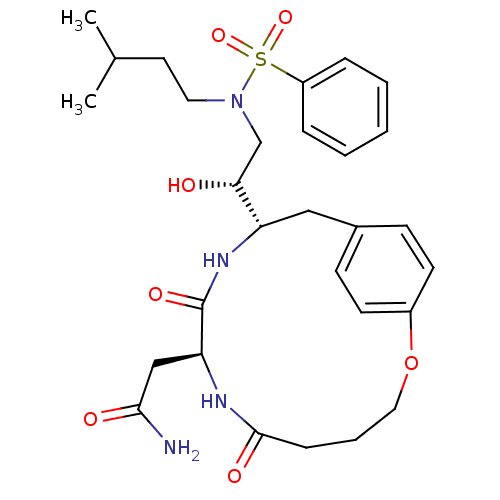 (2-[(8S,11S)-11-[(1R)-2-[benzene(3-methylbutyl)sulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086880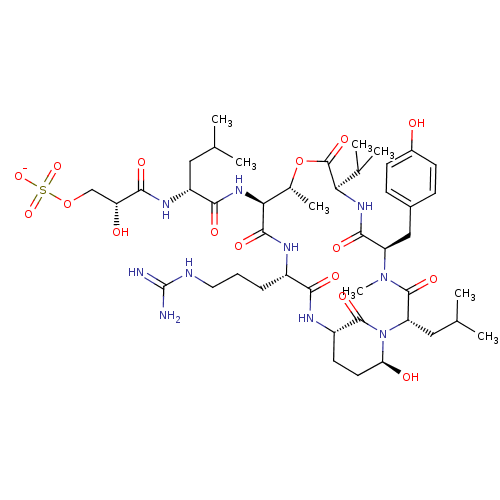 (Proteolytic Enzyme inhibitor) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086884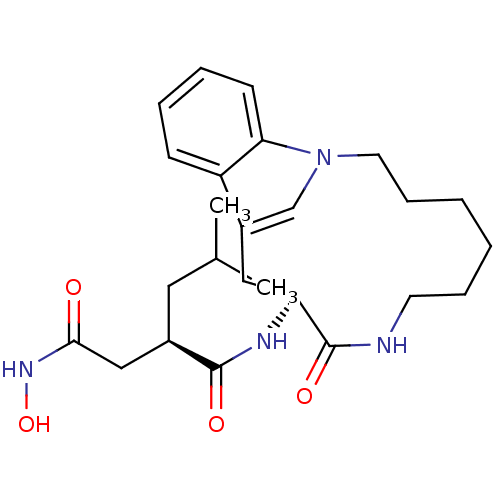 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-9-oxo-1,8-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151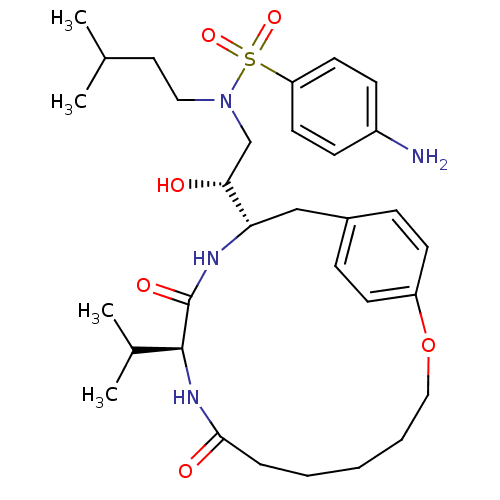 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibitory constant against HIV-1 protease | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13931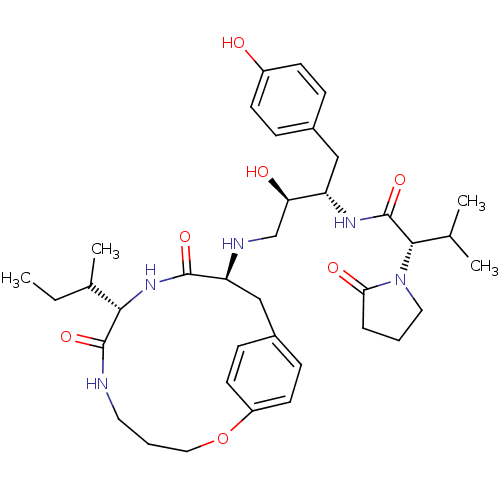 ((2S)-N-[(2S,3R)-4-{[(8S,11S)-8-[(2R)-butan-2-yl]-7...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.80 | -51.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13933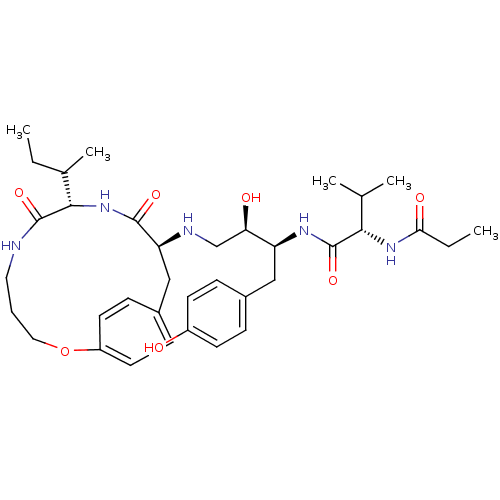 ((2S)-N-[(2S,3R)-4-{[(8S,11S)-8-[(2S)-butan-2-yl]-7...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369797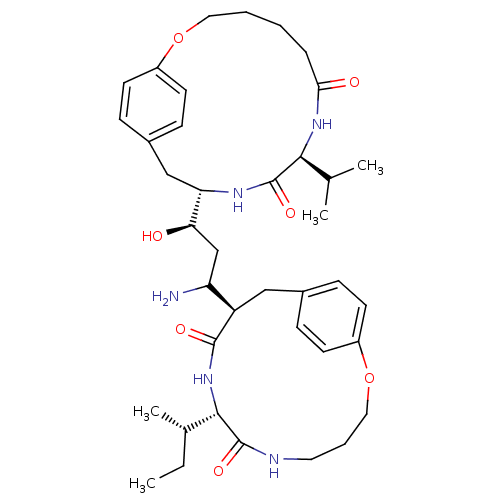 (CHEMBL1794029) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13929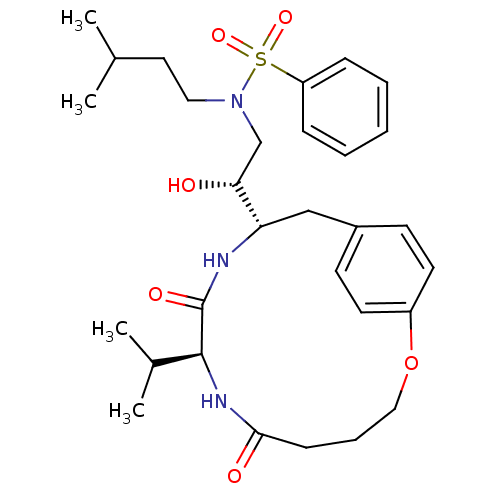 ((2R)-2-[(8S,11S)-6,9-dioxo-8-(propan-2-yl)-2-oxa-7...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369799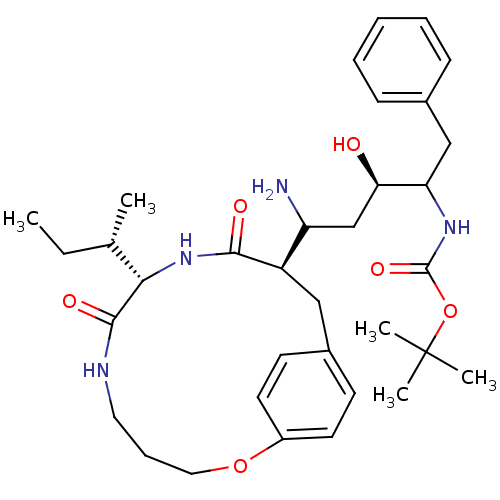 (CHEMBL1794024) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13932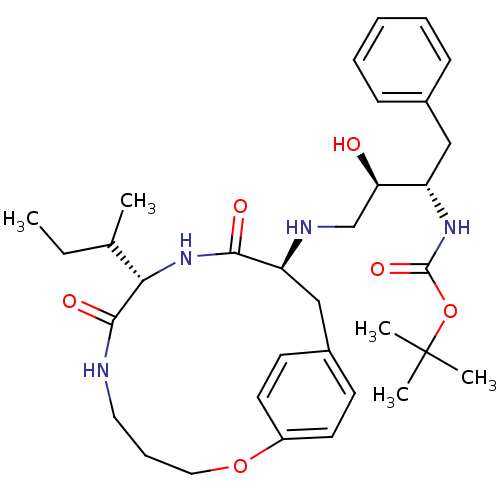 (Macrocyclic Peptidomimetic Inhibitor 6 | tert-buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13930 ((2S)-N-tert-butyl-1-[(2R)-2-[(8S,11S)-8-(carbamoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086886 (CHEMBL436149 | N-[14-Benzyl-18-(3-guanidino-propyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599,Q508K,L534I,L564I,C568A,C596A] (Human immunodeficiency virus type 1) | BDBM13927 ((2S,3S)-N-[(1S)-1-carbamoyl-2-methylpropyl]-2-{[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | 12 | -47.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
University of Queensland | Assay Description he Ki values were determined using fluorogenic substrate, 2-(aminobenzoyl)-Thr-Ile-Nle-Phe(p-NO2)-Gln-ArgNH2. A standard curve relating changes in fl... | Biochemistry 38: 7978-88 (1999) Article DOI: 10.1021/bi990174x BindingDB Entry DOI: 10.7270/Q20000B3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50086883 (2-{2-[2-(8-Carbamoylmethyl-6,9-dioxo-2-oxa-7,10-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369800 (CHEMBL1232357) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369798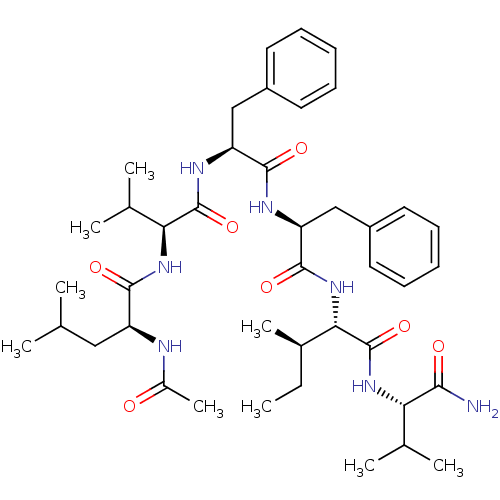 (CHEMBL1794028) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Queensland Curated by ChEMBL | Assay Description binding affinity towards HIV-1 Protease enzyme | J Med Chem 43: 1271-81 (2001) BindingDB Entry DOI: 10.7270/Q23779F5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093001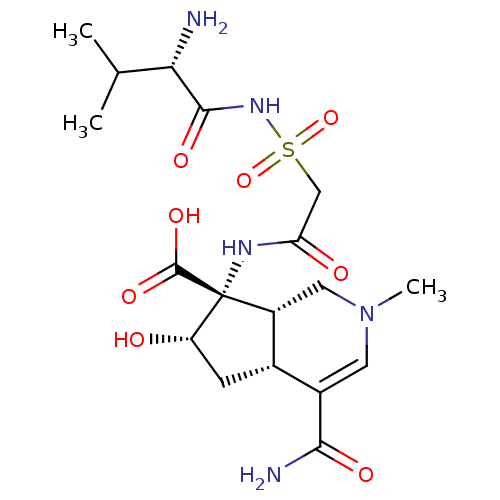 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093003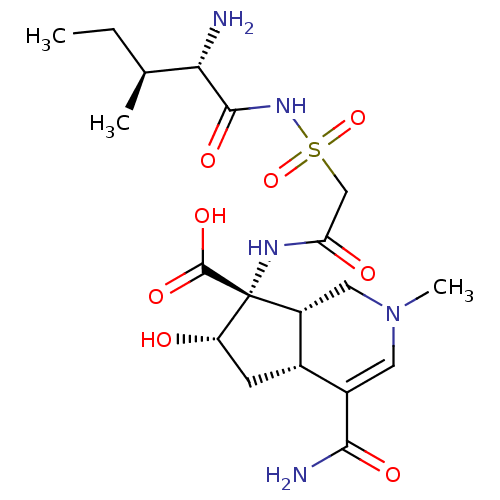 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093005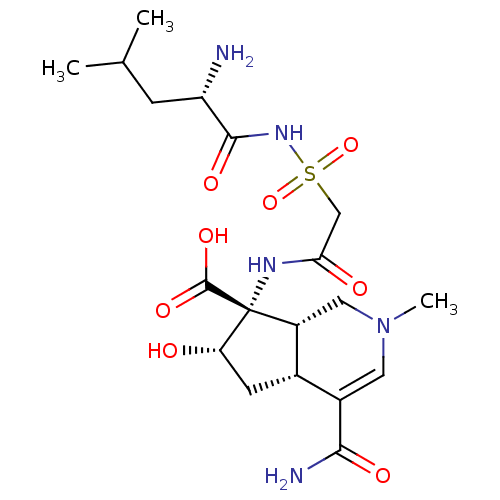 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in MT2 cells infected with HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in cord blood mononuclear cells (CBMC) infected with HIV -1 TC354... | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in PBMC cells infected with HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in cord blood mononuclear cells (CBMC) infected with HIV -1 TC354... | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in MT2 cells infected with HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093004 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in MT2 cells of HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in MT2 cells infected with HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50369584 (CHEMBL1790230) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in cord blood mononuclear cells (CBMC) infected with HIV -1 TC354... | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in MT2 cells infected with HIV-2 strain (ROD) | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM150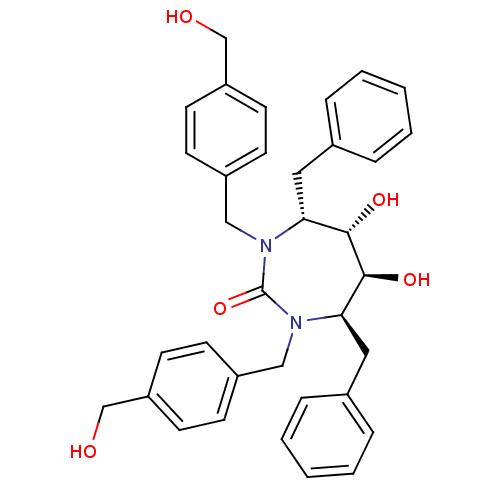 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in cord blood mononuclear cells (CBMC) infected with HIV -1 TC354... | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092152 (2-Benzyl-5-(10-sec-butyl-9,12-dioxo-2-oxa-8,11-dia...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in PBMC cells infected with HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093002 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Valine--tRNA ligase (Homo sapiens (Human)) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against VRS (valyl tRNA synthetase) from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in PBMC cells infected with HIV-1 237288 strain | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093006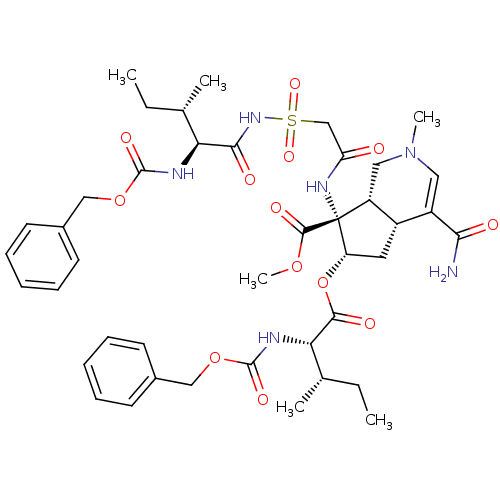 ((4aR,6S,7R,7aS)-6-((2S,3S)-2-Benzyloxycarbonylamin...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50092151 ((10S,13S,1'R)-13-[1'-HYDROXY-2'-(N-P-AMINOBENZENES...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland Curated by ChEMBL | Assay Description Inhibition of virion associated RT activity relative to untreated, infected control in MT2 cells infected with HIV-2 strain (ROD) | J Med Chem 43: 3495-504 (2000) BindingDB Entry DOI: 10.7270/Q2KH0P1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093005 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-4-methyl-pentano...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093003 ((4aR,6S,7R,7aS)-7-[2-((2S,3S)-2-Amino-3-methyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Homo sapiens (Human)) | BDBM50093001 ((4aR,6S,7R,7aS)-7-[2-((S)-2-Amino-3-methyl-butyryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Leucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucine--tRNA ligase (Staphylococcus aureus) | BDBM50093008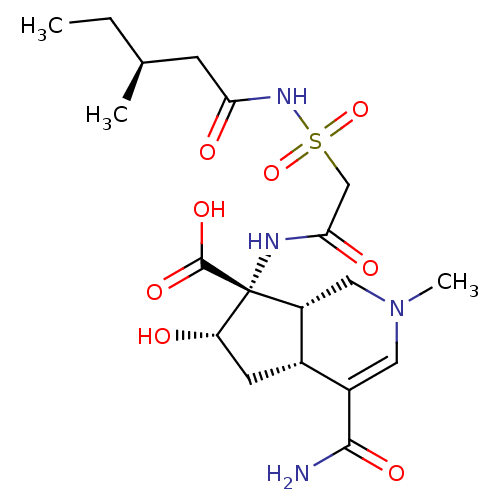 ((4aR,6S,7R,7aS)-4-Carbamoyl-6-hydroxy-2-methyl-7-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from staphylococcus aureus WCUH29 | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoleucyl-tRNA synthetase (Rattus norvegicus) | BDBM50093007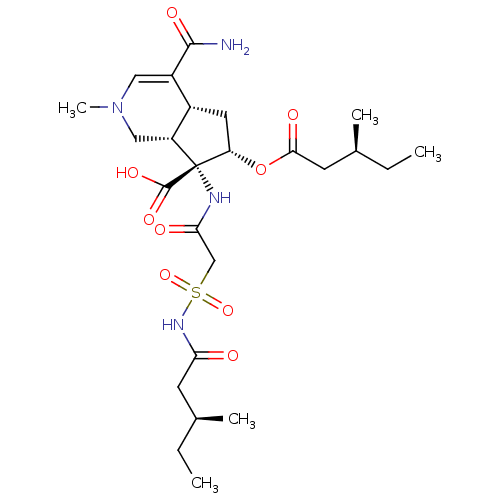 ((4aR,6S,7R,7aS)-4-Carbamoyl-2-methyl-6-((S)-3-meth...) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Australian National University Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against Isoleucyl-tRNA synthetase from rat liver | Bioorg Med Chem Lett 10: 2263-6 (2001) BindingDB Entry DOI: 10.7270/Q2PN94W3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |