

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315250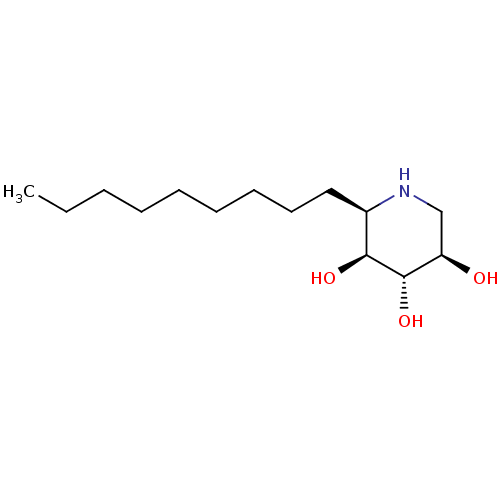 ((2R,3S,4S,5R)-2-nonylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant beta-glucocerebrosidase assessed as p-nitrophenolate accumulation preincubated for 10 mins before p-nitrophenyl-beta-... | Bioorg Med Chem 18: 2645-50 (2010) Article DOI: 10.1016/j.bmc.2010.02.027 BindingDB Entry DOI: 10.7270/Q24F1QVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18364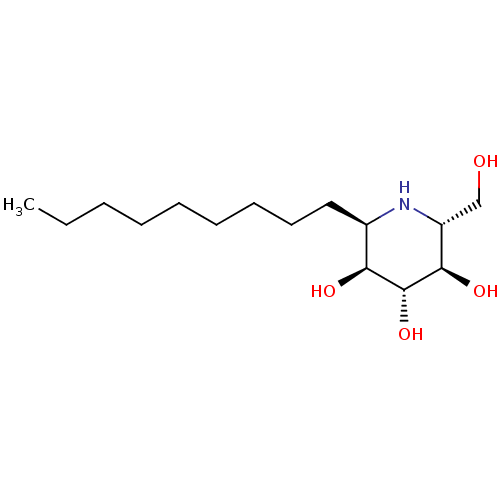 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-nonylpiperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | -39.8 | 270 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18363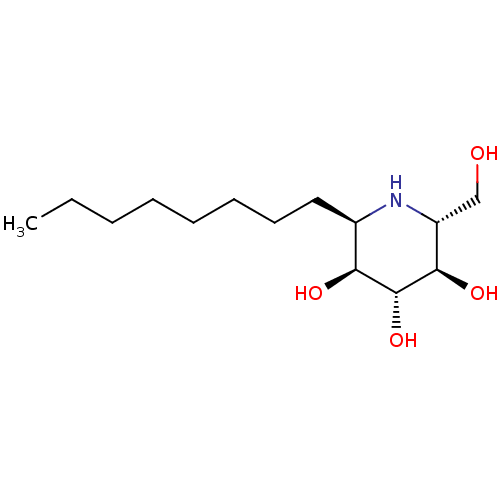 ((2R,3R,4R,5S,6R)-2-(hydroxymethyl)-6-octylpiperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 280 | -38.9 | 500 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358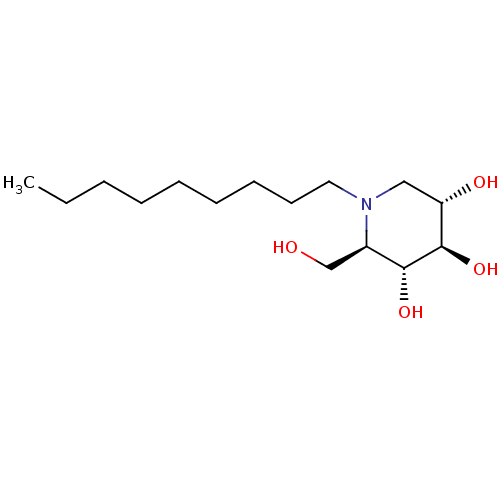 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 300 | -38.7 | 660 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18357 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-octylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 420 | -37.9 | 820 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18362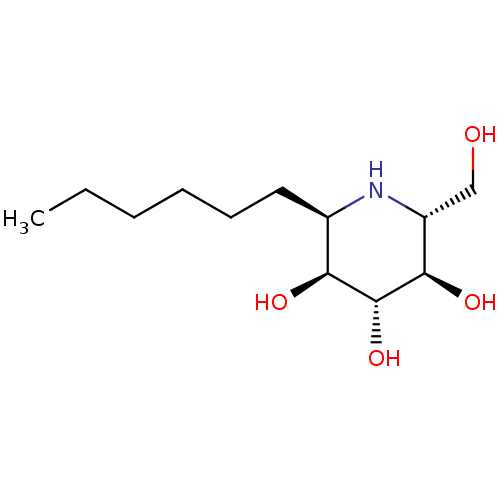 ((2R,3S,4R,5R,6R)-2-hexyl-6-(hydroxymethyl)piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | -33.5 | 4.20E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18356 ((2R,3R,4R,5S)-1-hexyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.50E+3 | -31.2 | 1.30E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.90E+4 | -24.4 | 2.40E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18361 ((2R,3S,4R,5R,6R)-2-butyl-6-(hydroxymethyl)piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+5 | -23.5 | 1.00E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.16E+5 | -23.4 | 2.70E+5 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Hokuriku University | Assay Description Enzyme activity was determined as the production of fluorescent 4-MU from the substrate. The fluorescence was measured (excitation 362 nm, emission 4... | Bioorg Med Chem 14: 7736-44 (2006) Article DOI: 10.1016/j.bmc.2006.08.003 BindingDB Entry DOI: 10.7270/Q2BZ649B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50469238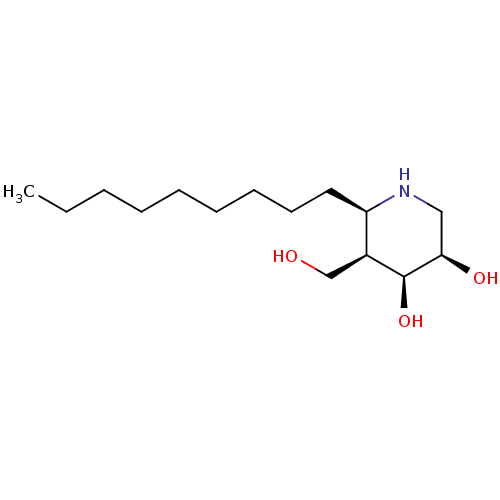 (CHEMBL4288931) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS Curated by ChEMBL | Assay Description Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... | Bioorg Med Chem 26: 5462-5469 (2018) Article DOI: 10.1016/j.bmc.2018.09.023 BindingDB Entry DOI: 10.7270/Q2G73HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156360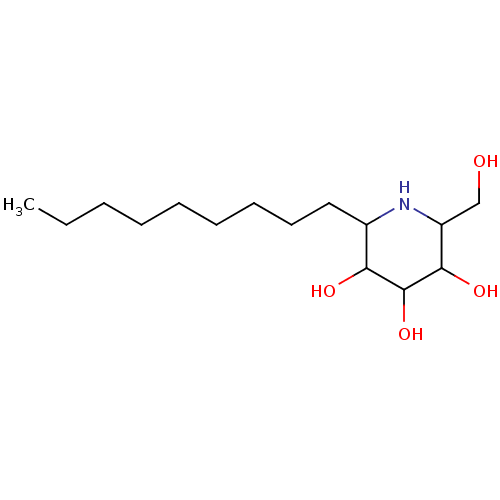 (2-Hydroxymethyl-6-nonyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50235812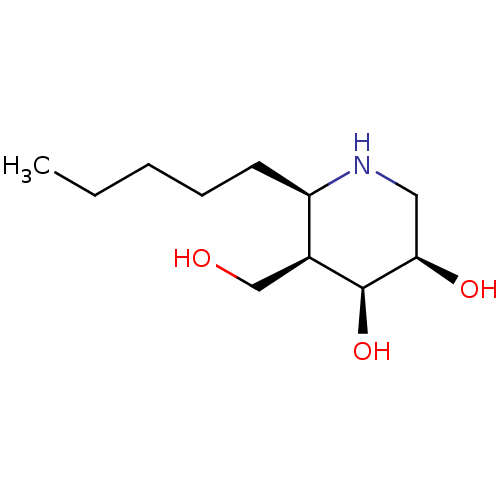 (CHEMBL4085739) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E | Eur J Med Chem 126: 160-170 (2017) Article DOI: 10.1016/j.ejmech.2016.09.095 BindingDB Entry DOI: 10.7270/Q21G0PHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50235812 (CHEMBL4085739) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS Curated by ChEMBL | Assay Description Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... | Bioorg Med Chem 26: 5462-5469 (2018) Article DOI: 10.1016/j.bmc.2018.09.023 BindingDB Entry DOI: 10.7270/Q2G73HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50469239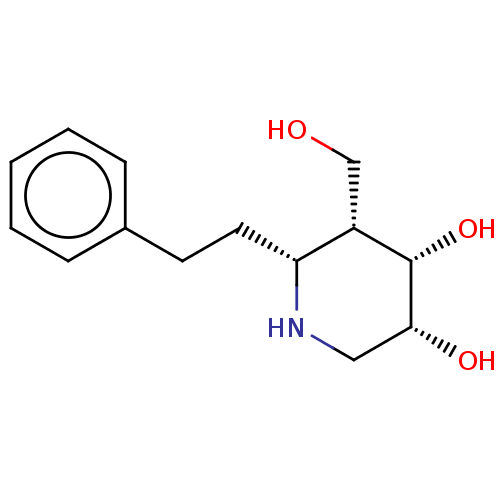 (CHEMBL4284436) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS Curated by ChEMBL | Assay Description Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... | Bioorg Med Chem 26: 5462-5469 (2018) Article DOI: 10.1016/j.bmc.2018.09.023 BindingDB Entry DOI: 10.7270/Q2G73HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315255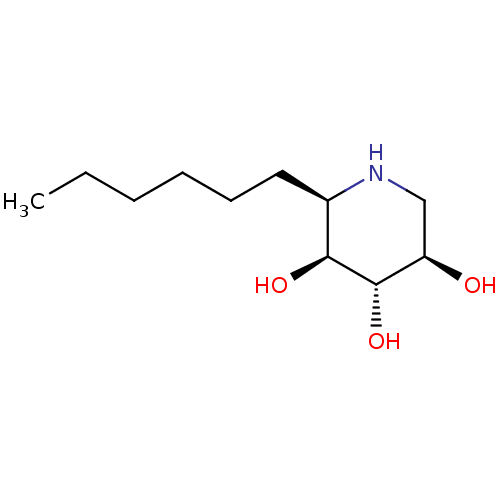 ((2R,3S,4S,5R)-2-hexylpiperidine-3,4,5-triol | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant beta-glucocerebrosidase assessed as p-nitrophenolate accumulation preincubated for 10 mins before p-nitrophenyl-beta-... | Bioorg Med Chem 18: 2645-50 (2010) Article DOI: 10.1016/j.bmc.2010.02.027 BindingDB Entry DOI: 10.7270/Q24F1QVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50182801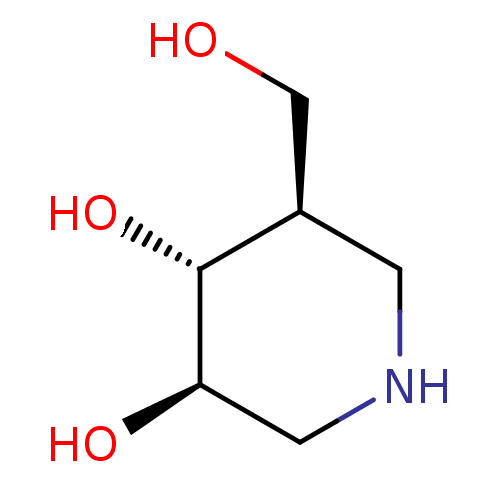 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS Curated by ChEMBL | Assay Description Inhibition of beta-glucosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-glucopyranoside as subst... | Bioorg Med Chem 26: 5462-5469 (2018) Article DOI: 10.1016/j.bmc.2018.09.023 BindingDB Entry DOI: 10.7270/Q2G73HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase C (Homo sapiens (Human)) | BDBM50082234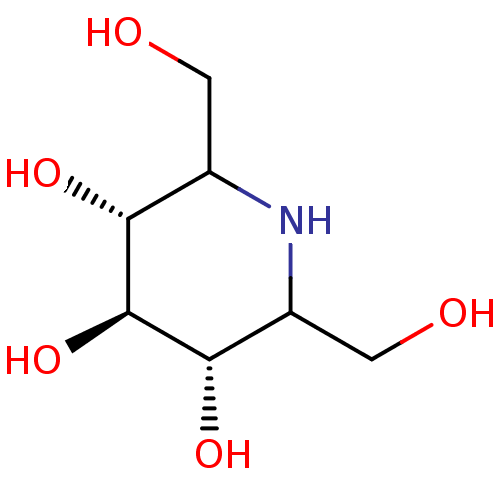 ((3R,4R,5S)-2,6-Bis-hydroxymethyl-piperidine-3,4,5-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Organique et Analytique (I.C.O.A.) Curated by ChEMBL | Assay Description Inhibitory activity against alpha-galactosidase from coffee bean. | Bioorg Med Chem Lett 9: 3171-4 (1999) BindingDB Entry DOI: 10.7270/Q2GT5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-lysosomal glucosylceramidase (Homo sapiens (Human)) | BDBM50469238 (CHEMBL4288931) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS Curated by ChEMBL | Assay Description Inhibition of beta-glucosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-glucopyranoside as subst... | Bioorg Med Chem 26: 5462-5469 (2018) Article DOI: 10.1016/j.bmc.2018.09.023 BindingDB Entry DOI: 10.7270/Q2G73HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM188519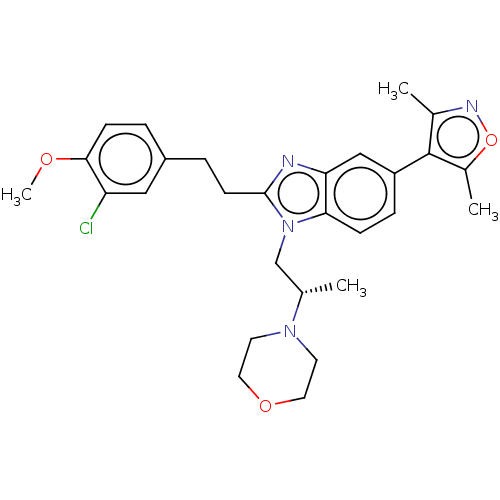 (SGC-CBP30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Alphascreen assay. Binding to CREBBPA (domain start/stop: R1081-G1197) by alphascreen assay | Proc Natl Acad Sci U S A 112: 10768-73 (2015) Article DOI: 10.1073/pnas.1501956112 BindingDB Entry DOI: 10.7270/Q2NG4V7B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM188519 (SGC-CBP30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Alphascreen assay. Binding to CREBBPA (domain start/stop: R1081-G1197) by alphascreen assay | Proc Natl Acad Sci U S A 112: 10768-73 (2015) Article DOI: 10.1073/pnas.1501956112 BindingDB Entry DOI: 10.7270/Q2NG4V7B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS (UMR 7509) Curated by ChEMBL | Assay Description Inhibition of human recombinant serum BuchE using butyrylthiocholine iodide as substrate by Ellman method | Bioorg Med Chem Lett 25: 830-3 (2015) Article DOI: 10.1016/j.bmcl.2014.12.071 BindingDB Entry DOI: 10.7270/Q22B90QH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM50156359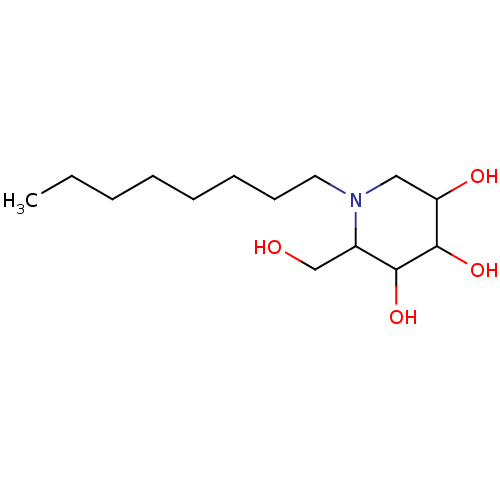 (2-Hydroxymethyl-1-octyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM50156357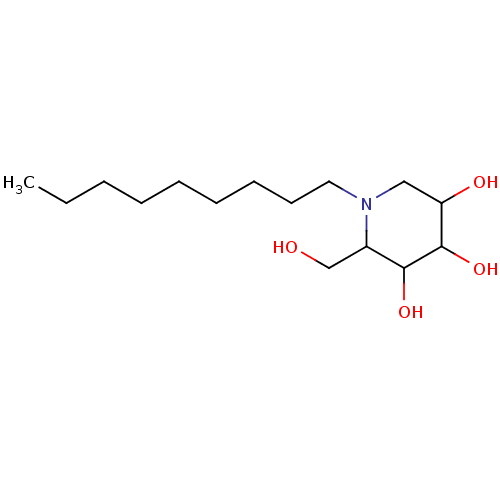 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156356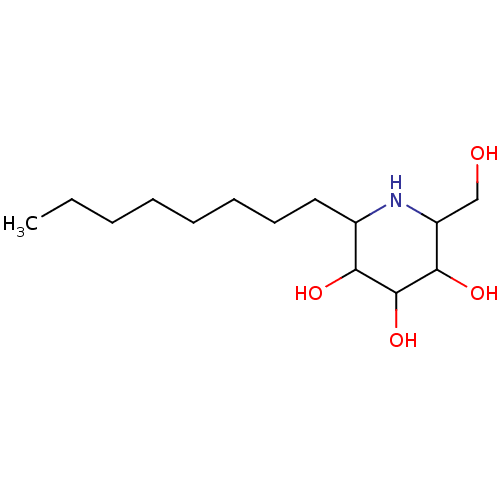 (2-Hydroxymethyl-6-octyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit alpha (Homo sapiens (Human)) | BDBM50235813 (CHEMBL4097052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E | Eur J Med Chem 126: 160-170 (2017) Article DOI: 10.1016/j.ejmech.2016.09.095 BindingDB Entry DOI: 10.7270/Q21G0PHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 136 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS (UMR 7509) Curated by ChEMBL | Assay Description Inhibition of electric eel AchE using acetylthiocholine iodide as substrate by Ellman method | Bioorg Med Chem Lett 25: 830-3 (2015) Article DOI: 10.1016/j.bmcl.2014.12.071 BindingDB Entry DOI: 10.7270/Q22B90QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156355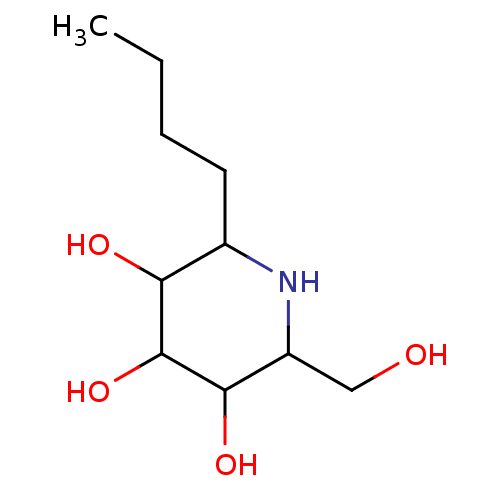 (2-Butyl-6-hydroxymethyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50235812 (CHEMBL4085739) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E | Eur J Med Chem 126: 160-170 (2017) Article DOI: 10.1016/j.ejmech.2016.09.095 BindingDB Entry DOI: 10.7270/Q21G0PHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against rat intestinal sucrase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50350758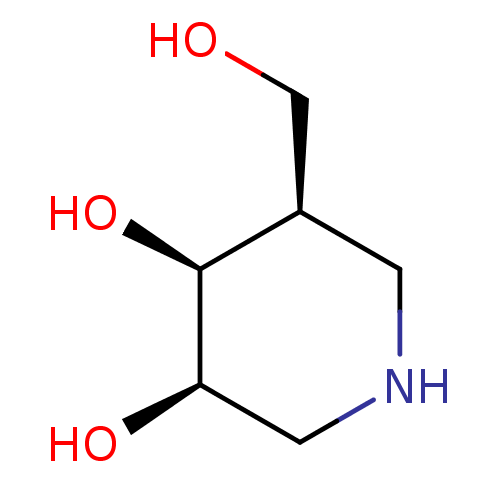 (CHEMBL1818433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibitory concentration against cyclin dependent kinase-2 (CDK2)/Cyclin E | Eur J Med Chem 126: 160-170 (2017) Article DOI: 10.1016/j.ejmech.2016.09.095 BindingDB Entry DOI: 10.7270/Q21G0PHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-galactosidase (Homo sapiens (Human)) | BDBM50469237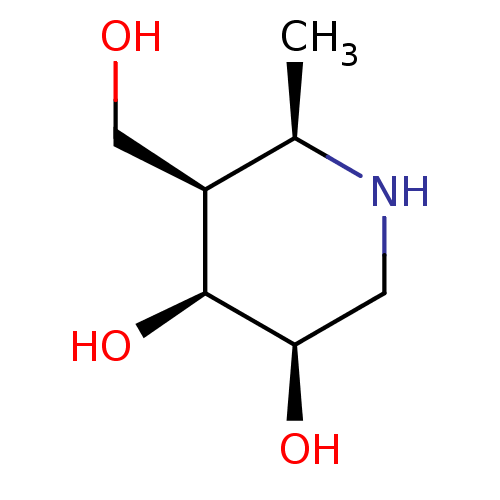 (CHEMBL4281031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans & CNRS Curated by ChEMBL | Assay Description Inhibition of beta-galactosidase derived from human peripheral blood mononuclear cell lysate using 4-methylumbelliferyl beta-D-galactopyranoside as s... | Bioorg Med Chem 26: 5462-5469 (2018) Article DOI: 10.1016/j.bmc.2018.09.023 BindingDB Entry DOI: 10.7270/Q2G73HFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156359 (2-Hydroxymethyl-1-octyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156353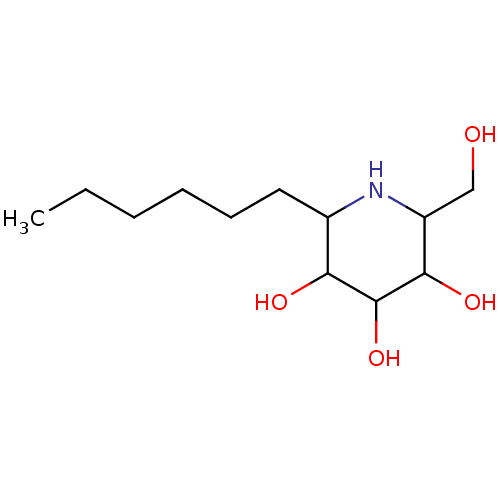 (2-Hexyl-6-hydroxymethyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase C (Mus musculus) | BDBM50259956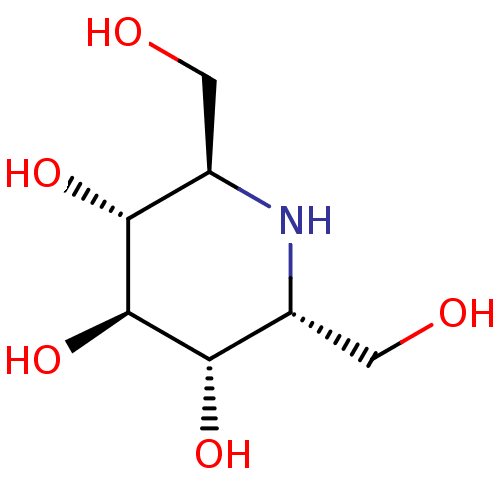 (2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie Organique et Analytique (I.C.O.A.) Curated by ChEMBL | Assay Description Inhibitory activity against alpha-glucosidase from rat intestinal maltase | Bioorg Med Chem Lett 9: 3171-4 (1999) BindingDB Entry DOI: 10.7270/Q2GT5MDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against rat intestinal maltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM18355 ((2R,3R,4R,5S)-1-butyl-2-(hydroxymethyl)piperidine-...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156354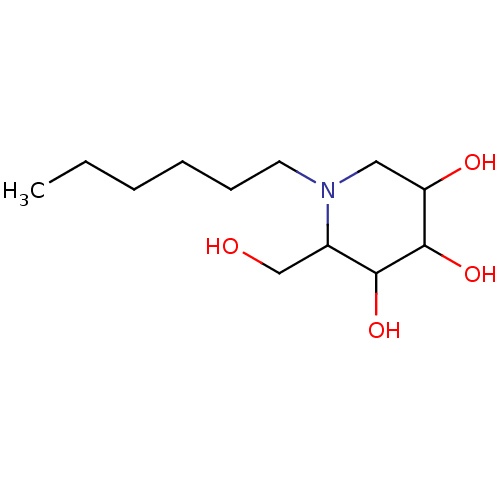 (1-Hexyl-2-hydroxymethyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156354 (1-Hexyl-2-hydroxymethyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg/CNRS (UMR 7509) Curated by ChEMBL | Assay Description Inhibition of human recombinant AchE using acetylthiocholine iodide as substrate by Ellman method | Bioorg Med Chem Lett 25: 830-3 (2015) Article DOI: 10.1016/j.bmcl.2014.12.071 BindingDB Entry DOI: 10.7270/Q22B90QH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156358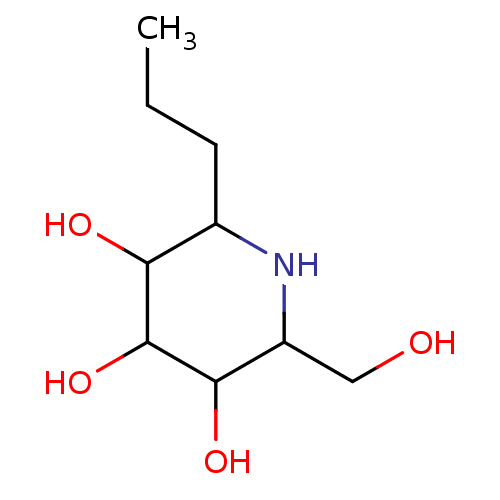 (2-Hydroxymethyl-6-propyl-piperidine-3,4,5-triol | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal isomaltase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50315252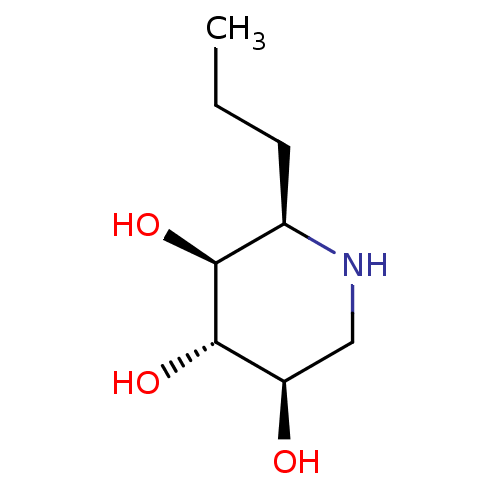 ((2R,3S,4S,5R)-2-propylpiperidine-3,4,5-triol | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans& CNRS Curated by ChEMBL | Assay Description Inhibition of human recombinant beta-glucocerebrosidase assessed as p-nitrophenolate accumulation preincubated for 10 mins before p-nitrophenyl-beta-... | Bioorg Med Chem 18: 2645-50 (2010) Article DOI: 10.1016/j.bmc.2010.02.027 BindingDB Entry DOI: 10.7270/Q24F1QVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM50156356 (2-Hydroxymethyl-6-octyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156359 (2-Hydroxymethyl-1-octyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sucrase-isomaltase, intestinal (Rattus norvegicus (Rat)) | BDBM50156357 (2-Hydroxymethyl-1-nonyl-piperidine-3,4,5-triol | C...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibition of rat intestinal sucrase using disaccharide | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM50156354 (1-Hexyl-2-hydroxymethyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Putative alpha-glucosidase (Oryza sativa subsp. japonica) | BDBM50156353 (2-Hexyl-6-hydroxymethyl-piperidine-3,4,5-triol | C...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ d'Orl£ans Curated by ChEMBL | Assay Description Inhibitory activity against alpha-Glucosidase from rice | Bioorg Med Chem Lett 14: 5991-5 (2004) Article DOI: 10.1016/j.bmcl.2004.09.086 BindingDB Entry DOI: 10.7270/Q27P904W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 155 total ) | Next | Last >> |