 Found 390 hits with Last Name = 'mathea' and Initial = 's'
Found 390 hits with Last Name = 'mathea' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92912
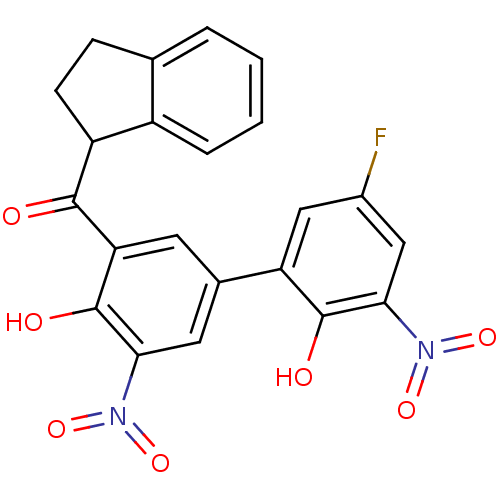
(Aryl 1-indanylketone, 1)Show SMILES Oc1c(cc(cc1N(=O)=O)-c1cc(F)cc(c1O)N(=O)=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92915

(Aryl 1-indanylketone, 4)Show SMILES COc1ccc(cc1C(=O)C1CCc2ccccc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H19F3O3/c1-29-22-13-9-17(15-6-10-18(11-7-15)30-24(25,26)27)14-21(22)23(28)20-12-8-16-4-2-3-5-19(16)20/h2-7,9-11,13-14,20H,8,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+3 | -31.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92914

(Aryl 1-indanylketone, 3)Show SMILES COc1ccc(F)cc1-c1cc(N)c(O)c(c1)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C23H20FNO3/c1-28-21-9-7-15(24)12-18(21)14-10-19(23(27)20(25)11-14)22(26)17-8-6-13-4-2-3-5-16(13)17/h2-5,7,9-12,17,27H,6,8,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | -30.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92915

(Aryl 1-indanylketone, 4)Show SMILES COc1ccc(cc1C(=O)C1CCc2ccccc12)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C24H19F3O3/c1-29-22-13-9-17(15-6-10-18(11-7-15)30-24(25,26)27)14-21(22)23(28)20-12-8-16-4-2-3-5-19(16)20/h2-7,9-11,13-14,20H,8,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase D
(Homo sapiens (Human)) | BDBM92912

(Aryl 1-indanylketone, 1)Show SMILES Oc1c(cc(cc1N(=O)=O)-c1cc(F)cc(c1O)N(=O)=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.42E+3 | -29.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase D
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.28E+3 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92914

(Aryl 1-indanylketone, 3)Show SMILES COc1ccc(F)cc1-c1cc(N)c(O)c(c1)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C23H20FNO3/c1-28-21-9-7-15(24)12-18(21)14-10-19(23(27)20(25)11-14)22(26)17-8-6-13-4-2-3-5-16(13)17/h2-5,7,9-12,17,27H,6,8,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+3 | -27.0 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92916
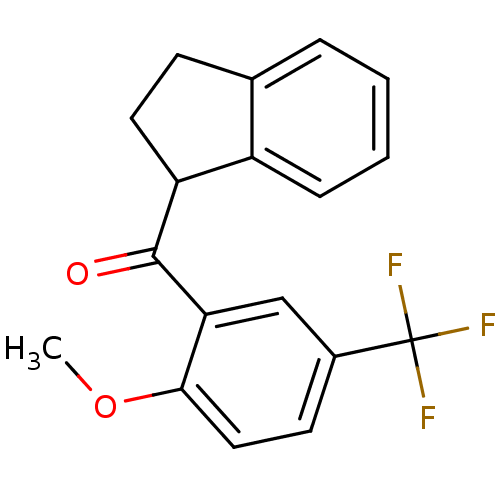
(Aryl 1-indanylketone, 5)Show InChI InChI=1S/C18H15F3O2/c1-23-16-9-7-12(18(19,20)21)10-15(16)17(22)14-8-6-11-4-2-3-5-13(11)14/h2-5,7,9-10,14H,6,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+4 | -26.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | -26.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92918
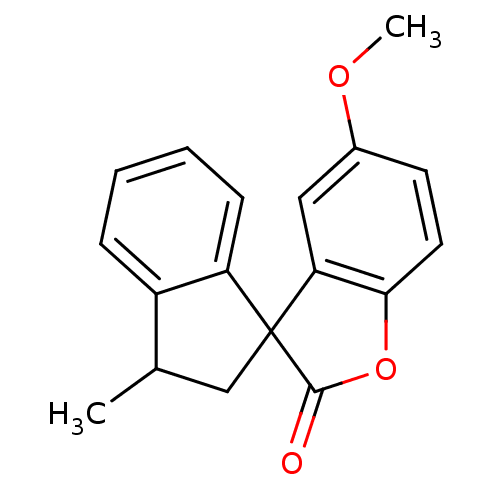
(Benzofuranone, 7)Show InChI InChI=1S/C18H16O3/c1-11-10-18(14-6-4-3-5-13(11)14)15-9-12(20-2)7-8-16(15)21-17(18)19/h3-9,11H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10E+4 | -24.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase-like 1
(Homo sapiens (Human)) | BDBM92912

(Aryl 1-indanylketone, 1)Show SMILES Oc1c(cc(cc1N(=O)=O)-c1cc(F)cc(c1O)N(=O)=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92920

(Benzofuranone, 9)Show InChI InChI=1S/C17H14O3/c1-19-13-6-7-15-14(8-13)16(18)17(20-15)9-11-4-2-3-5-12(11)10-17/h2-8H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30E+4 | -22.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92916

(Aryl 1-indanylketone, 5)Show InChI InChI=1S/C18H15F3O2/c1-23-16-9-7-12(18(19,20)21)10-15(16)17(22)14-8-6-11-4-2-3-5-13(11)14/h2-5,7,9-10,14H,6,8H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92917
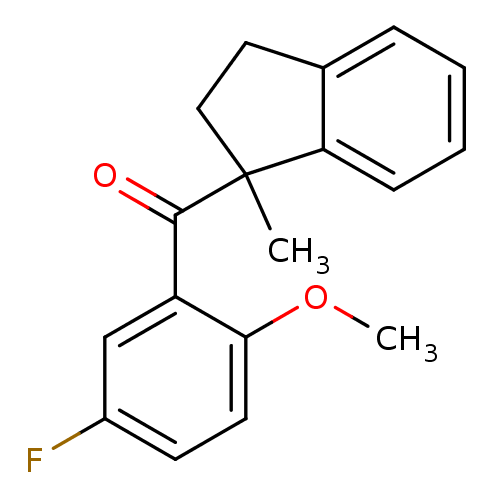
(Aryl 1-indanylketone, 6)Show InChI InChI=1S/C18H17FO2/c1-18(10-9-12-5-3-4-6-15(12)18)17(20)14-11-13(19)7-8-16(14)21-2/h3-8,11H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92918

(Benzofuranone, 7)Show InChI InChI=1S/C18H16O3/c1-11-10-18(14-6-4-3-5-13(11)14)15-9-12(20-2)7-8-16(15)21-17(18)19/h3-9,11H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92919
(Benzofuranone, 8)Show InChI InChI=1S/C16H12O2/c17-15-16(13-7-3-4-8-14(13)18-15)9-11-5-1-2-6-12(11)10-16/h1-8H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase C
(Homo sapiens (Human)) | BDBM92912

(Aryl 1-indanylketone, 1)Show SMILES Oc1c(cc(cc1N(=O)=O)-c1cc(F)cc(c1O)N(=O)=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92919

(Benzofuranone, 8)Show InChI InChI=1S/C16H12O2/c17-15-16(13-7-3-4-8-14(13)18-15)9-11-5-1-2-6-12(11)10-16/h1-8H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase H
(Homo sapiens (Human)) | BDBM92912

(Aryl 1-indanylketone, 1)Show SMILES Oc1c(cc(cc1N(=O)=O)-c1cc(F)cc(c1O)N(=O)=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase C
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase-like 1
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92917

(Aryl 1-indanylketone, 6)Show InChI InChI=1S/C18H17FO2/c1-18(10-9-12-5-3-4-6-15(12)18)17(20)14-11-13(19)7-8-16(14)21-2/h3-8,11H,9-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase B
(Homo sapiens (Human)) | BDBM92912

(Aryl 1-indanylketone, 1)Show SMILES Oc1c(cc(cc1N(=O)=O)-c1cc(F)cc(c1O)N(=O)=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM92920

(Benzofuranone, 9)Show InChI InChI=1S/C17H14O3/c1-19-13-6-7-15-14(8-13)16(18)17(20-15)9-11-4-2-3-5-12(11)10-17/h2-8H,9-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase H
(Homo sapiens (Human)) | BDBM92913

(Aryl 1-indanylketone, 2)Show SMILES Nc1cc(F)cc(-c2ccc(O)c(c2)C(=O)C2CCc3ccccc23)c1O Show InChI InChI=1S/C22H18FNO3/c23-14-10-17(22(27)19(24)11-14)13-6-8-20(25)18(9-13)21(26)16-7-5-12-3-1-2-4-15(12)16/h1-4,6,8-11,16,25,27H,5,7,24H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | >-21.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 5 |
Max Planck Research
| Assay Description
PPIase activity assay were performed at 283 K in quartz cuvettes with a path length of 1 cm under vigorous stirring with a Hewlett-Packard 8453A UV-v... |
Biochemistry 48: 6268-77 (2009)
Article DOI: 10.1021/bi9007287
BindingDB Entry DOI: 10.7270/Q2X34W26 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CSF1R (unknown origin) by enzymatic assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00440
BindingDB Entry DOI: 10.7270/Q27S7SND |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50044004
(CHEMBL3356433)Show SMILES CN(C)C(=O)Oc1cccc(NC(=O)C2(CN)CCN(CC2)c2ncnc3[nH]cc(C)c23)c1 Show InChI InChI=1S/C23H29N7O3/c1-15-12-25-19-18(15)20(27-14-26-19)30-9-7-23(13-24,8-10-30)21(31)28-16-5-4-6-17(11-16)33-22(32)29(2)3/h4-6,11-12,14H,7-10,13,24H2,1-3H3,(H,28,31)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50586579
(CHEMBL5081039)Show SMILES CS(=O)(=O)N1CCC[C@@H](C1)NC(=O)[C@H](CCC1CCCCC1)NC(=O)c1ccc(CNC(=O)c2cnn(c2N)-c2ccccc2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p38alpha (unknown origin) expressed in HEK293T cells using NanoBRET NanoGlo substrate incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00868
BindingDB Entry DOI: 10.7270/Q2X352CP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17A
(Homo sapiens (Human)) | BDBM50585203
(CHEMBL5085430)Show SMILES Brc1cnc(Nc2cccc(NC(=O)N3CCCC3)c2)nc1NCCCNC(=O)C1CCC1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of tracer K9 from NLuc fused DRAK1 (unknown origin) expressed in HEK293 cells by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00440
BindingDB Entry DOI: 10.7270/Q27S7SND |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50586590
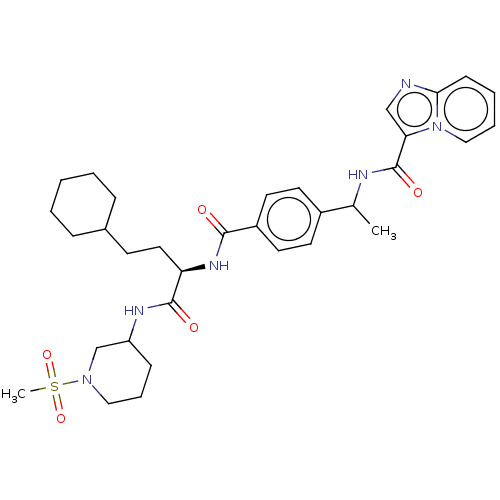
(CHEMBL5087073)Show SMILES CC(NC(=O)c1cnc2ccccn12)c1ccc(cc1)C(=O)N[C@H](CCC1CCCCC1)C(=O)NC1CCCN(C1)S(C)(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DDR2 (unknown origin) expressed in HEK293T cells using NanoBRET NanoGlo substrate incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00868
BindingDB Entry DOI: 10.7270/Q2X352CP |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50591090

(CHEMBL2141887)Show SMILES CC(C)C(=O)Nc1ncc(s1)-c1cc(nn1-c1c(Cl)cccc1Cl)C(F)F |(.04,3.6,;-1.29,2.83,;-2.62,3.6,;-1.29,1.29,;.04,.52,;-2.62,.52,;-2.62,-1.02,;-1.38,-1.93,;-1.85,-3.39,;-3.39,-3.39,;-3.87,-1.93,;-4.3,-4.64,;-5.84,-4.64,;-6.31,-6.1,;-5.07,-7.01,;-3.82,-6.1,;-2.36,-6.58,;-1.21,-5.55,;-1.53,-4.04,;.25,-6.03,;.57,-7.53,;-.57,-8.56,;-2.04,-8.09,;-3.18,-9.12,;-7.78,-6.58,;-8.92,-5.55,;-8.1,-8.09,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50591091

(CHEMBL5169387)Show SMILES CC(C)C(=O)Nc1ncc(s1)C(=O)NCCN(Cc1ccccc1)C(=O)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50591090

(CHEMBL2141887)Show SMILES CC(C)C(=O)Nc1ncc(s1)-c1cc(nn1-c1c(Cl)cccc1Cl)C(F)F |(.04,3.6,;-1.29,2.83,;-2.62,3.6,;-1.29,1.29,;.04,.52,;-2.62,.52,;-2.62,-1.02,;-1.38,-1.93,;-1.85,-3.39,;-3.39,-3.39,;-3.87,-1.93,;-4.3,-4.64,;-5.84,-4.64,;-6.31,-6.1,;-5.07,-7.01,;-3.82,-6.1,;-2.36,-6.58,;-1.21,-5.55,;-1.53,-4.04,;.25,-6.03,;.57,-7.53,;-.57,-8.56,;-2.04,-8.09,;-3.18,-9.12,;-7.78,-6.58,;-8.92,-5.55,;-8.1,-8.09,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50591091

(CHEMBL5169387)Show SMILES CC(C)C(=O)Nc1ncc(s1)C(=O)NCCN(Cc1ccccc1)C(=O)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50591090

(CHEMBL2141887)Show SMILES CC(C)C(=O)Nc1ncc(s1)-c1cc(nn1-c1c(Cl)cccc1Cl)C(F)F |(.04,3.6,;-1.29,2.83,;-2.62,3.6,;-1.29,1.29,;.04,.52,;-2.62,.52,;-2.62,-1.02,;-1.38,-1.93,;-1.85,-3.39,;-3.39,-3.39,;-3.87,-1.93,;-4.3,-4.64,;-5.84,-4.64,;-6.31,-6.1,;-5.07,-7.01,;-3.82,-6.1,;-2.36,-6.58,;-1.21,-5.55,;-1.53,-4.04,;.25,-6.03,;.57,-7.53,;-.57,-8.56,;-2.04,-8.09,;-3.18,-9.12,;-7.78,-6.58,;-8.92,-5.55,;-8.1,-8.09,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50591091

(CHEMBL5169387)Show SMILES CC(C)C(=O)Nc1ncc(s1)C(=O)NCCN(Cc1ccccc1)C(=O)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50556597
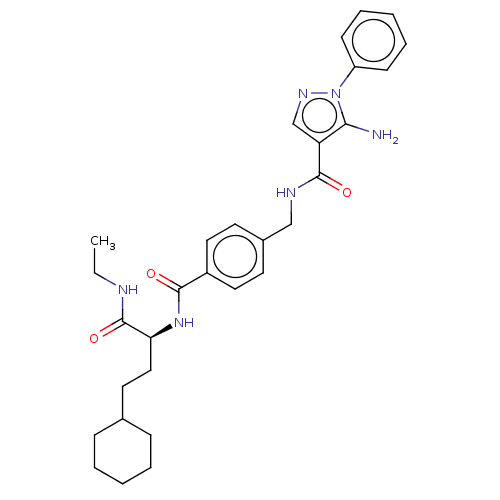
(CHEMBL4756586)Show SMILES CCNC(=O)[C@H](CCC1CCCCC1)NC(=O)c1ccc(CNC(=O)c2cnn(c2N)-c2ccccc2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p38alpha (unknown origin) expressed in HEK293T cells using NanoBRET NanoGlo substrate incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00868
BindingDB Entry DOI: 10.7270/Q2X352CP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17A
(Homo sapiens (Human)) | BDBM50606011
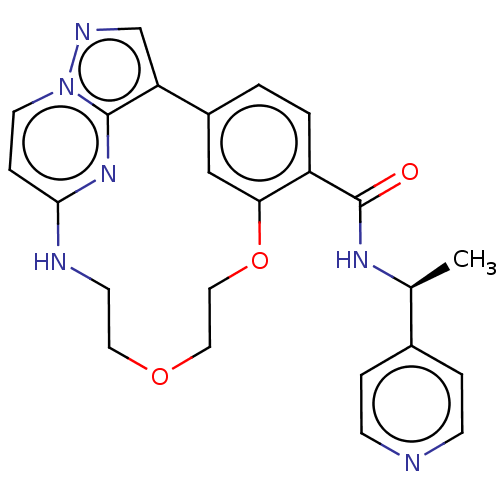
(CHEMBL5191892)Show SMILES C[C@H](NC(=O)c1ccc-2cc1OCCOCCNc1ccn3ncc-2c3n1)c1ccncc1 |r| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00173
BindingDB Entry DOI: 10.7270/Q2NC6587 |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50591082
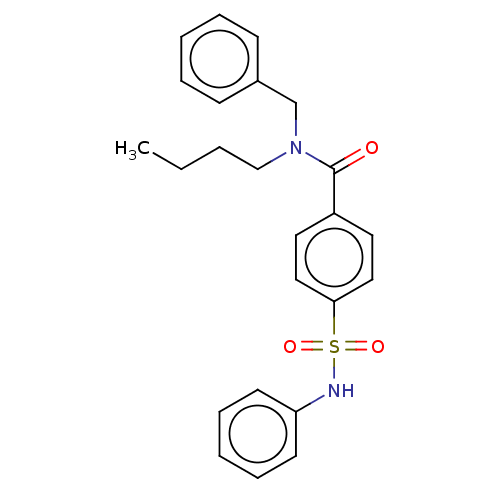
(CHEMBL4790618)Show SMILES CCCCN(Cc1ccccc1)C(=O)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50591090

(CHEMBL2141887)Show SMILES CC(C)C(=O)Nc1ncc(s1)-c1cc(nn1-c1c(Cl)cccc1Cl)C(F)F |(.04,3.6,;-1.29,2.83,;-2.62,3.6,;-1.29,1.29,;.04,.52,;-2.62,.52,;-2.62,-1.02,;-1.38,-1.93,;-1.85,-3.39,;-3.39,-3.39,;-3.87,-1.93,;-4.3,-4.64,;-5.84,-4.64,;-6.31,-6.1,;-5.07,-7.01,;-3.82,-6.1,;-2.36,-6.58,;-1.21,-5.55,;-1.53,-4.04,;.25,-6.03,;.57,-7.53,;-.57,-8.56,;-2.04,-8.09,;-3.18,-9.12,;-7.78,-6.58,;-8.92,-5.55,;-8.1,-8.09,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50556598
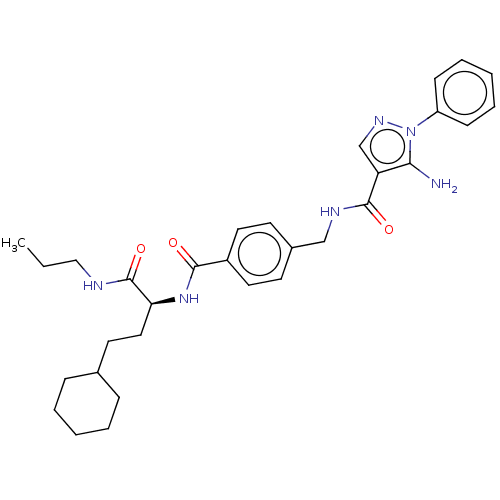
(CHEMBL4778587)Show SMILES CCCNC(=O)[C@H](CCC1CCCCC1)NC(=O)c1ccc(CNC(=O)c2cnn(c2N)-c2ccccc2)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p38alpha (unknown origin) expressed in HEK293T cells using NanoBRET NanoGlo substrate incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00868
BindingDB Entry DOI: 10.7270/Q2X352CP |
More data for this
Ligand-Target Pair | |
LIM domain kinase 1
(Homo sapiens (Human)) | BDBM50591090

(CHEMBL2141887)Show SMILES CC(C)C(=O)Nc1ncc(s1)-c1cc(nn1-c1c(Cl)cccc1Cl)C(F)F |(.04,3.6,;-1.29,2.83,;-2.62,3.6,;-1.29,1.29,;.04,.52,;-2.62,.52,;-2.62,-1.02,;-1.38,-1.93,;-1.85,-3.39,;-3.39,-3.39,;-3.87,-1.93,;-4.3,-4.64,;-5.84,-4.64,;-6.31,-6.1,;-5.07,-7.01,;-3.82,-6.1,;-2.36,-6.58,;-1.21,-5.55,;-1.53,-4.04,;.25,-6.03,;.57,-7.53,;-.57,-8.56,;-2.04,-8.09,;-3.18,-9.12,;-7.78,-6.58,;-8.92,-5.55,;-8.1,-8.09,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of NanoLuc-fused CSF1R (unknown origin) expressed in HEK293 cells incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00440
BindingDB Entry DOI: 10.7270/Q27S7SND |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50586589
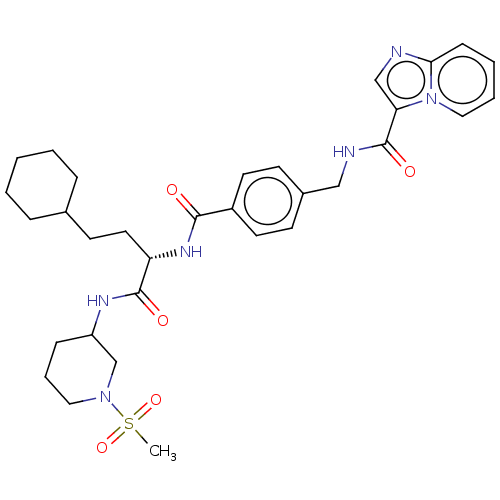
(SR-302)Show SMILES CS(=O)(=O)N1CCCC(C1)NC(=O)[C@H](CCC1CCCCC1)NC(=O)c1ccc(CNC(=O)c2cnc3ccccn23)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DDR2 (unknown origin) expressed in HEK293T cells using NanoBRET NanoGlo substrate incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00868
BindingDB Entry DOI: 10.7270/Q2X352CP |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM50585187
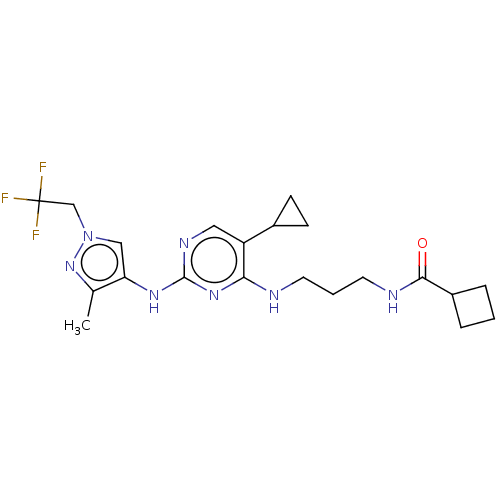
(CHEMBL5079601)Show SMILES Cc1nn(CC(F)(F)F)cc1Nc1ncc(C2CC2)c(NCCCNC(=O)C2CCC2)n1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human LRRK2 (970 to end residues) using RLGRDKYKTLRQIRQ as substrate incubated for 40 mins in presence of [gamma-33P-ATP] b... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00440
BindingDB Entry DOI: 10.7270/Q27S7SND |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50591091

(CHEMBL5169387)Show SMILES CC(C)C(=O)Nc1ncc(s1)C(=O)NCCN(Cc1ccccc1)C(=O)c1ccc(cc1)S(=O)(=O)Nc1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50586589

(SR-302)Show SMILES CS(=O)(=O)N1CCCC(C1)NC(=O)[C@H](CCC1CCCCC1)NC(=O)c1ccc(CNC(=O)c2cnc3ccccn23)cc1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DDR1 (unknown origin) expressed in HEK293T cells using NanoBRET NanoGlo substrate incubated for 2 hrs by NanoBRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00868
BindingDB Entry DOI: 10.7270/Q2X352CP |
More data for this
Ligand-Target Pair | |
LIM domain kinase 2
(Homo sapiens (Human)) | BDBM50072664
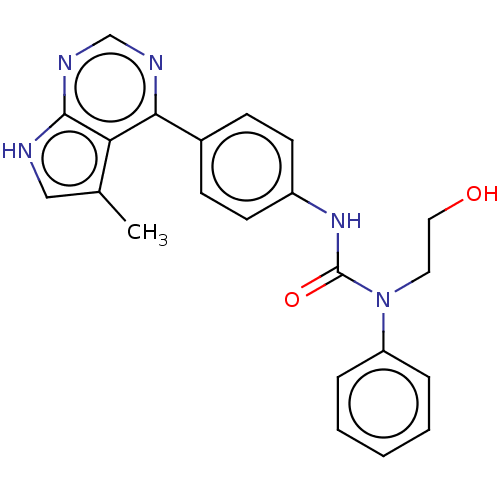
(CHEMBL3410036)Show SMILES Cc1c[nH]c2ncnc(-c3ccc(NC(=O)N(CCO)c4ccccc4)cc3)c12 Show InChI InChI=1S/C22H21N5O2/c1-15-13-23-21-19(15)20(24-14-25-21)16-7-9-17(10-8-16)26-22(29)27(11-12-28)18-5-3-2-4-6-18/h2-10,13-14,28H,11-12H2,1H3,(H,26,29)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01106
BindingDB Entry DOI: 10.7270/Q23R0XVH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17A
(Homo sapiens (Human)) | BDBM50606014
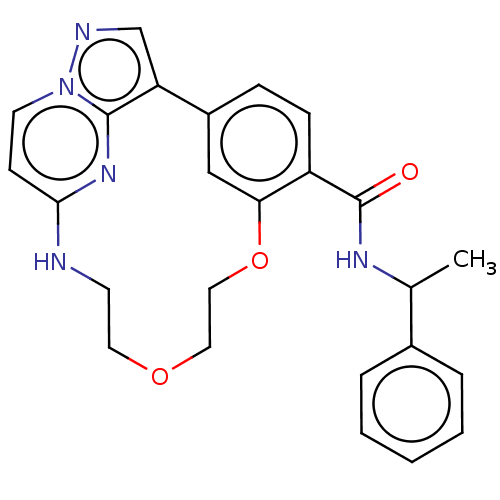
(CHEMBL5193635)Show SMILES CC(NC(=O)c1ccc-2cc1OCCOCCNc1ccn3ncc-2c3n1)c1ccccc1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00173
BindingDB Entry DOI: 10.7270/Q2NC6587 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data