

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17468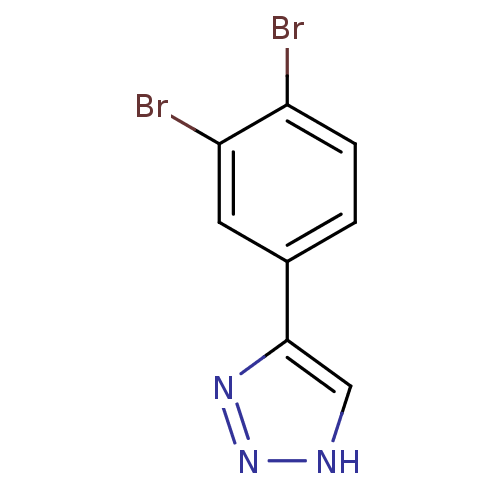 (1,2,3-triazole analogue, 24 | 5-(3,4-dibromophenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17461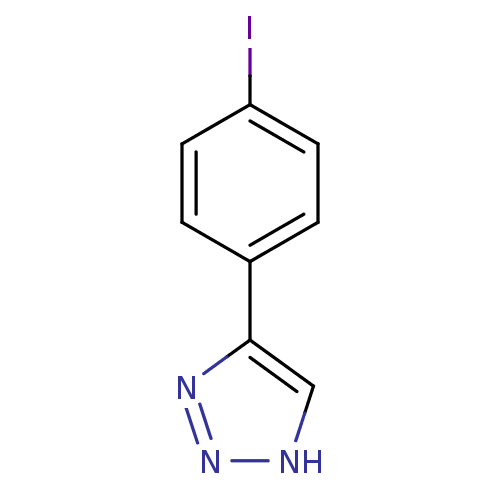 (1,2,3-triazole analogue, 17 | 5-(4-iodophenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17460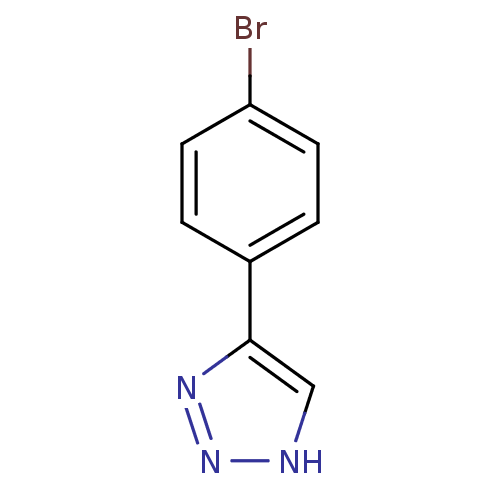 (1,2,3-triazole analogue, 16 | 5-(4-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17474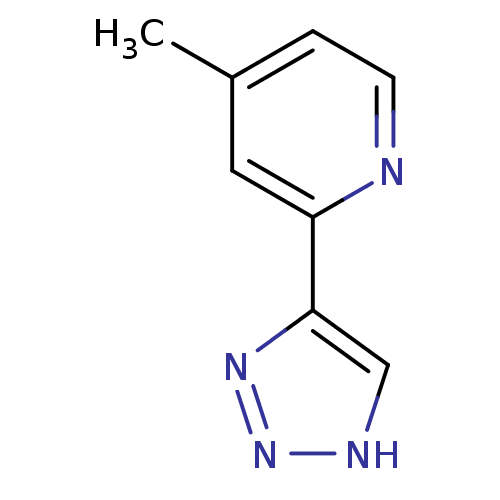 (1,2,3-triazole analogue, 30 | 4-methyl-2-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17467 (1,2,3-triazole analogue, 23 | 5-(3-bromophenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17473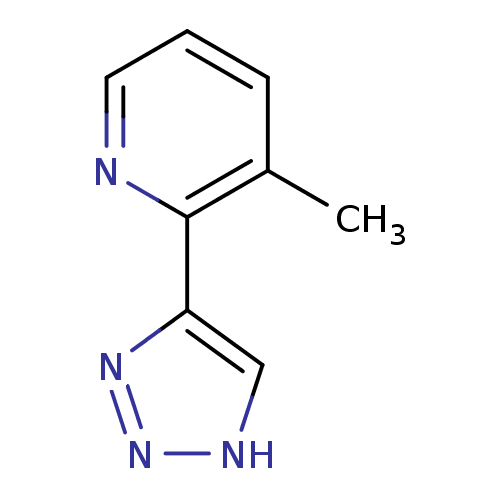 (1,2,3-triazole analogue, 29 | 3-methyl-2-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17459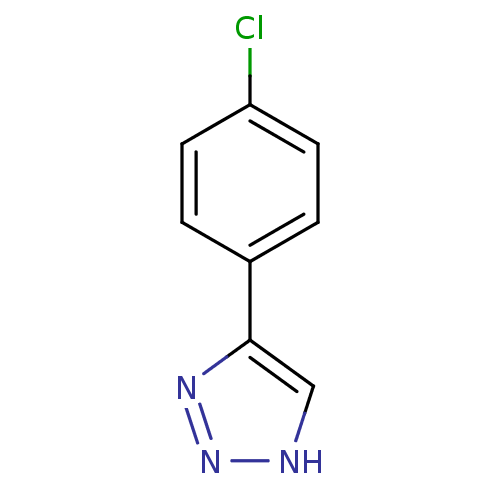 (1,2,3-triazole analogue, 15 | 5-(4-chlorophenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17475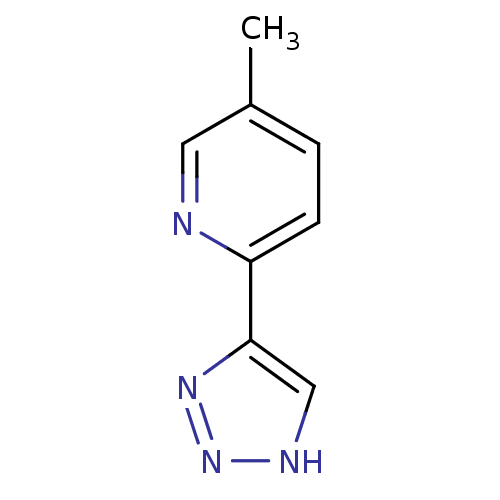 (1,2,3-triazole analogue, 31 | 5-methyl-2-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 8 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17469 (1,2,3-triazole analogue, 25 | 5-(1-benzofuran-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | -44.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17447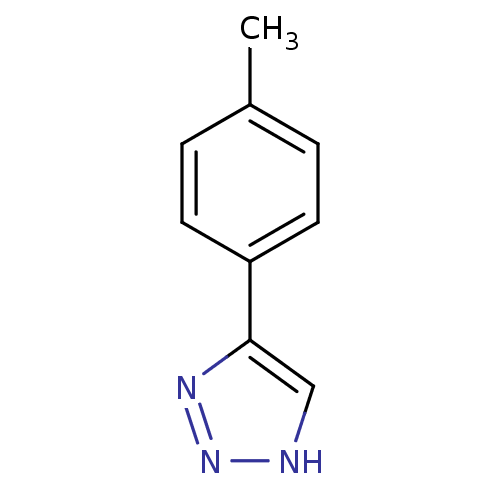 (1,2,3-triazole analogue, 3 | 5-(4-methylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17466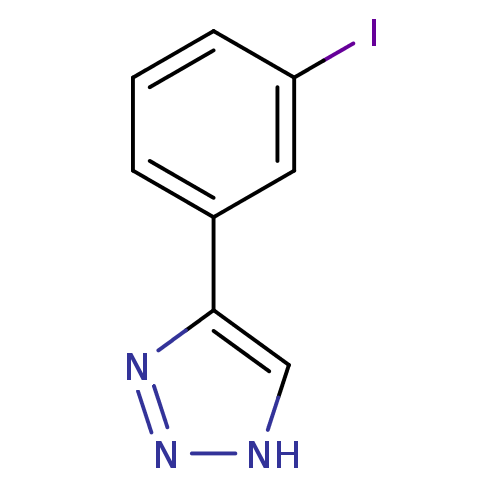 (1,2,3-triazole analogue, 22 | 5-(3-iodophenyl)-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17462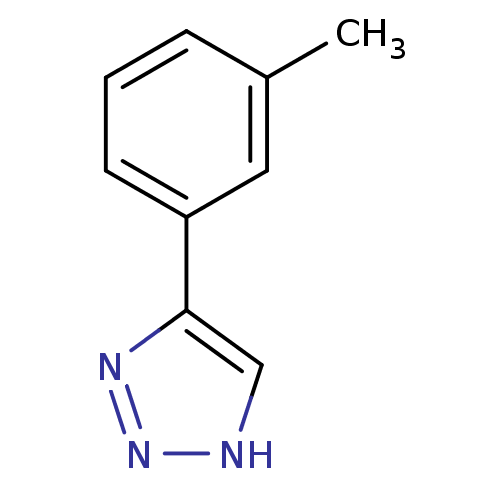 (1,2,3-triazole analogue, 18 | 5-(3-methylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 18 | -43.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17465 (1,2,3-triazole analogue, 21 | N-benzyl-3-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17455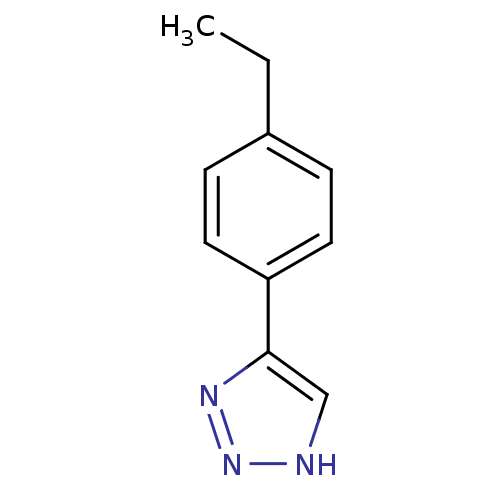 (1,2,3-triazole analogue, 11 | 5-(4-ethylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17470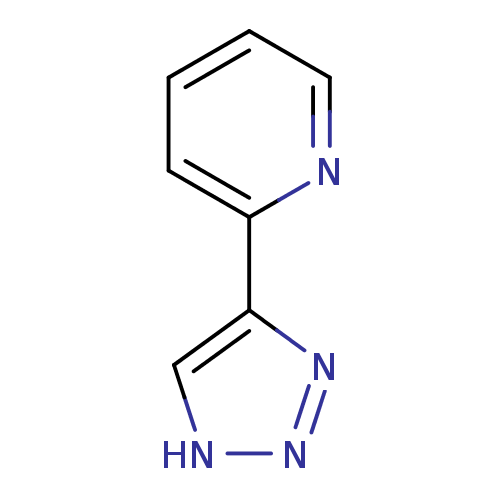 (1,2,3-triazole analogue, 26 | 2-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17449 (1,2,3-triazole analogue, 5 | 1H,4H-indeno[1,2-d][1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17476 (1,2,3-triazole analogue, 32 | 2-methyl-6-(1H-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 52 | -41.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17448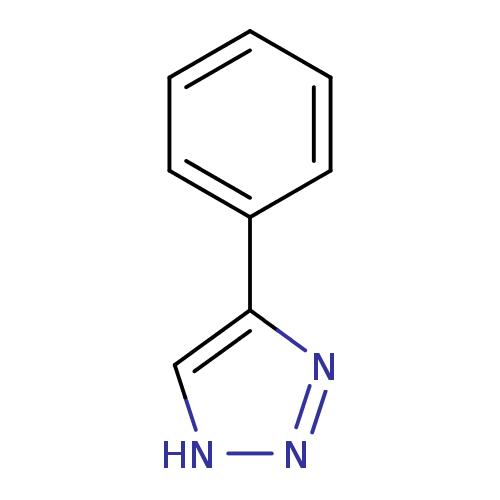 (1,2,3-triazole analogue, 4 | 5-phenyl-1H-1,2,3-tri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 70 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17452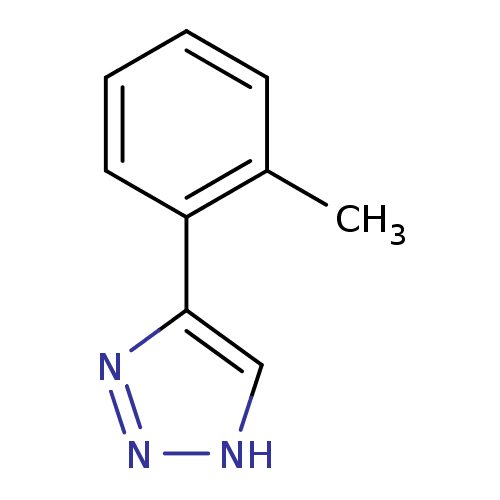 (1,2,3-triazole analogue, 8 | 5-(2-methylphenyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 112 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17453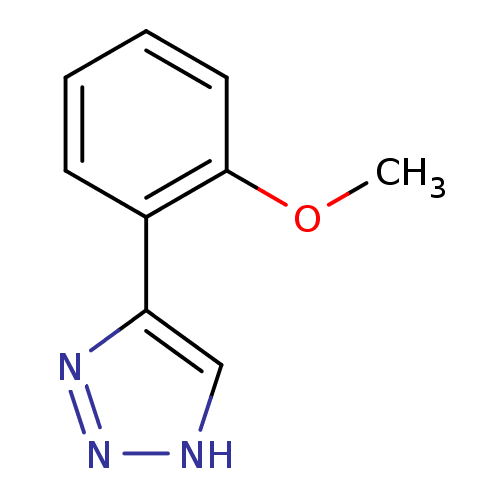 (1,2,3-triazole analogue, 9 | 5-(2-methoxyphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 150 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17471 (1,2,3-triazole analogue, 27 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | 260 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17464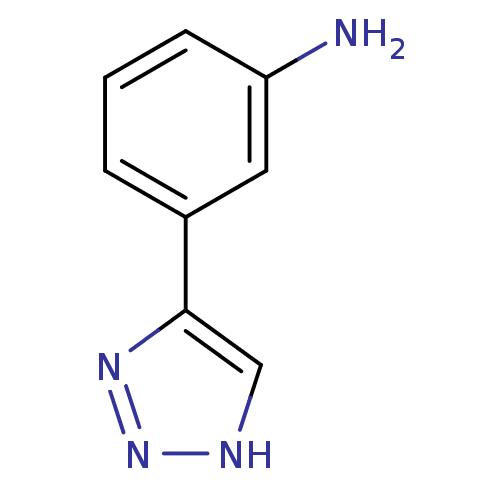 (1,2,3-triazole analogue, 20 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17450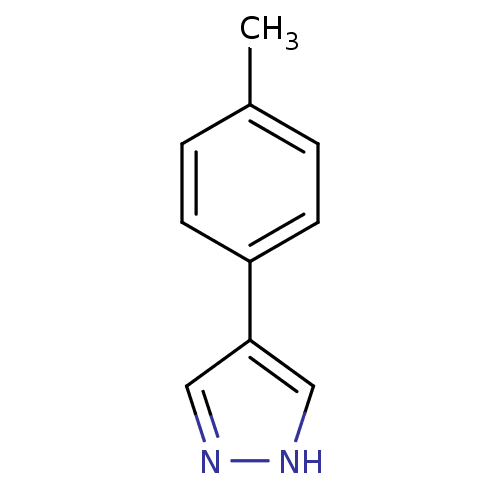 (4-(4-methylphenyl)-1H-pyrazole | pyrazole analogue...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17463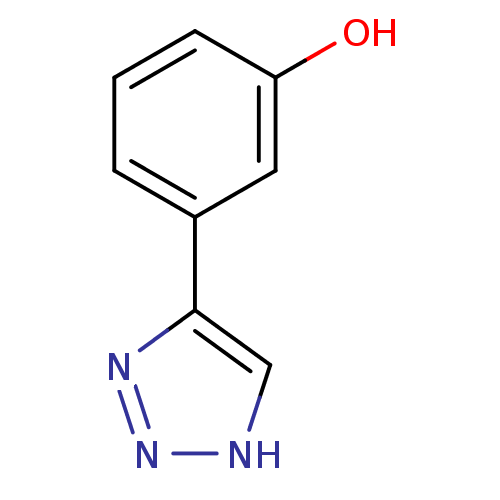 (1,2,3-triazole analogue, 19 | 3-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17458 (1,2,3-triazole analogue, 14 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 337 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17454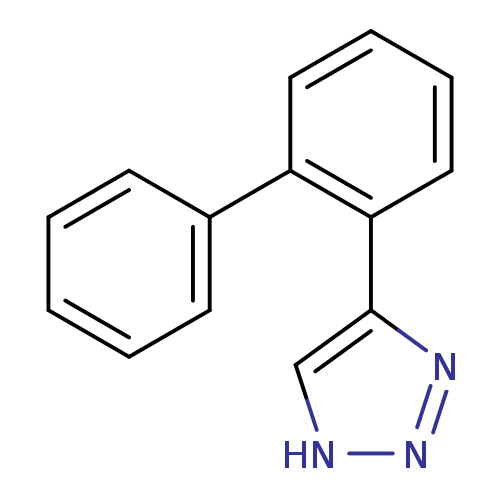 (1,2,3-triazole analogue, 10 | 5-(2-phenylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17456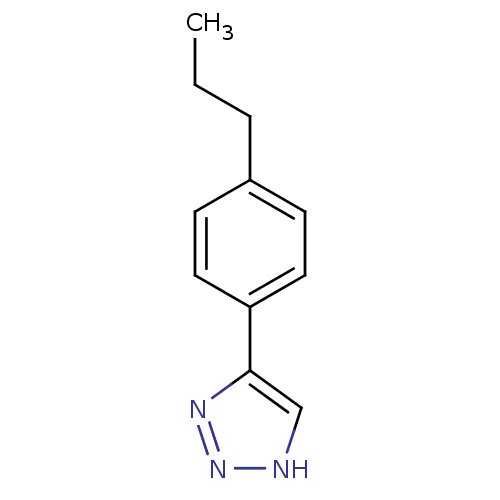 (1,2,3-triazole analogue, 12 | 5-(4-propylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17457 (1,2,3-triazole analogue, 13 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17472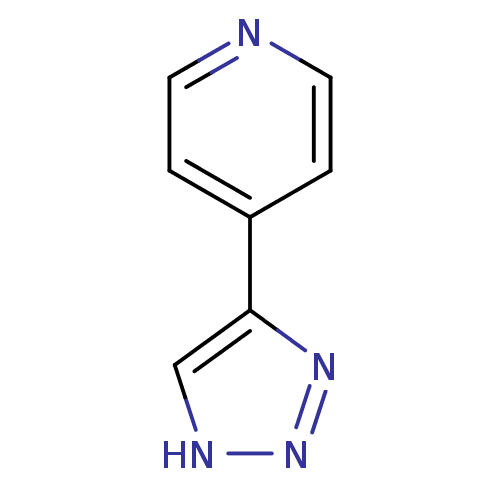 (1,2,3-triazole analogue, 28 | 4-(1H-1,2,3-triazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | >-33.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 48: 5644-7 (2005) Article DOI: 10.1021/jm050408c BindingDB Entry DOI: 10.7270/Q26M3537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50259819 (CHEMBL481635 | Calceolarioside | Calceolarioside A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50269517 (CHEMBL504363 | Forsythiaside | forsythoside A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50478448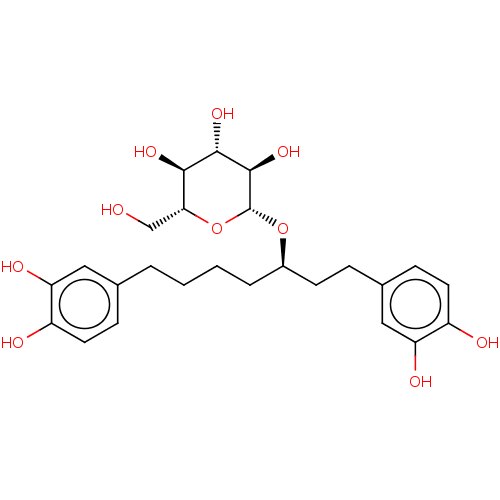 (CHEMBL464362) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1407-9 (1998) Article DOI: 10.1021/np9801460 BindingDB Entry DOI: 10.7270/Q2CN76PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50478445 (Dehydrohirsutanonol | Hirsutanone | Hirsutenone | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1407-9 (1998) Article DOI: 10.1021/np9801460 BindingDB Entry DOI: 10.7270/Q2CN76PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50269516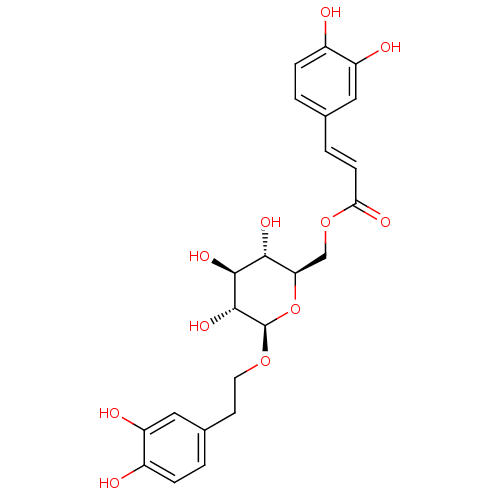 (CHEMBL518414 | Calceolarioside B) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50478446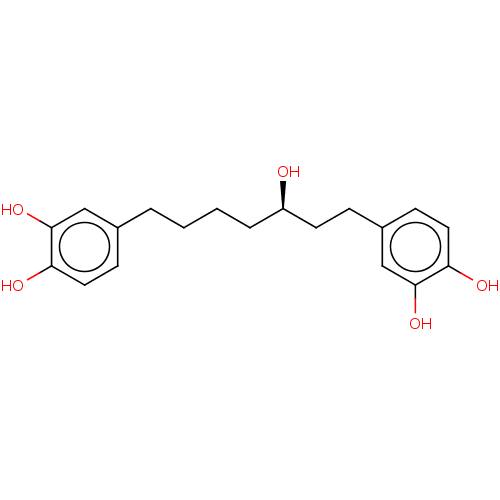 (CHEMBL464363) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1407-9 (1998) Article DOI: 10.1021/np9801460 BindingDB Entry DOI: 10.7270/Q2CN76PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Saccharomyces cerevisiae S288c) | BDBM50090044 ((Z)-2',3'-dihydroxy-3,4,4',5-tetramethoxystilbene ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in galactose medium by microtiter plate assay | J Nat Prod 63: 457-60 (2000) Article DOI: 10.1021/np9904410 BindingDB Entry DOI: 10.7270/Q2930WZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Saccharomyces cerevisiae S288c) | BDBM50090044 ((Z)-2',3'-dihydroxy-3,4,4',5-tetramethoxystilbene ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in glucose medium by microtiter plate assay | J Nat Prod 63: 457-60 (2000) Article DOI: 10.1021/np9904410 BindingDB Entry DOI: 10.7270/Q2930WZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50377909 (ACETOSIDE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50478447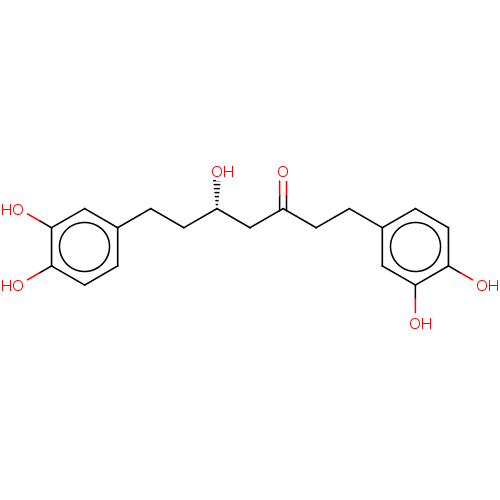 (Hirsutanonol) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1407-9 (1998) Article DOI: 10.1021/np9801460 BindingDB Entry DOI: 10.7270/Q2CN76PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50269518 (CHEMBL452413 | Plantainoside D) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50478444 (Oregonin) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1407-9 (1998) Article DOI: 10.1021/np9801460 BindingDB Entry DOI: 10.7270/Q2CN76PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50250350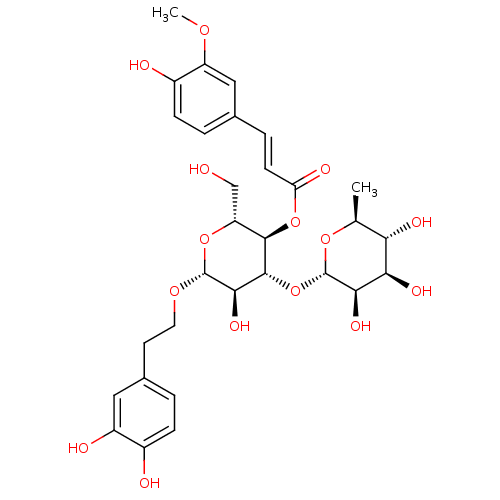 (CHEMBL450121 | Leucosceptoside A) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Saccharomyces cerevisiae S288c) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in galactose medium by microtiter plate assay | J Nat Prod 63: 457-60 (2000) Article DOI: 10.1021/np9904410 BindingDB Entry DOI: 10.7270/Q2930WZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Saccharomyces cerevisiae S288c) | BDBM50005480 ((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in glucose medium by microtiter plate assay | J Nat Prod 63: 457-60 (2000) Article DOI: 10.1021/np9904410 BindingDB Entry DOI: 10.7270/Q2930WZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50241873 (CHEMBL499145 | Poliumoside) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50269515 (2-(3-hydroxy-4-methoxy-phenyl)-ethyl-O-(alpha-L-rh...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of human recombinant PKCalpha | J Nat Prod 61: 1410-2 (1999) Article DOI: 10.1021/np980147s BindingDB Entry DOI: 10.7270/Q2348M80 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Saccharomyces cerevisiae S288c) | BDBM50478907 (CHEMBL497532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.38E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in galactose medium by microtiter plate assay | J Nat Prod 63: 457-60 (2000) Article DOI: 10.1021/np9904410 BindingDB Entry DOI: 10.7270/Q2930WZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Saccharomyces cerevisiae S288c) | BDBM50478907 (CHEMBL497532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Polytechnic Institute and State University Curated by ChEMBL | Assay Description Inhibition of topoisomerase 1 in Saccharomyces cerevisiae RS321N in glucose medium by microtiter plate assay | J Nat Prod 63: 457-60 (2000) Article DOI: 10.1021/np9904410 BindingDB Entry DOI: 10.7270/Q2930WZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50393440 (CHEMBL2159498) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant USP7 expressed in Sf9 cells by Ub-CHOP reporter assay | ACS Med Chem Lett 3: 789-792 (2012) Article DOI: 10.1021/ml200276j BindingDB Entry DOI: 10.7270/Q2154J51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 7 (Homo sapiens (Human)) | BDBM50393441 (CHEMBL2159499) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of recombinant USP7 expressed in Sf9 cells by Ub-CHOP reporter assay | ACS Med Chem Lett 3: 789-792 (2012) Article DOI: 10.1021/ml200276j BindingDB Entry DOI: 10.7270/Q2154J51 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |