

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50095296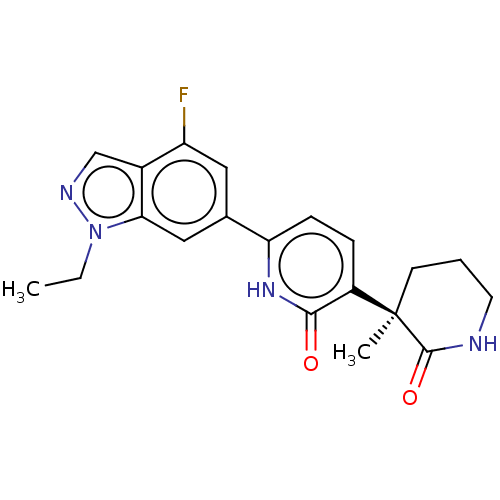 (CHEMBL3589082 | US9278953, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335698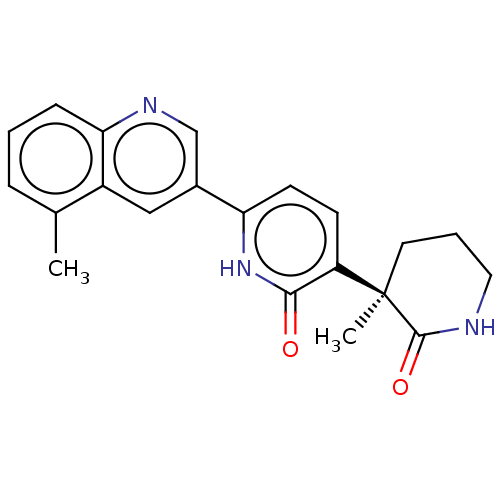 ((R)-3-(3-Methyl-2-oxopiperidin-3-yl)-6-(5-methylqu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50095295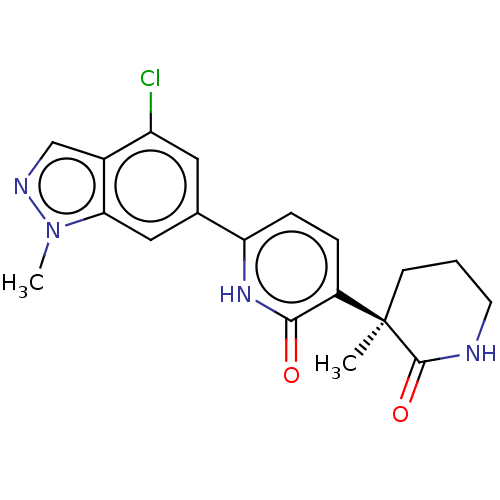 (CHEMBL3589083 | US9278953, 2) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 3.60 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50032651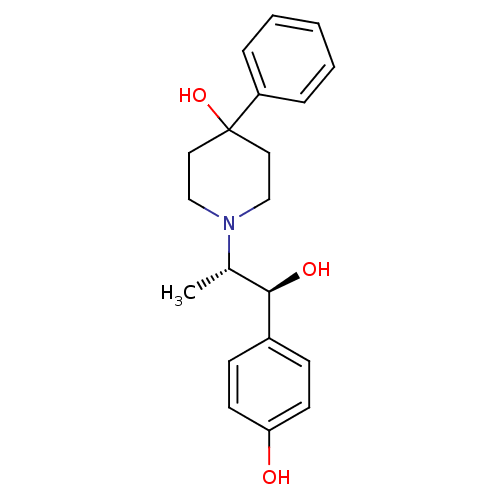 (1-((1S,2S)-1-hydroxy-1-(4-hydroxyphenyl)propan-2-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335700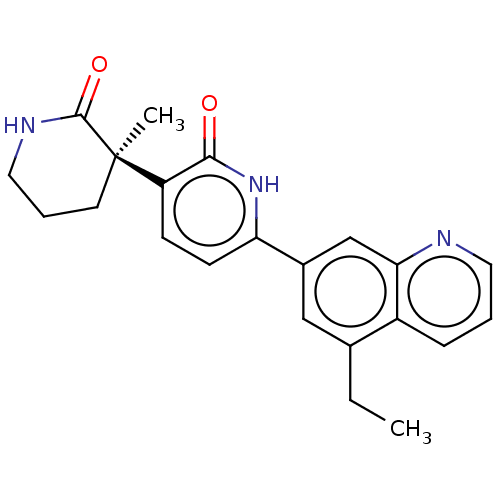 ((R)-6-(5-Ethylquinolin-7-yl)-3-(3-methyl-2-oxopipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250387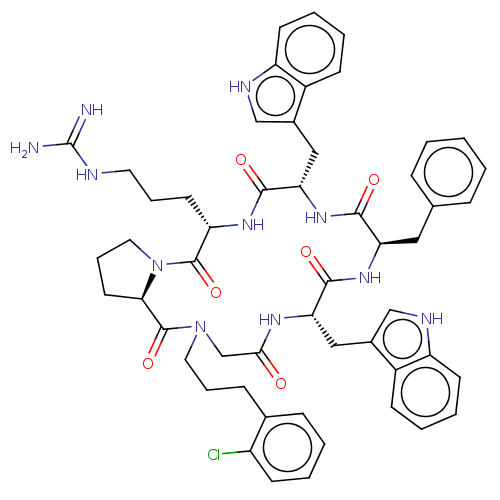 (CHEMBL4102791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250387 (CHEMBL4102791) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335699 ((R)-3-(3-Methyl-2-oxopiperidin-3-yl)-6-(5-methylqu...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335702 ((R)-6-(5-Cyclopropylquinolin-7-yl)-3-(3-methyl-2-o...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50095294 (CHEMBL3589084 | US9278953, 7) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 7.80 | -46.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250402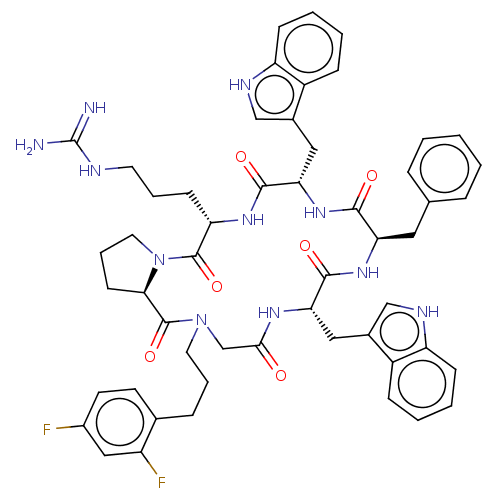 (CHEMBL4084835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264278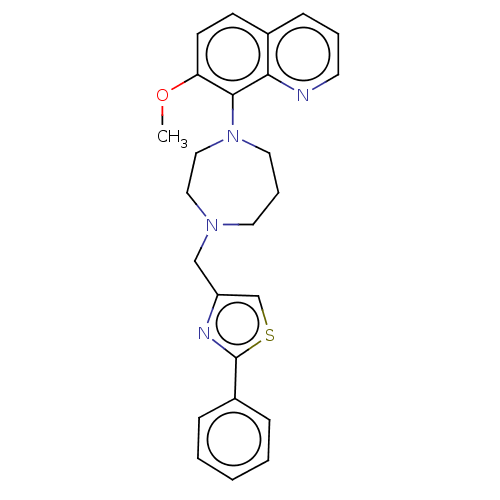 (CHEMBL4064803) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264278 (CHEMBL4064803) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250402 (CHEMBL4084835) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335701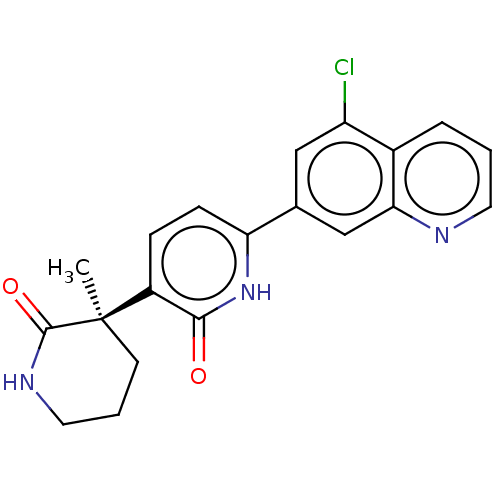 ((R)-6-(5-Chloroquinolin-7-yl)-3-(3-methyl-2-oxopip...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250375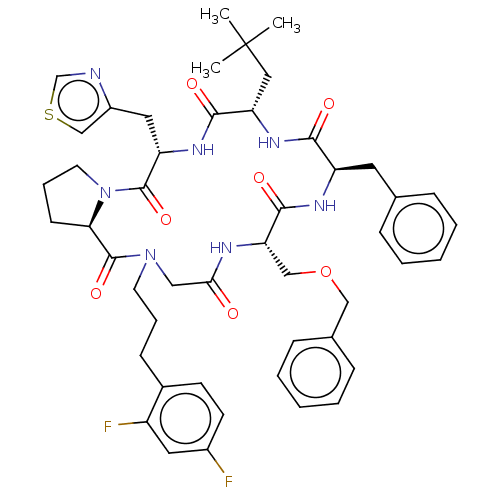 (CHEMBL4077017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250375 (CHEMBL4077017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250395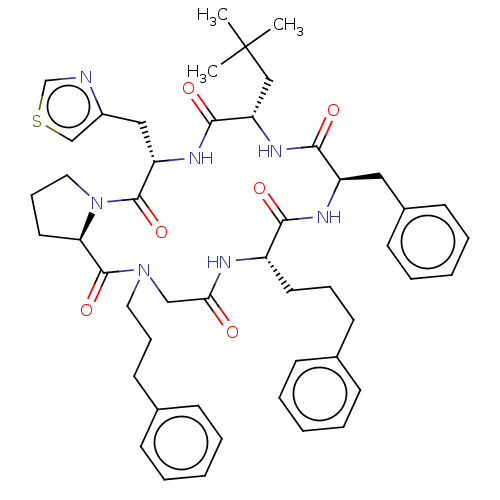 (CHEMBL4103373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250395 (CHEMBL4103373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264278 (CHEMBL4064803) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to CXCR7 (unknown origin) assessed as inhibition constant | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128320 BindingDB Entry DOI: 10.7270/Q2Q2441F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212596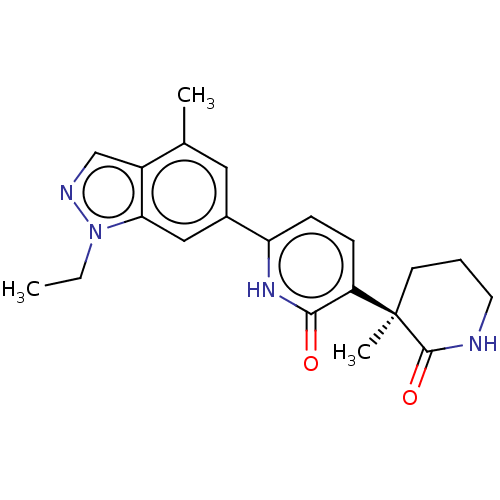 (US9278953, 3) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.5 | -45.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212598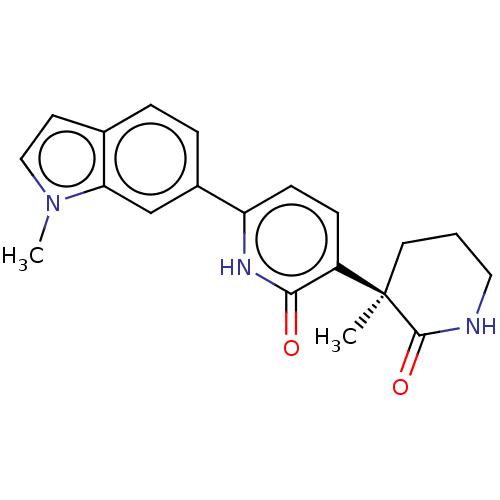 (US9278953, 4) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11.2 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212605 (US9278953, 9) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11.9 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344250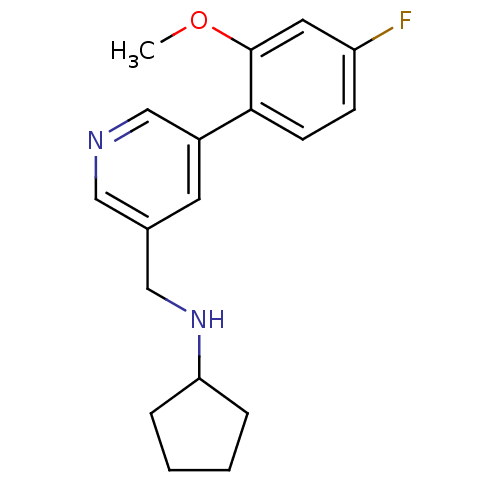 (CHEMBL1779003 | N-((5-(4-fluoro-2-methoxyphenyl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212600 (US9278953, 5) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.2 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264279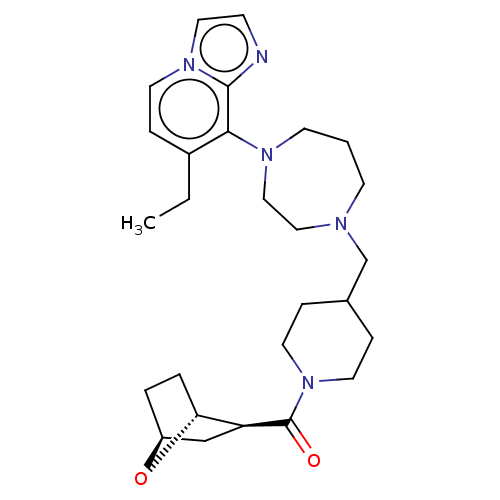 (CHEMBL4072602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264279 (CHEMBL4072602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264279 (CHEMBL4072602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264279 (CHEMBL4072602) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264280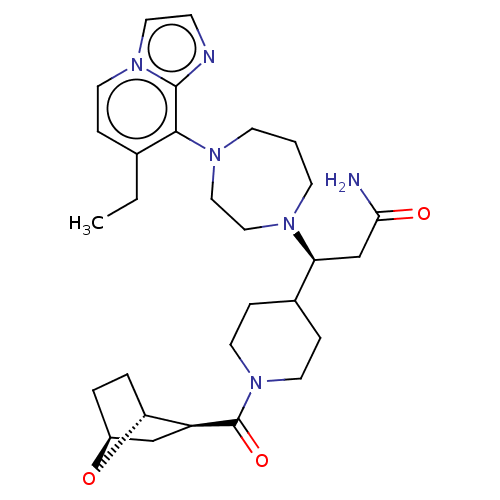 (CHEMBL4084050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264280 (CHEMBL4084050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50264278 (CHEMBL4064803) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ERG by membrane-competitive-binding assay | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212607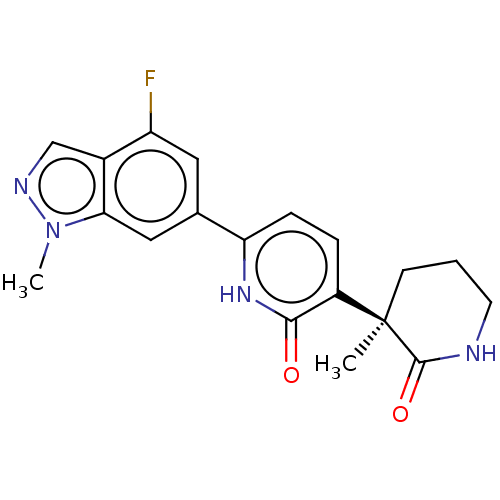 (US9278953, 11) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.9 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212604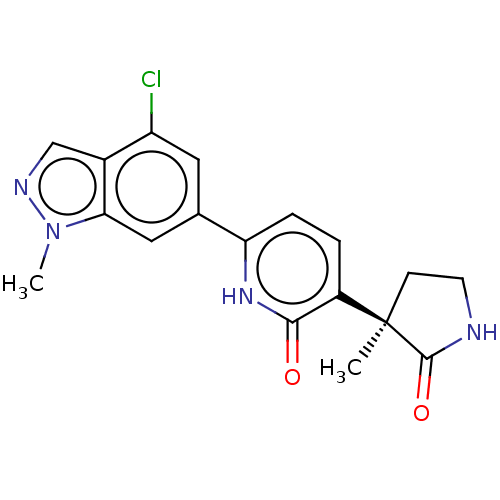 (US9278953, 8) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14.2 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50567153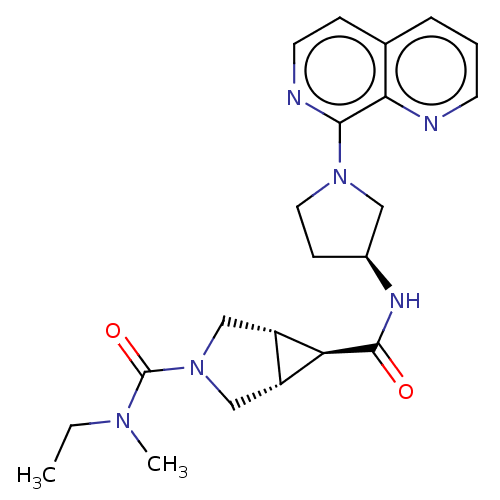 (CHEMBL4855707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to CXCR7 (unknown origin) assessed as inhibition constant | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128320 BindingDB Entry DOI: 10.7270/Q2Q2441F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Mus musculus) | BDBM50264280 (CHEMBL4084050) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from mouse CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM335704 ((R)-3-(3-Methyl-2-oxopyrrolidin-3-yl)-6-(5-methylq...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description To measure the ability of test compounds in the present invention to bind to the human EP3 receptor, and therefore have the potential to antagonize P... | US Patent US9738626 (2017) BindingDB Entry DOI: 10.7270/Q2CF9S7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212606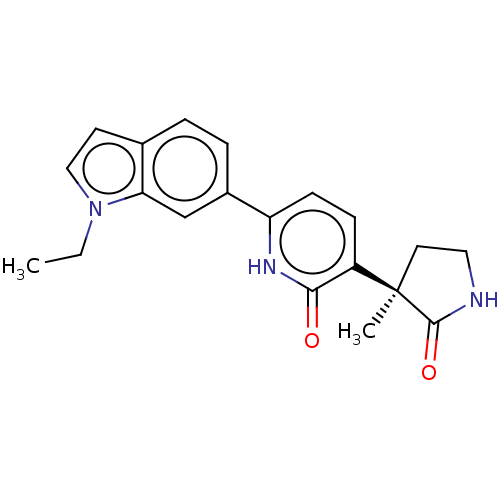 (US9278953, 10) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19.9 | -44.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250391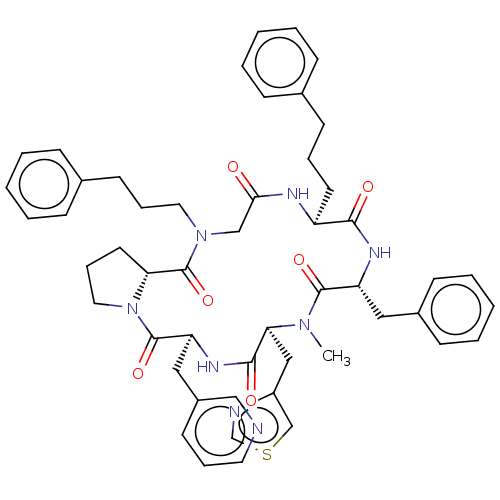 (CHEMBL4064492) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250391 (CHEMBL4064492) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250380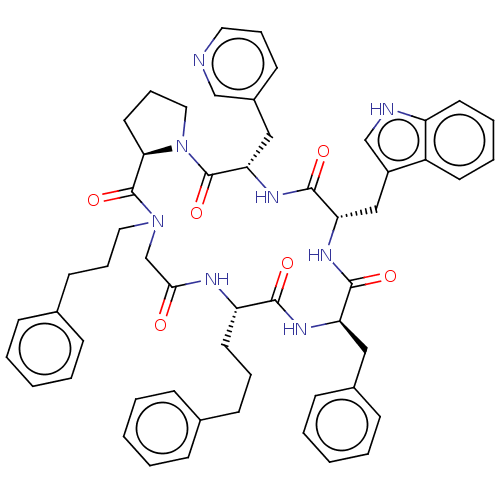 (CHEMBL4070766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250401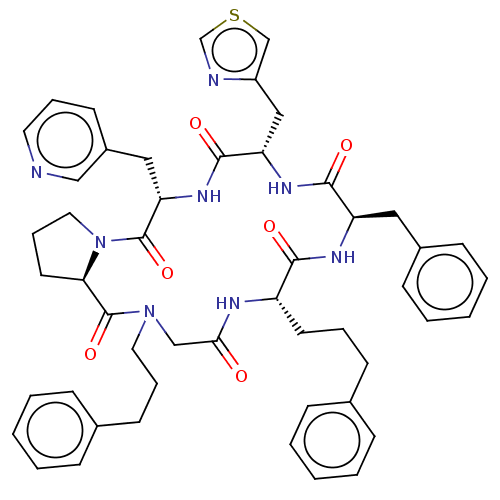 (CHEMBL4067701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250380 (CHEMBL4070766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250393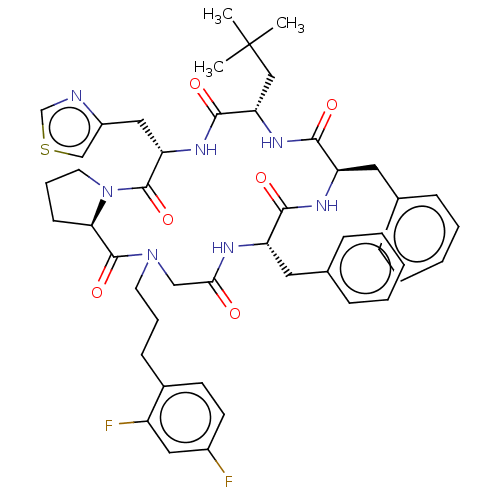 (CHEMBL4097577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Modulation of ProLink-fused human CXCR7 expressed in CHOK1 cell membranes assessed as induction of EA-tagged beta-arrestin recruitment after 30 mins ... | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250401 (CHEMBL4067701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212608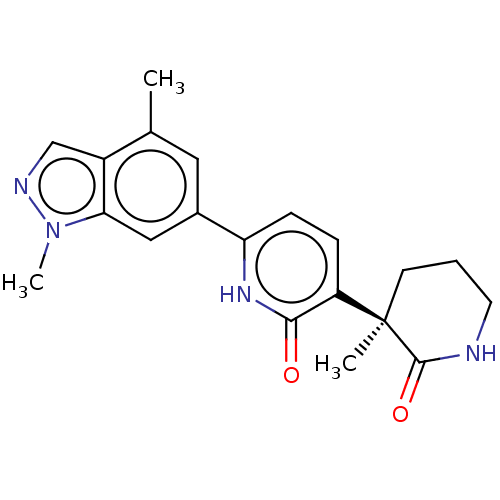 (US9278953, 12) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 25 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50250393 (CHEMBL4097577) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-CXCL12 from human CXCR7 expressed in CHOK1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 60: 9653-9663 (2017) Article DOI: 10.1021/acs.jmedchem.7b01028 BindingDB Entry DOI: 10.7270/Q2ZG6VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Rattus norvegicus (Rat)) | BDBM50344232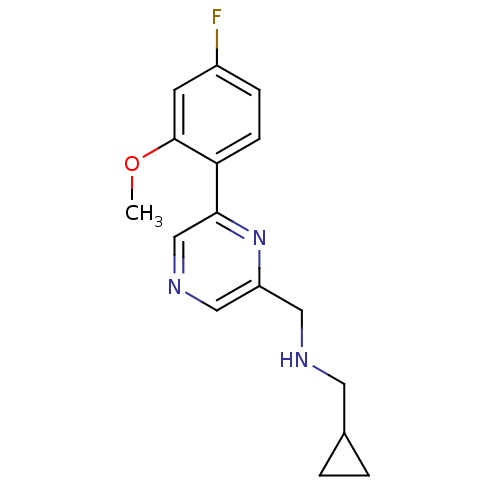 (1-cyclopropyl-N-((6-(4-fluoro-2-methoxyphenyl)pyra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]CP101606 from NR2B in rat brain minus cerebellum membrane | Bioorg Med Chem Lett 21: 3399-403 (2011) Article DOI: 10.1016/j.bmcl.2011.03.117 BindingDB Entry DOI: 10.7270/Q29887BK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Atypical chemokine receptor 3 (Homo sapiens (Human)) | BDBM50264271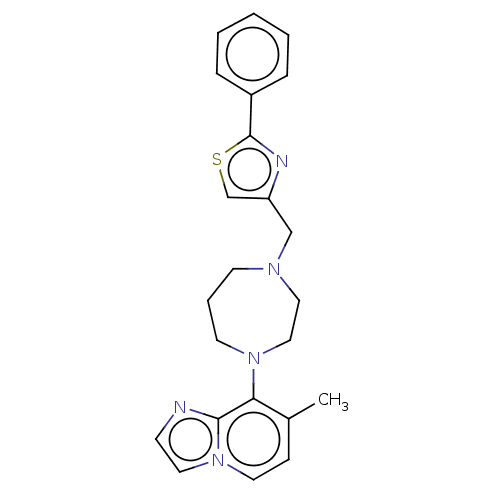 (CHEMBL4086432) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CXCL12 from human CXCR7 expressed in CHO-K1 cell membranes after 2 hrs by scintillation counting method | J Med Chem 61: 3685-3696 (2018) Article DOI: 10.1021/acs.jmedchem.8b00190 BindingDB Entry DOI: 10.7270/Q2KD21C4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM212609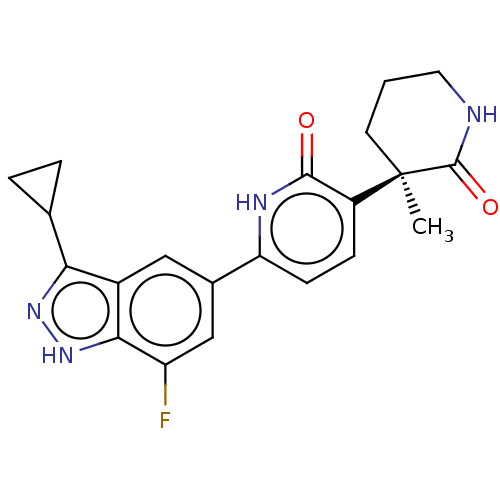 (US9278953, 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 29.2 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc. US Patent | Assay Description Test compounds were half log serially diluted in 100% DMSO (J. T. Baker #922401). 1 uL of each compound was added to appropriate wells of a 384-well ... | US Patent US9278953 (2016) BindingDB Entry DOI: 10.7270/Q2W37V5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 274 total ) | Next | Last >> |