 Found 2977 hits with Last Name = 'miller' and Initial = 'w'
Found 2977 hits with Last Name = 'miller' and Initial = 'w' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121249

(CHEMBL415845 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-Aib-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-7-49(69(120)106-57(37-60(85)112)75(126)100-50(65(86)116)28-29-59(84)111)99-74(125)55(34-42(2)3)105-72(123)51(24-14-16-30-81)103-71(122)54(27-19-33-92-80(89)90)102-67(118)44(5)97-77(128)58(41-109)107-73(124)52(25-15-17-31-82)104-70(121)53(26-18-32-91-79(87)88)101-66(117)43(4)96-62(114)40-95-78(129)64(45(6)110)108-76(127)56(36-47-22-12-9-13-23-47)98-63(115)39-93-61(113)38-94-68(119)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,109-110H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,119)(H,95,129)(H,96,114)(H,97,128)(H,98,115)(H,99,125)(H,100,126)(H,101,117)(H,102,118)(H,103,122)(H,104,121)(H,105,123)(H,106,120)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121247
(CHEMBL414542 | F-G-G-F-T-G-Aib-R-K-S-A-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-7-49(98-63(115)40-95-78(129)64(45(6)110)108-76(127)56(36-47-22-12-9-13-23-47)99-62(114)39-93-61(113)38-94-68(119)48(83)35-46-20-10-8-11-21-46)69(120)102-54(27-19-33-92-80(89)90)71(122)104-52(25-15-17-31-82)73(124)107-58(41-109)77(128)97-43(4)66(117)101-53(26-18-32-91-79(87)88)70(121)103-51(24-14-16-30-81)72(123)106-55(34-42(2)3)74(125)96-44(5)67(118)105-57(37-60(85)112)75(126)100-50(65(86)116)28-29-59(84)111/h8-13,20-23,42-45,48-58,64,109-110H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,119)(H,95,129)(H,96,125)(H,97,128)(H,98,115)(H,99,114)(H,100,126)(H,101,117)(H,102,120)(H,103,121)(H,104,122)(H,105,118)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121245
(CHEMBL266191 | F-G-G-F-T-G-Aib-R-K-S-Aib-R-K-L-A-N...)Show SMILES CC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C81H134N28O21/c1-7-49(98-64(116)41-96-79(130)65(45(6)111)109-77(128)57(37-47-23-13-10-14-24-47)99-63(115)40-94-62(114)39-95-68(119)48(84)36-46-21-11-9-12-22-46)69(120)102-54(27-19-33-92-80(88)89)71(122)105-53(26-16-18-32-83)74(125)108-59(42-110)78(129)100-50(8-2)70(121)103-55(28-20-34-93-81(90)91)72(123)104-52(25-15-17-31-82)73(124)107-56(35-43(3)4)75(126)97-44(5)67(118)106-58(38-61(86)113)76(127)101-51(66(87)117)29-30-60(85)112/h9-14,21-24,43-45,48-59,65,110-111H,7-8,15-20,25-42,82-84H2,1-6H3,(H2,85,112)(H2,86,113)(H2,87,117)(H,94,114)(H,95,119)(H,96,130)(H,97,126)(H,98,116)(H,99,115)(H,100,129)(H,101,127)(H,102,120)(H,103,121)(H,104,123)(H,105,122)(H,106,118)(H,107,124)(H,108,125)(H,109,128)(H4,88,89,92)(H4,90,91,93)/t44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121246
(CHEMBL438537 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-MeA-N-Q...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-42(2)34-57(78(129)108(7)45(5)68(119)104-56(37-60(85)112)74(125)99-50(65(86)116)28-29-59(84)111)105-72(123)51(24-14-16-30-81)102-71(122)54(27-19-33-92-80(89)90)101-67(118)44(4)97-76(127)58(41-109)106-73(124)52(25-15-17-31-82)103-70(121)53(26-18-32-91-79(87)88)100-66(117)43(3)96-62(114)40-95-77(128)64(46(6)110)107-75(126)55(36-48-22-12-9-13-23-48)98-63(115)39-93-61(113)38-94-69(120)49(83)35-47-20-10-8-11-21-47/h8-13,20-23,42-46,49-58,64,109-110H,14-19,24-41,81-83H2,1-7H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,120)(H,95,128)(H,96,114)(H,97,127)(H,98,115)(H,99,125)(H,100,117)(H,101,118)(H,102,122)(H,103,121)(H,104,119)(H,105,123)(H,106,124)(H,107,126)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121253
(CHEMBL408356 | F-G-G-F-T-G-A-R-K-S-Aib-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C80H132N28O21/c1-7-49(69(120)102-54(27-19-33-92-80(89)90)71(122)103-51(24-14-16-30-81)72(123)106-55(34-42(2)3)74(125)97-44(5)67(118)105-57(37-60(85)112)75(126)100-50(65(86)116)28-29-59(84)111)99-77(128)58(41-109)107-73(124)52(25-15-17-31-82)104-70(121)53(26-18-32-91-79(87)88)101-66(117)43(4)96-62(114)40-95-78(129)64(45(6)110)108-76(127)56(36-47-22-12-9-13-23-47)98-63(115)39-93-61(113)38-94-68(119)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,109-110H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,111)(H2,85,112)(H2,86,116)(H,93,113)(H,94,119)(H,95,129)(H,96,114)(H,97,125)(H,98,115)(H,99,128)(H,100,126)(H,101,117)(H,102,120)(H,103,122)(H,104,121)(H,105,118)(H,106,123)(H,107,124)(H,108,127)(H4,87,88,91)(H4,89,90,92)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121244
(CHEMBL264084 | F-G-G-F-T-G-Aib-R-K-S-A-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C80H131N27O22/c1-7-49(97-63(114)40-94-77(127)64(45(6)109)107-75(125)56(36-47-22-12-9-13-23-47)98-62(113)39-92-61(112)38-93-67(117)48(83)35-46-20-10-8-11-21-46)68(118)100-53(27-19-33-91-80(88)89)70(120)102-51(25-15-17-31-82)72(122)106-58(41-108)76(126)96-43(4)65(115)99-52(26-18-32-90-79(86)87)69(119)101-50(24-14-16-30-81)71(121)105-55(34-42(2)3)73(123)95-44(5)66(116)104-57(37-60(85)111)74(124)103-54(78(128)129)28-29-59(84)110/h8-13,20-23,42-45,48-58,64,108-109H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,117)(H,94,127)(H,95,123)(H,96,126)(H,97,114)(H,98,113)(H,99,115)(H,100,118)(H,101,119)(H,102,120)(H,103,124)(H,104,116)(H,105,121)(H,106,122)(H,107,125)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017686

(4-[4-(4-Chloro-3-trifluoromethyl-phenyl)-4-hydroxy...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)c(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C30H32ClF3N2O2/c1-35(2)27(37)29(22-9-5-3-6-10-22,23-11-7-4-8-12-23)17-20-36-18-15-28(38,16-19-36)24-13-14-26(31)25(21-24)30(32,33)34/h3-14,21,38H,15-20H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153608
(4-[4-Hydroxy-4-(3-trifluoromethyl-phenyl)-piperidi...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1cccc(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H30F3NO3/c1-36-26(34)28(22-9-4-2-5-10-22,23-11-6-3-7-12-23)17-20-33-18-15-27(35,16-19-33)24-13-8-14-25(21-24)29(30,31)32/h2-14,21,35H,15-20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121256
(CHEMBL410979 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-Aib-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C80H131N27O22/c1-7-49(68(118)105-57(37-60(85)111)74(124)103-54(78(128)129)28-29-59(84)110)98-73(123)55(34-42(2)3)104-71(121)50(24-14-16-30-81)101-70(120)53(27-19-33-91-80(88)89)100-66(116)44(5)96-76(126)58(41-108)106-72(122)51(25-15-17-31-82)102-69(119)52(26-18-32-90-79(86)87)99-65(115)43(4)95-62(113)40-94-77(127)64(45(6)109)107-75(125)56(36-47-22-12-9-13-23-47)97-63(114)39-92-61(112)38-93-67(117)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,108-109H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,117)(H,94,127)(H,95,113)(H,96,126)(H,97,114)(H,98,123)(H,99,115)(H,100,116)(H,101,120)(H,102,119)(H,103,124)(H,104,121)(H,105,118)(H,106,122)(H,107,125)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153614
(4-[4-Hydroxy-4-(3-trifluoromethyl-phenyl)-piperidi...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1cccc(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C30H33F3N2O2/c1-34(2)27(36)29(23-10-5-3-6-11-23,24-12-7-4-8-13-24)18-21-35-19-16-28(37,17-20-35)25-14-9-15-26(22-25)30(31,32)33/h3-15,22,37H,16-21H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86492

(CAS_170713-75-4 | NSC_6324645 | Nociceptin)Show SMILES [#6]-[#6](-[#8])-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178

(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nociceptin binding to human Opioid receptor like 1 |
Bioorg Med Chem Lett 14: 5045-50 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.001
BindingDB Entry DOI: 10.7270/Q2TM7BV8 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
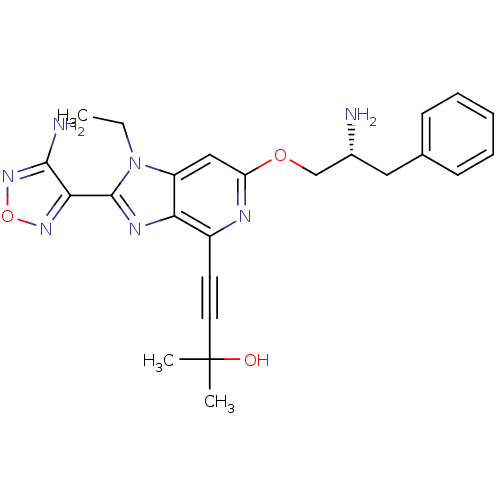
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153611

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-2...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30ClNO3/c1-33-26(31)28(23-8-4-2-5-9-23,24-10-6-3-7-11-24)18-21-30-19-16-27(32,17-20-30)22-12-14-25(29)15-13-22/h2-15,32H,16-21H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50153613

(4-[4-(4-Chloro-3-trifluoromethyl-phenyl)-4-hydroxy...)Show SMILES COC(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)c(c1)C(F)(F)F)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H29ClF3NO3/c1-37-26(35)28(21-8-4-2-5-9-21,22-10-6-3-7-11-22)16-19-34-17-14-27(36,15-18-34)23-12-13-25(30)24(20-23)29(31,32)33/h2-13,20,36H,14-19H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM21842
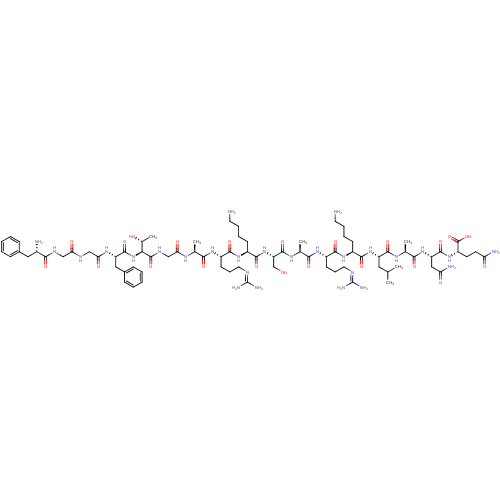
((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...)Show SMILES [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-c1ccccc1)-[#6@@H](-[#6])-[#8])-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#7])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6](-[#7])=O)-[#6](-[#8])=O Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
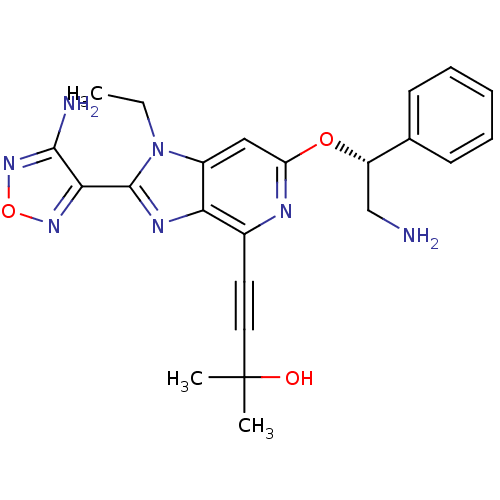
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121250

(CHEMBL415584 | F-G-G-F-T-G-A-R-K-S-Aib-R-K-L-A-N-Q...)Show SMILES CC[C@H](NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C80H131N27O22/c1-7-49(68(118)100-53(27-19-33-91-80(88)89)70(120)101-50(24-14-16-30-81)71(121)105-55(34-42(2)3)73(123)96-44(5)66(116)104-57(37-60(85)111)74(124)103-54(78(128)129)28-29-59(84)110)98-76(126)58(41-108)106-72(122)51(25-15-17-31-82)102-69(119)52(26-18-32-90-79(86)87)99-65(115)43(4)95-62(113)40-94-77(127)64(45(6)109)107-75(125)56(36-47-22-12-9-13-23-47)97-63(114)39-92-61(112)38-93-67(117)48(83)35-46-20-10-8-11-21-46/h8-13,20-23,42-45,48-58,64,108-109H,7,14-19,24-41,81-83H2,1-6H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,117)(H,94,127)(H,95,113)(H,96,123)(H,97,114)(H,98,126)(H,99,115)(H,100,118)(H,101,120)(H,102,119)(H,103,124)(H,104,116)(H,105,121)(H,106,122)(H,107,125)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698
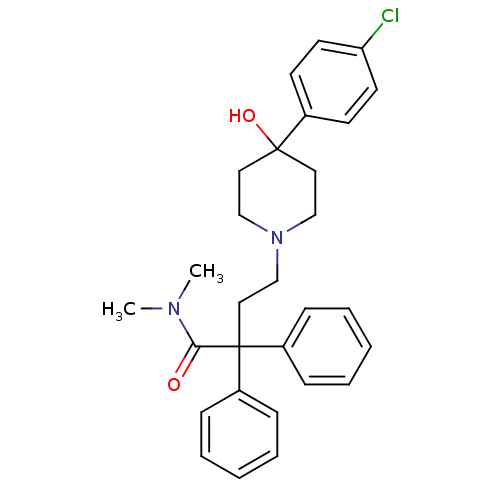
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from human vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM86491

(DiPOA | [8-(3,3-Diphenyl-propyl)-4-oxo-1-phenyl-1,...)Show SMILES OC(=O)CN1CN(c2ccccc2)C2(CCN(CCC(c3ccccc3)c3ccccc3)CC2)C1=O Show InChI InChI=1S/C30H33N3O3/c34-28(35)22-32-23-33(26-14-8-3-9-15-26)30(29(32)36)17-20-31(21-18-30)19-16-27(24-10-4-1-5-11-24)25-12-6-2-7-13-25/h1-15,27H,16-23H2,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083761
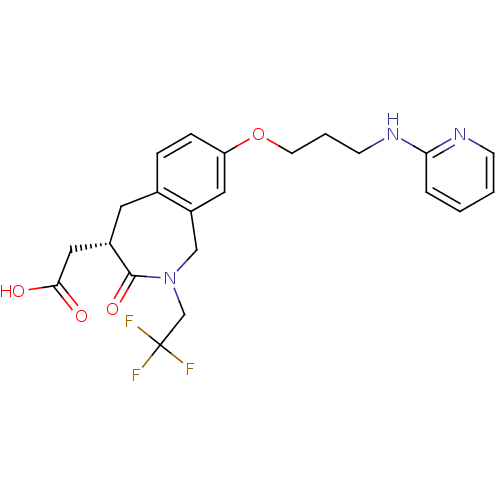
(CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288109
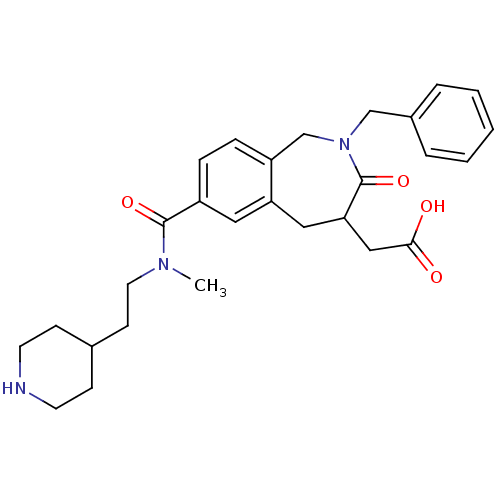
(CHEMBL82123 | {2-Benzyl-7-[methyl-(2-piperidin-4-y...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(Cc3ccccc3)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C28H35N3O4/c1-30(14-11-20-9-12-29-13-10-20)27(34)22-7-8-23-19-31(18-21-5-3-2-4-6-21)28(35)25(17-26(32)33)16-24(23)15-22/h2-8,15,20,25,29H,9-14,16-19H2,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM50299343

((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...)Show SMILES COc1ccc(c(OC)c1)S(=O)(=O)N1C(=O)[C@@](N2C[C@H](O)C[C@H]2C(=O)N(C)C)(c2cc(Cl)ccc12)c1ccccc1OC |r| Show InChI InChI=1S/C30H32ClN3O8S/c1-32(2)28(36)24-15-19(35)17-33(24)30(21-8-6-7-9-25(21)41-4)22-14-18(31)10-12-23(22)34(29(30)37)43(38,39)27-13-11-20(40-3)16-26(27)42-5/h6-14,16,19,24,35H,15,17H2,1-5H3/t19-,24+,30+/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter |
Bioorg Med Chem Lett 19: 6018-22 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.050
BindingDB Entry DOI: 10.7270/Q2J9679W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132647
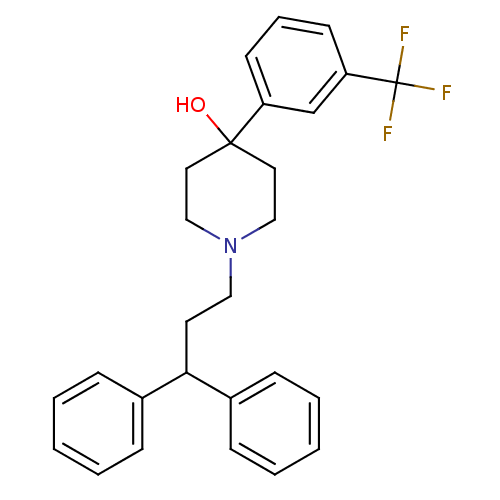
(1-(3,3-Diphenyl-propyl)-4-(3-trifluoromethyl-pheny...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H28F3NO/c28-27(29,30)24-13-7-12-23(20-24)26(32)15-18-31(19-16-26)17-14-25(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-13,20,25,32H,14-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Binding affinity for recombinant human mu-opioid receptor was determined by using [3H]- diprenophine radioligand |
Bioorg Med Chem Lett 14: 5275-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.08.032
BindingDB Entry DOI: 10.7270/Q2K073RN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50132647

(1-(3,3-Diphenyl-propyl)-4-(3-trifluoromethyl-pheny...)Show SMILES OC1(CCN(CCC(c2ccccc2)c2ccccc2)CC1)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H28F3NO/c28-27(29,30)24-13-7-12-23(20-24)26(32)15-18-31(19-16-26)17-14-25(21-8-3-1-4-9-21)22-10-5-2-6-11-22/h1-13,20,25,32H,14-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L.P.
Curated by ChEMBL
| Assay Description
Antagonistic activity against opioid receptor mu1 |
Bioorg Med Chem Lett 13: 3247-52 (2003)
BindingDB Entry DOI: 10.7270/Q2K936XQ |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
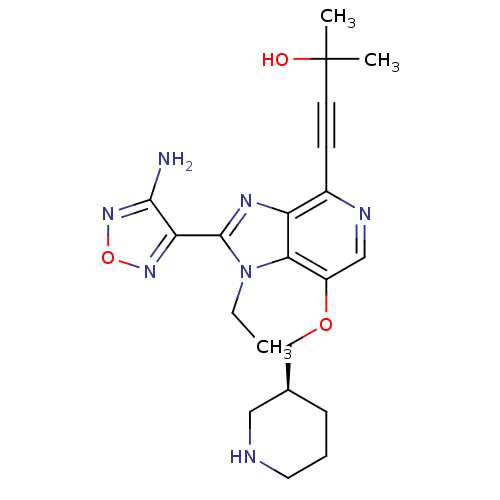
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50121248

(CHEMBL436732 | F-G-G-F-T-G-A-R-K-S-A-R-K-L-MeA-N-Q...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N(C)[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C80H131N27O22/c1-42(2)34-57(77(127)107(7)45(5)67(117)103-56(37-60(85)111)73(123)102-54(78(128)129)28-29-59(84)110)104-71(121)50(24-14-16-30-81)100-70(120)53(27-19-33-91-80(88)89)99-66(116)44(4)96-75(125)58(41-108)105-72(122)51(25-15-17-31-82)101-69(119)52(26-18-32-90-79(86)87)98-65(115)43(3)95-62(113)40-94-76(126)64(46(6)109)106-74(124)55(36-48-22-12-9-13-23-48)97-63(114)39-92-61(112)38-93-68(118)49(83)35-47-20-10-8-11-21-47/h8-13,20-23,42-46,49-58,64,108-109H,14-19,24-41,81-83H2,1-7H3,(H2,84,110)(H2,85,111)(H,92,112)(H,93,118)(H,94,126)(H,95,113)(H,96,125)(H,97,114)(H,98,115)(H,99,116)(H,100,120)(H,101,119)(H,102,123)(H,103,117)(H,104,121)(H,105,122)(H,106,124)(H,128,129)(H4,86,87,90)(H4,88,89,91)/t43-,44-,45-,46+,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,64-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma L. P.
Curated by ChEMBL
| Assay Description
Affinity for human Opioid receptor like 1 (ORL-1) expressed in HEK293 cells |
J Med Chem 45: 5280-6 (2002)
BindingDB Entry DOI: 10.7270/Q2474BKJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083763
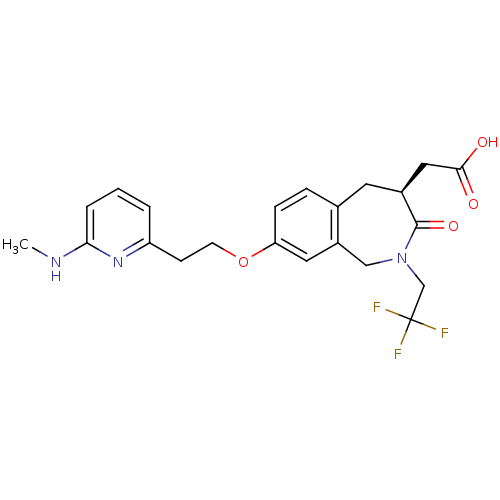
(CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...)Show SMILES CNc1cccc(CCOc2ccc3C[C@@H](CC(O)=O)C(=O)N(CC(F)(F)F)Cc3c2)n1 Show InChI InChI=1S/C22H24F3N3O4/c1-26-19-4-2-3-17(27-19)7-8-32-18-6-5-14-9-15(11-20(29)30)21(31)28(12-16(14)10-18)13-22(23,24)25/h2-6,10,15H,7-9,11-13H2,1H3,(H,26,27)(H,29,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083764
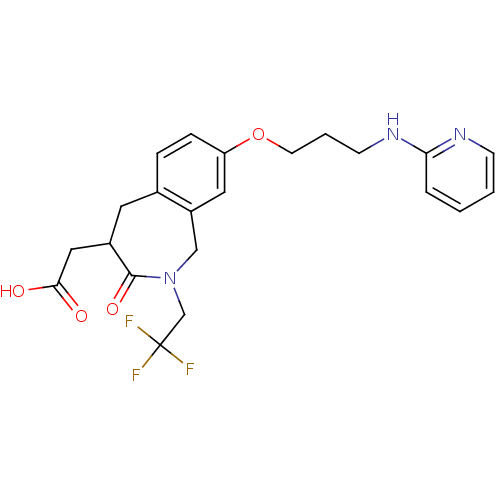
(CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...)Show SMILES OC(=O)CC1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288120

(CHEMBL313768 | {2-Cyclohexyl-7-[methyl-(2-piperidi...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(C3CCCCC3)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C27H39N3O4/c1-29(14-11-19-9-12-28-13-10-19)26(33)20-7-8-21-18-30(24-5-3-2-4-6-24)27(34)23(17-25(31)32)16-22(21)15-20/h7-8,15,19,23-24,28H,2-6,9-14,16-18H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714
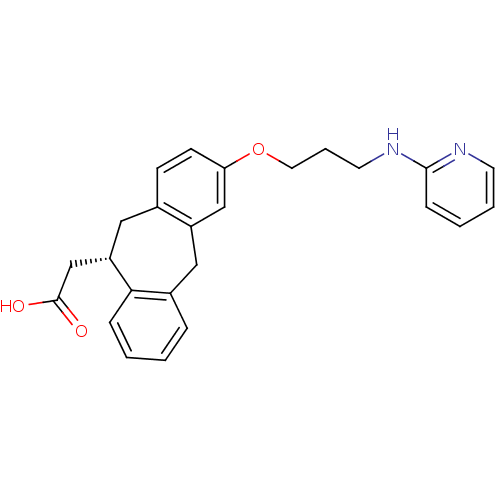
(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) |
Bioorg Med Chem Lett 9: 1807-12 (1999)
BindingDB Entry DOI: 10.7270/Q2Q23ZFC |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50074035
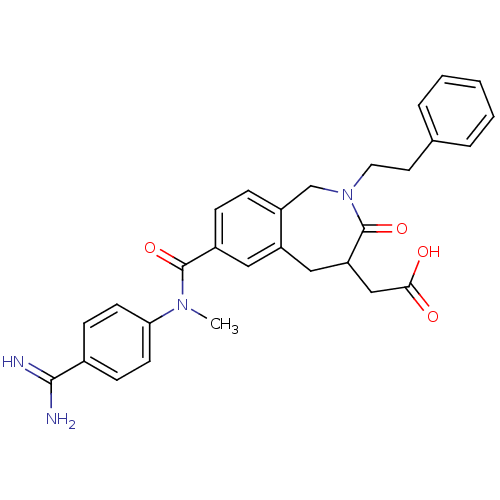
(CHEMBL82980 | {7-[(4-Carbamimidoyl-phenyl)-methyl-...)Show SMILES CN(C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Cc2c1)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C29H30N4O4/c1-32(25-11-9-20(10-12-25)27(30)31)28(36)21-7-8-22-18-33(14-13-19-5-3-2-4-6-19)29(37)24(17-26(34)35)16-23(22)15-21/h2-12,15,24H,13-14,16-18H2,1H3,(H3,30,31)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288119
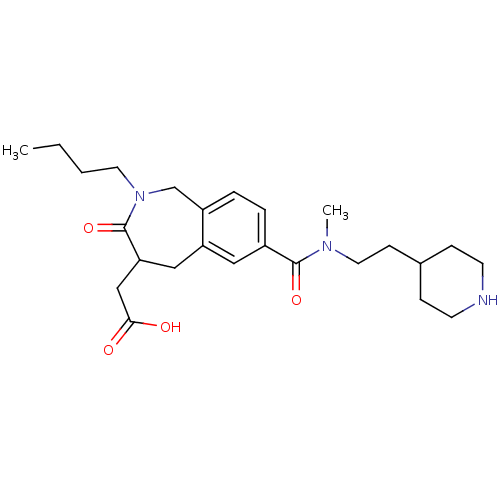
(CHEMBL84073 | {2-Butyl-7-[methyl-(2-piperidin-4-yl...)Show SMILES CCCCN1Cc2ccc(cc2CC(CC(O)=O)C1=O)C(=O)N(C)CCC1CCNCC1 Show InChI InChI=1S/C25H37N3O4/c1-3-4-12-28-17-20-6-5-19(14-21(20)15-22(25(28)32)16-23(29)30)24(31)27(2)13-9-18-7-10-26-11-8-18/h5-6,14,18,22,26H,3-4,7-13,15-17H2,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50054826
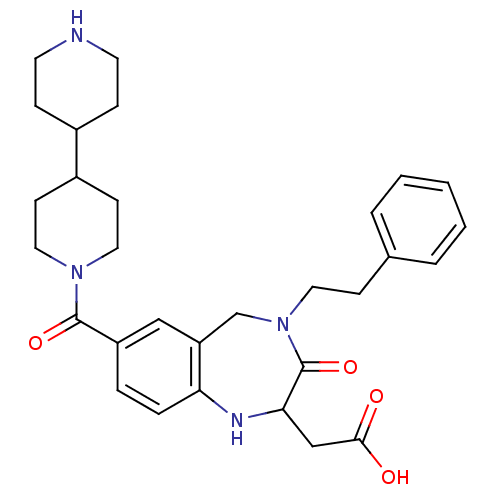
(CHEMBL144474 | [7-([4,4']Bipiperidinyl-1-carbonyl)...)Show SMILES OC(=O)CC1Nc2ccc(cc2CN(CCc2ccccc2)C1=O)C(=O)N1CCC(CC1)C1CCNCC1 Show InChI InChI=1S/C30H38N4O4/c35-28(36)19-27-30(38)34(15-10-21-4-2-1-3-5-21)20-25-18-24(6-7-26(25)32-27)29(37)33-16-11-23(12-17-33)22-8-13-31-14-9-22/h1-7,18,22-23,27,31-32H,8-17,19-20H2,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of binding to purified integrin alphaIIb-beta3 of human platelets |
J Med Chem 39: 4867-70 (1997)
Article DOI: 10.1021/jm960558a
BindingDB Entry DOI: 10.7270/Q2GH9H16 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288118
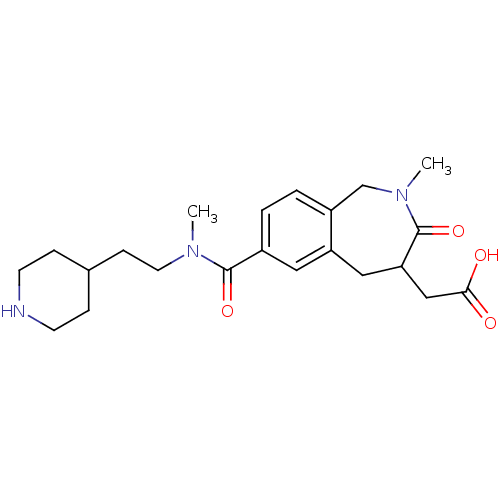
(CHEMBL312122 | {2-Methyl-7-[methyl-(2-piperidin-4-...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(C)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C22H31N3O4/c1-24(10-7-15-5-8-23-9-6-15)21(28)16-3-4-17-14-25(2)22(29)19(13-20(26)27)12-18(17)11-16/h3-4,11,15,19,23H,5-10,12-14H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288115
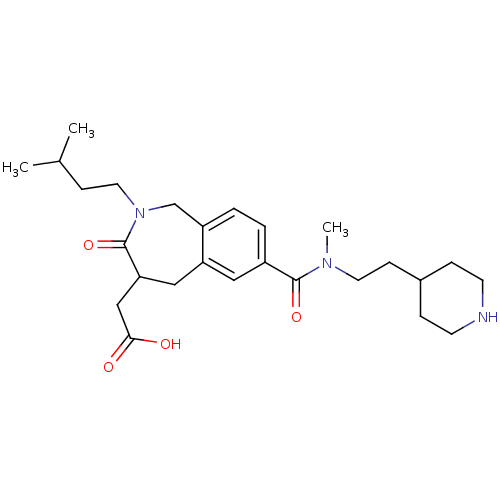
(CHEMBL82746 | {2-(3-Methyl-butyl)-7-[methyl-(2-pip...)Show SMILES CC(C)CCN1Cc2ccc(cc2CC(CC(O)=O)C1=O)C(=O)N(C)CCC1CCNCC1 Show InChI InChI=1S/C26H39N3O4/c1-18(2)8-13-29-17-21-5-4-20(14-22(21)15-23(26(29)33)16-24(30)31)25(32)28(3)12-9-19-6-10-27-11-7-19/h4-5,14,18-19,23,27H,6-13,15-17H2,1-3H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50078697
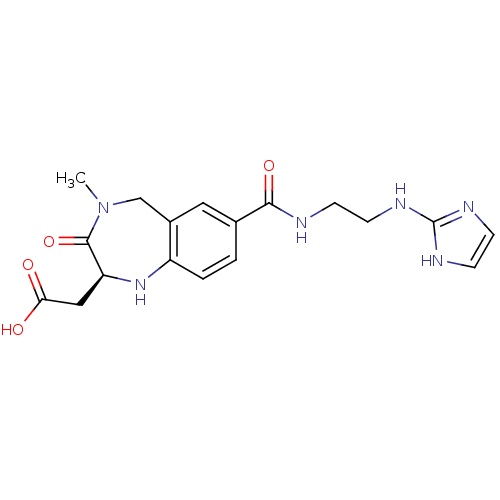
(CHEMBL54138 | {(S)-7-[2-(1H-Imidazol-2-ylamino)-et...)Show SMILES CN1Cc2cc(ccc2N[C@@H](CC(O)=O)C1=O)C(=O)NCCNc1ncc[nH]1 Show InChI InChI=1S/C18H22N6O4/c1-24-10-12-8-11(16(27)19-4-5-20-18-21-6-7-22-18)2-3-13(12)23-14(17(24)28)9-15(25)26/h2-3,6-8,14,23H,4-5,9-10H2,1H3,(H,19,27)(H,25,26)(H2,20,21,22)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity towards human Vitronectin receptor. |
Bioorg Med Chem Lett 9: 1801-6 (1999)
BindingDB Entry DOI: 10.7270/Q2TT4Q4B |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50074035

(CHEMBL82980 | {7-[(4-Carbamimidoyl-phenyl)-methyl-...)Show SMILES CN(C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Cc2c1)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C29H30N4O4/c1-32(25-11-9-20(10-12-25)27(30)31)28(36)21-7-8-22-18-33(14-13-19-5-3-2-4-6-19)29(37)24(17-26(34)35)16-23(22)15-21/h2-12,15,24H,13-14,16-18H2,1H3,(H3,30,31)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... |
J Med Chem 42: 545-59 (1999)
Article DOI: 10.1021/jm980166z
BindingDB Entry DOI: 10.7270/Q2QZ295H |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50054827

(CHEMBL85094 | SB-208651 | {8-[(4-Carbamimidoyl-phe...)Show SMILES CN(C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Nc2c1)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H29N5O4/c1-32(22-11-9-19(10-12-22)26(29)30)27(36)20-7-8-21-17-33(14-13-18-5-3-2-4-6-18)28(37)24(16-25(34)35)31-23(21)15-20/h2-12,15,24,31H,13-14,16-17H2,1H3,(H3,29,30)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... |
J Med Chem 42: 545-59 (1999)
Article DOI: 10.1021/jm980166z
BindingDB Entry DOI: 10.7270/Q2QZ295H |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50054827

(CHEMBL85094 | SB-208651 | {8-[(4-Carbamimidoyl-phe...)Show SMILES CN(C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Nc2c1)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H29N5O4/c1-32(22-11-9-19(10-12-22)26(29)30)27(36)20-7-8-21-17-33(14-13-18-5-3-2-4-6-18)28(37)24(16-25(34)35)31-23(21)15-20/h2-12,15,24,31H,13-14,16-17H2,1H3,(H3,29,30)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50054827

(CHEMBL85094 | SB-208651 | {8-[(4-Carbamimidoyl-phe...)Show SMILES CN(C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Nc2c1)c1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H29N5O4/c1-32(22-11-9-19(10-12-22)26(29)30)27(36)20-7-8-21-17-33(14-13-18-5-3-2-4-6-18)28(37)24(16-25(34)35)31-23(21)15-20/h2-12,15,24,31H,13-14,16-17H2,1H3,(H3,29,30)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of binding to purified integrin alphaIIb-beta3 of human platelets |
J Med Chem 39: 4867-70 (1997)
Article DOI: 10.1021/jm960558a
BindingDB Entry DOI: 10.7270/Q2GH9H16 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50072493
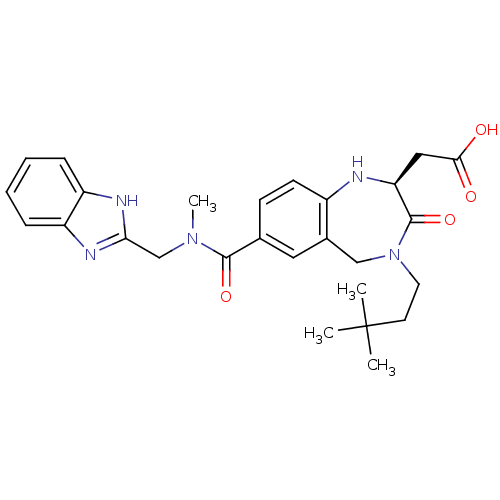
(CHEMBL108490 | [(S)-7-[(1H-Benzoimidazol-2-ylmethy...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(CCC(C)(C)C)Cc2c1 Show InChI InChI=1S/C27H33N5O4/c1-27(2,3)11-12-32-15-18-13-17(9-10-19(18)28-22(26(32)36)14-24(33)34)25(35)31(4)16-23-29-20-7-5-6-8-21(20)30-23/h5-10,13,22,28H,11-12,14-16H2,1-4H3,(H,29,30)(H,33,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Affinity for alphaIIb-beta3 receptor |
Bioorg Med Chem Lett 8: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2NG4PSF |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50288117
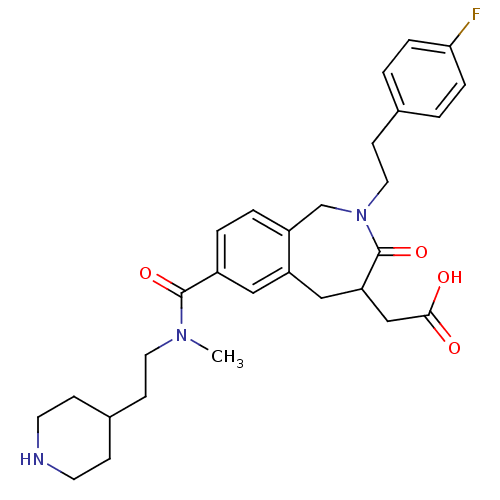
(CHEMBL83756 | {2-[2-(4-Fluoro-phenyl)-ethyl]-7-[me...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(CCc3ccc(F)cc3)C(=O)C(CC(O)=O)Cc2c1 Show InChI InChI=1S/C29H36FN3O4/c1-32(14-10-21-8-12-31-13-9-21)28(36)22-4-5-23-19-33(15-11-20-2-6-26(30)7-3-20)29(37)25(18-27(34)35)17-24(23)16-22/h2-7,16,21,25,31H,8-15,17-19H2,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro binding affinity against human alpha IIb beta3 integrin |
Bioorg Med Chem Lett 6: 2481-2486 (1996)
Article DOI: 10.1016/0960-894X(96)00432-5
BindingDB Entry DOI: 10.7270/Q25M65Q4 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083762
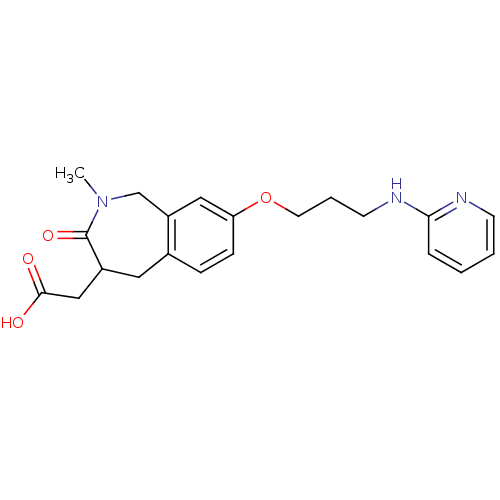
(CHEMBL314022 | {2-Methyl-3-oxo-8-[3-(pyridin-2-yla...)Show InChI InChI=1S/C21H25N3O4/c1-24-14-17-12-18(28-10-4-9-23-19-5-2-3-8-22-19)7-6-15(17)11-16(21(24)27)13-20(25)26/h2-3,5-8,12,16H,4,9-11,13-14H2,1H3,(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50072492
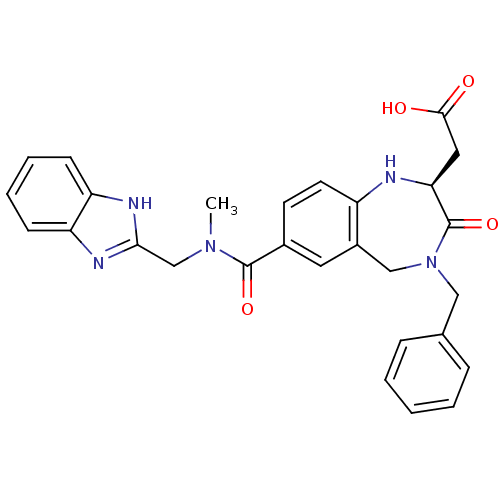
(CHEMBL419180 | {(S)-7-[(1H-Benzoimidazol-2-ylmethy...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(Cc3ccccc3)Cc2c1 Show InChI InChI=1S/C28H27N5O4/c1-32(17-25-30-22-9-5-6-10-23(22)31-25)27(36)19-11-12-21-20(13-19)16-33(15-18-7-3-2-4-8-18)28(37)24(29-21)14-26(34)35/h2-13,24,29H,14-17H2,1H3,(H,30,31)(H,34,35)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effect against adhesion of HEK 293 cells transfected with human alpha-v beta-3 to vitronectin coated plates |
Bioorg Med Chem Lett 8: 3165-70 (1999)
BindingDB Entry DOI: 10.7270/Q2NG4PSF |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
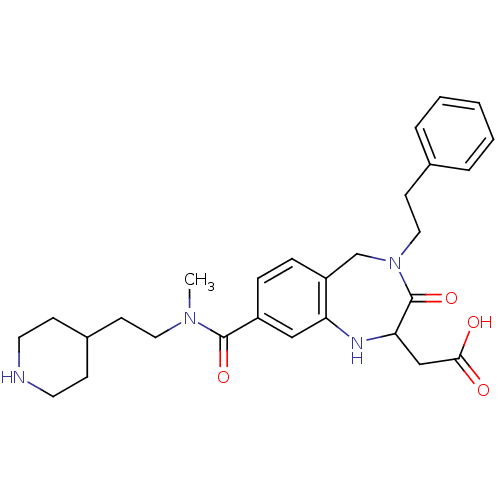
(Homo sapiens (Human)) | BDBM50074030
(CHEMBL356020 | {8-[Methyl-(2-piperidin-4-yl-ethyl)...)Show SMILES CN(CCC1CCNCC1)C(=O)c1ccc2CN(CCc3ccccc3)C(=O)C(CC(O)=O)Nc2c1 Show InChI InChI=1S/C28H36N4O4/c1-31(15-11-21-9-13-29-14-10-21)27(35)22-7-8-23-19-32(16-12-20-5-3-2-4-6-20)28(36)25(18-26(33)34)30-24(23)17-22/h2-8,17,21,25,29-30H,9-16,18-19H2,1H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Fibrinogen receptor binding affinity was determined by assaying for inhibition of [3H]-1 binding to purified Fibrinogen Receptor isolated from human... |
J Med Chem 42: 545-59 (1999)
Article DOI: 10.1021/jm980166z
BindingDB Entry DOI: 10.7270/Q2QZ295H |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185
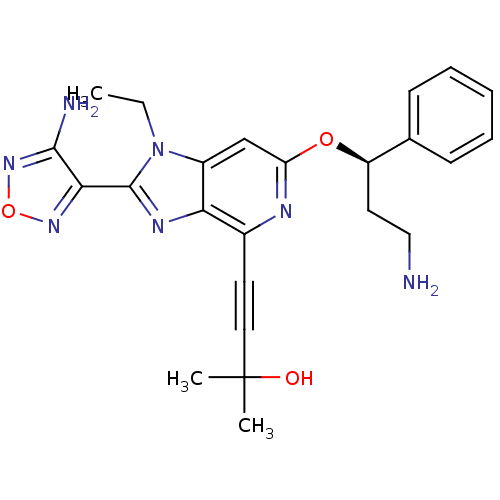
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data