 Found 125 hits with Last Name = 'moormann' and Initial = 'ae'
Found 125 hits with Last Name = 'moormann' and Initial = 'ae' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181844
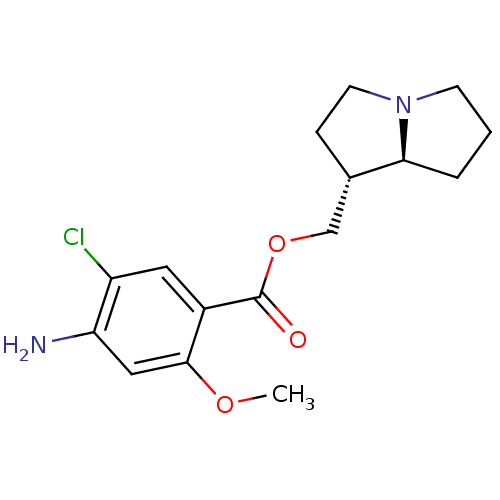
((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 4-amin...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H21ClN2O3/c1-21-15-8-13(18)12(17)7-11(15)16(20)22-9-10-4-6-19-5-2-3-14(10)19/h7-8,10,14H,2-6,9,18H2,1H3/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181840
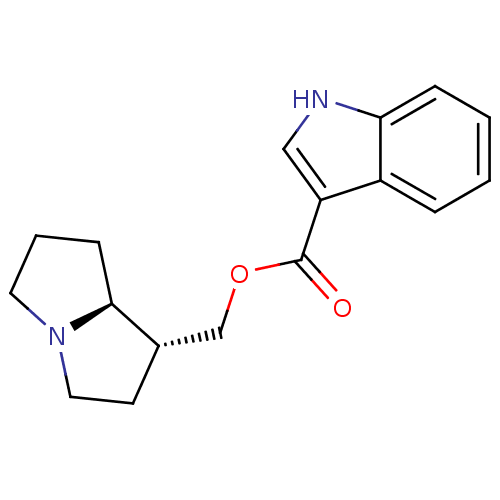
((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-Meth...)Show SMILES O=C(OC[C@@H]1CCN2CCC[C@@H]12)c1c[nH]c2ccccc12 Show InChI InChI=1S/C17H20N2O2/c20-17(14-10-18-15-5-2-1-4-13(14)15)21-11-12-7-9-19-8-3-6-16(12)19/h1-2,4-5,10,12,16,18H,3,6-9,11H2/t12-,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181845
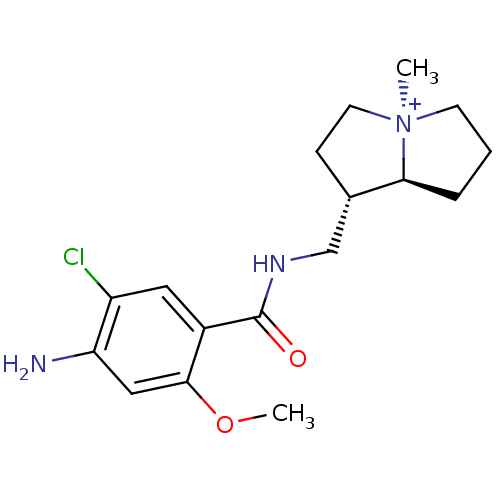
((1S,7aS)-1-{[(4-amino-5-chloro-2-methoxybenzoyl)am...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CC[N@@+]2(C)CCC[C@@H]12 Show InChI InChI=1S/C17H24ClN3O2/c1-21-6-3-4-15(21)11(5-7-21)10-20-17(22)12-8-13(18)14(19)9-16(12)23-2/h8-9,11,15H,3-7,10H2,1-2H3,(H2-,19,20,22)/p+1/t11-,15-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181837
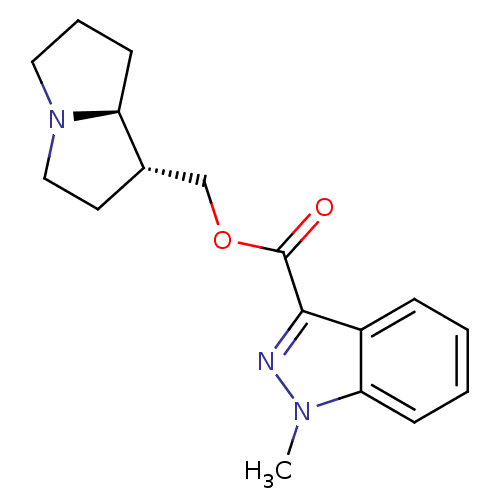
((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-meth...)Show SMILES Cn1nc(C(=O)OC[C@@H]2CCN3CCC[C@@H]23)c2ccccc12 Show InChI InChI=1S/C17H21N3O2/c1-19-15-6-3-2-5-13(15)16(18-19)17(21)22-11-12-8-10-20-9-4-7-14(12)20/h2-3,5-6,12,14H,4,7-11H2,1H3/t12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181842
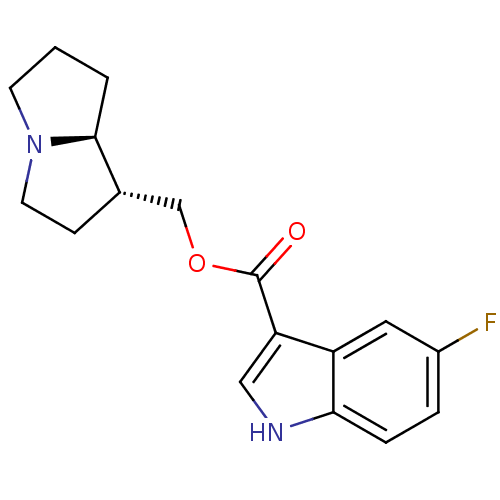
((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 5-Fluo...)Show SMILES Fc1ccc2[nH]cc(C(=O)OC[C@@H]3CCN4CCC[C@@H]34)c2c1 Show InChI InChI=1S/C17H19FN2O2/c18-12-3-4-15-13(8-12)14(9-19-15)17(21)22-10-11-5-7-20-6-1-2-16(11)20/h3-4,8-9,11,16,19H,1-2,5-7,10H2/t11-,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181841
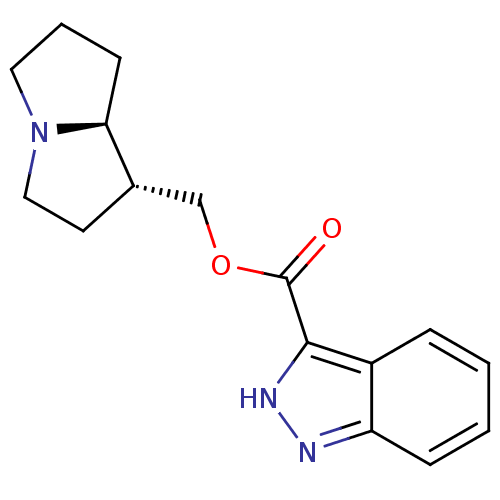
((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1H-ind...)Show SMILES O=C(OC[C@@H]1CCN2CCC[C@@H]12)c1[nH]nc2ccccc12 Show InChI InChI=1S/C16H19N3O2/c20-16(15-12-4-1-2-5-13(12)17-18-15)21-10-11-7-9-19-8-3-6-14(11)19/h1-2,4-5,11,14H,3,6-10H2,(H,17,18)/t11-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181836
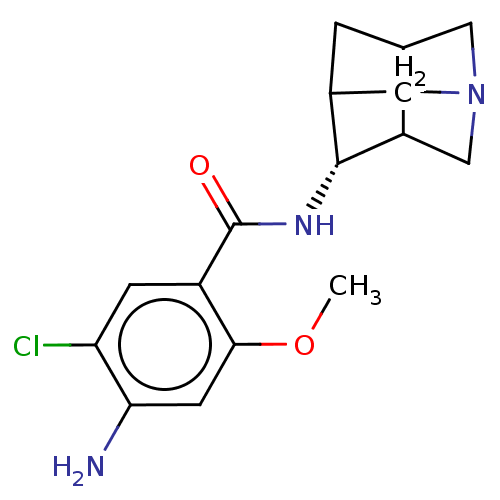
(4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)N[C@@H]1C2CC3CN(CC13)C2 |r,TLB:12:13:16.15:18.19.21,15:14:19:16.17,THB:17:18:13:16.15,17:16:13:18.19.21,12:13:19:16.17,15:16:19:13.14.21| Show InChI InChI=1S/C16H20ClN3O2/c1-22-14-4-13(18)12(17)3-10(14)16(21)19-15-9-2-8-5-20(6-9)7-11(8)15/h3-4,8-9,11,15H,2,5-7,18H2,1H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181839
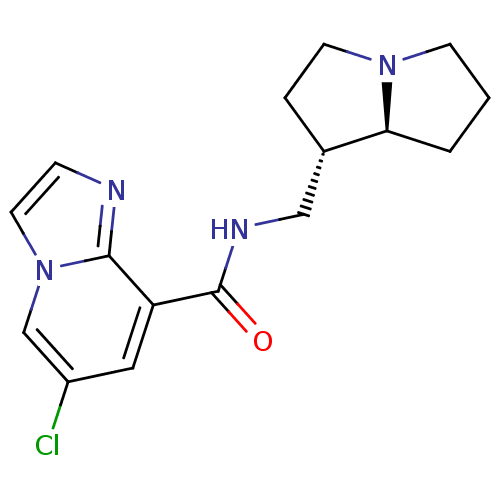
(CHEMBL202434 | N-exo-((4S,7alphaS)-tetrahydro-1H-p...)Show SMILES Clc1cc(C(=O)NC[C@@H]2CCN3CCC[C@@H]23)c2nccn2c1 Show InChI InChI=1S/C16H19ClN4O/c17-12-8-13(15-18-4-7-21(15)10-12)16(22)19-9-11-3-6-20-5-1-2-14(11)20/h4,7-8,10-11,14H,1-3,5-6,9H2,(H,19,22)/t11-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181840

((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-Meth...)Show SMILES O=C(OC[C@@H]1CCN2CCC[C@@H]12)c1c[nH]c2ccccc12 Show InChI InChI=1S/C17H20N2O2/c20-17(14-10-18-15-5-2-1-4-13(14)15)21-11-12-7-9-19-8-3-6-16(12)19/h1-2,4-5,10,12,16,18H,3,6-9,11H2/t12-,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50005833
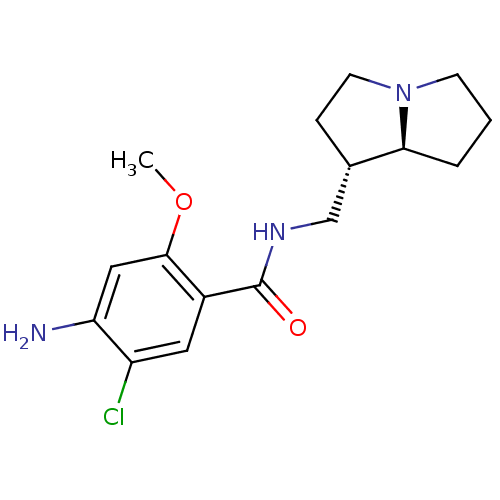
((exo)4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181843
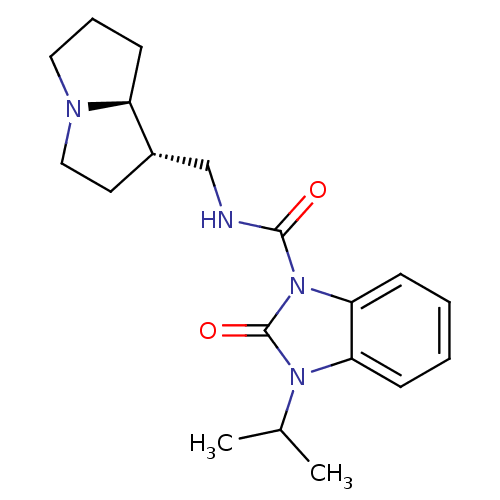
(CHEMBL201414 | cis-N-[(hexahydro-1H-pyrrolizin-l-y...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC[C@@H]2CCN3CCC[C@@H]23)c1=O Show InChI InChI=1S/C19H26N4O2/c1-13(2)22-16-6-3-4-7-17(16)23(19(22)25)18(24)20-12-14-9-11-21-10-5-8-15(14)21/h3-4,6-7,13-15H,5,8-12H2,1-2H3,(H,20,24)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181842

((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 5-Fluo...)Show SMILES Fc1ccc2[nH]cc(C(=O)OC[C@@H]3CCN4CCC[C@@H]34)c2c1 Show InChI InChI=1S/C17H19FN2O2/c18-12-3-4-15-13(8-12)14(9-19-15)17(21)22-10-11-5-7-20-6-1-2-16(11)20/h3-4,8-9,11,16,19H,1-2,5-7,10H2/t11-,16-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181843

(CHEMBL201414 | cis-N-[(hexahydro-1H-pyrrolizin-l-y...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC[C@@H]2CCN3CCC[C@@H]23)c1=O Show InChI InChI=1S/C19H26N4O2/c1-13(2)22-16-6-3-4-7-17(16)23(19(22)25)18(24)20-12-14-9-11-21-10-5-8-15(14)21/h3-4,6-7,13-15H,5,8-12H2,1-2H3,(H,20,24)/t14-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181837

((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1-meth...)Show SMILES Cn1nc(C(=O)OC[C@@H]2CCN3CCC[C@@H]23)c2ccccc12 Show InChI InChI=1S/C17H21N3O2/c1-19-15-6-3-2-5-13(15)16(18-19)17(21)22-11-12-8-10-20-9-4-7-14(12)20/h2-3,5-6,12,14H,4,7-11H2,1H3/t12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181838
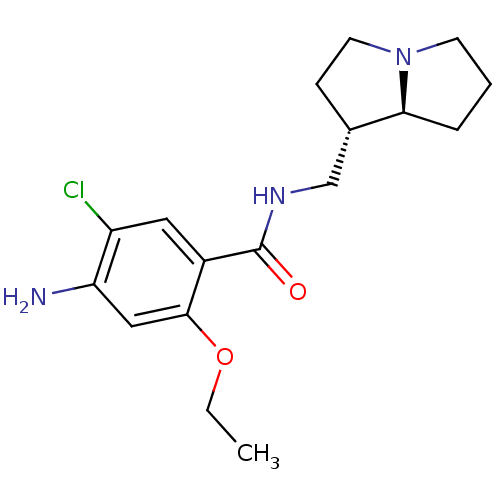
(4-amino-5-chloro-2-ethoxy-N-[(1S,7aS)-hexahydro-1H...)Show SMILES CCOc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C17H24ClN3O2/c1-2-23-16-9-14(19)13(18)8-12(16)17(22)20-10-11-5-7-21-6-3-4-15(11)21/h8-9,11,15H,2-7,10,19H2,1H3,(H,20,22)/t11-,15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(GUINEA PIG) | BDBM50181836

(4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)N[C@@H]1C2CC3CN(CC13)C2 |r,TLB:12:13:16.15:18.19.21,15:14:19:16.17,THB:17:18:13:16.15,17:16:13:18.19.21,12:13:19:16.17,15:16:19:13.14.21| Show InChI InChI=1S/C16H20ClN3O2/c1-22-14-4-13(18)12(17)3-10(14)16(21)19-15-9-2-8-5-20(6-9)7-11(8)15/h3-4,8-9,11,15H,2,5-7,18H2,1H3,(H,19,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181844

((1R,7aS)-hexahydro-1H-pyrrolizin-1-ylmethyl 4-amin...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H21ClN2O3/c1-21-15-8-13(18)12(17)7-11(15)16(20)22-9-10-4-6-19-5-2-3-14(10)19/h7-8,10,14H,2-6,9,18H2,1H3/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50005841

((exo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-y...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@H]1CCN2CCC[C@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181841

((1S,7aR)-hexahydro-1H-pyrrolizin-1-ylmethyl 1H-ind...)Show SMILES O=C(OC[C@@H]1CCN2CCC[C@@H]12)c1[nH]nc2ccccc12 Show InChI InChI=1S/C16H19N3O2/c20-16(15-12-4-1-2-5-13(12)17-18-15)21-10-11-7-9-19-8-3-6-14(11)19/h1-2,4-5,11,14H,3,6-10H2,(H,17,18)/t11-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50005833

((exo)4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A/3B
(Rattus norvegicus-RAT) | BDBM50181839

(CHEMBL202434 | N-exo-((4S,7alphaS)-tetrahydro-1H-p...)Show SMILES Clc1cc(C(=O)NC[C@@H]2CCN3CCC[C@@H]23)c2nccn2c1 Show InChI InChI=1S/C16H19ClN4O/c17-12-8-13(15-18-4-7-21(15)10-12)16(22)19-9-11-3-6-20-5-1-2-14(11)20/h4,7-8,10-11,14H,1-3,5-6,9H2,(H,19,22)/t11-,14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 259 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]GR65630 from 5HT3 receptor in brain cortex from Wistar rat |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2 receptor |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha-1 receptor |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104649
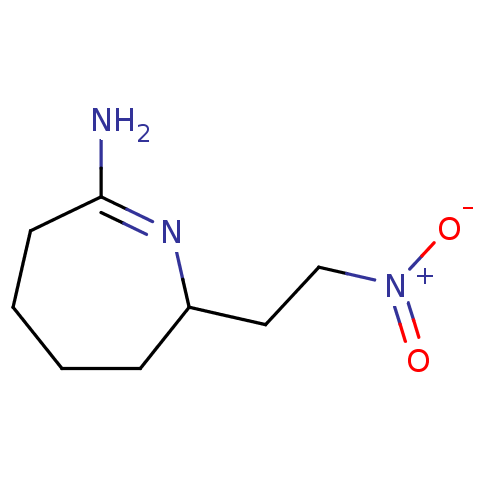
(7-(2-Nitro-ethyl)-azepan-(2Z)-ylideneamine | CHEMB...)Show InChI InChI=1S/C8H14N3O2/c9-8-4-2-1-3-7(10-8)5-6-11(12)13/h7H,1-6H2,(H-,9,10)/q-1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine receptor D2 |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50104649

(7-(2-Nitro-ethyl)-azepan-(2Z)-ylideneamine | CHEMB...)Show InChI InChI=1S/C8H14N3O2/c9-8-4-2-1-3-7(10-8)5-6-11(12)13/h7H,1-6H2,(H-,9,10)/q-1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 339 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50064011
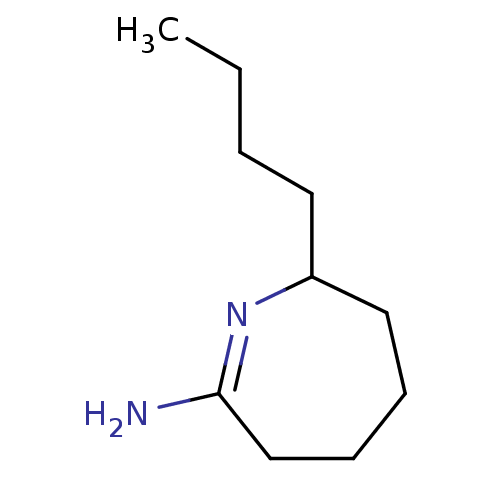
(7-Butyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C10H20N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 517 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104654
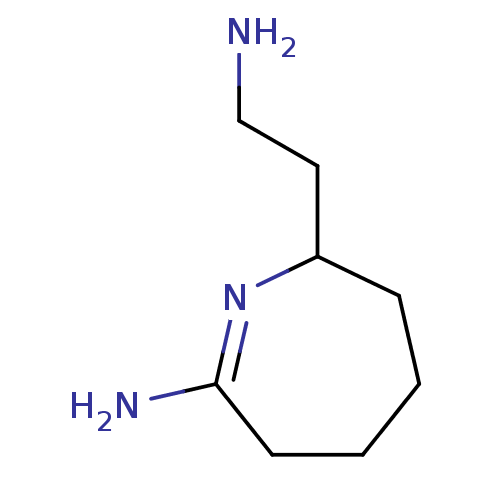
(2-(2-Amino-ethyl)-7-imino-azepane | CHEMBL92475)Show InChI InChI=1S/C8H17N3/c9-6-5-7-3-1-2-4-8(10)11-7/h7H,1-6,9H2,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 701 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50064011

(7-Butyl-azepan-(2Z)-ylideneamine; hydrochloride | ...)Show InChI InChI=1S/C10H20N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h9H,2-8H2,1H3,(H2,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104655

(((E)-7-But-2-enyl)-azepan-(2Z)-ylideneamine | CHEM...)Show InChI InChI=1S/C10H18N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h2-3,9H,4-8H2,1H3,(H2,11,12)/b3-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50104654

(2-(2-Amino-ethyl)-7-imino-azepane | CHEMBL92475)Show InChI InChI=1S/C8H17N3/c9-6-5-7-3-1-2-4-8(10)11-7/h7H,1-6,9H2,(H2,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 932 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104648
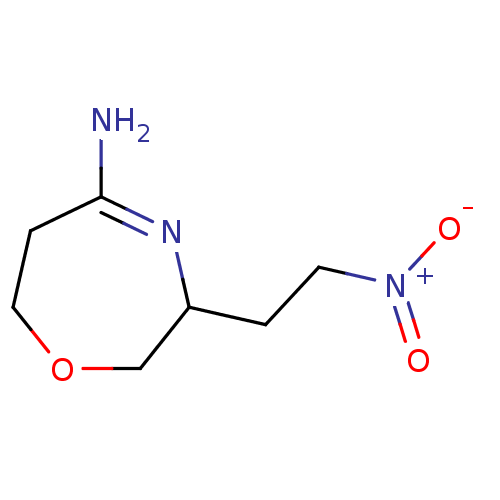
(3-(2-Nitro-ethyl)-[1,4]oxazepan-(5Z)-ylideneamine ...)Show InChI InChI=1S/C7H12N3O3/c8-7-2-4-13-5-6(9-7)1-3-10(11)12/h6H,1-5H2,(H-,8,9)/q-1/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 979 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine receptor D1 |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049257

(Azepan-(2Z)-ylideneamine | CHEMBL315857 | CHEMBL54...)Show InChI InChI=1S/C6H12N2/c7-6-4-2-1-3-5-8-6/h1-5H2,(H2,7,8) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104656
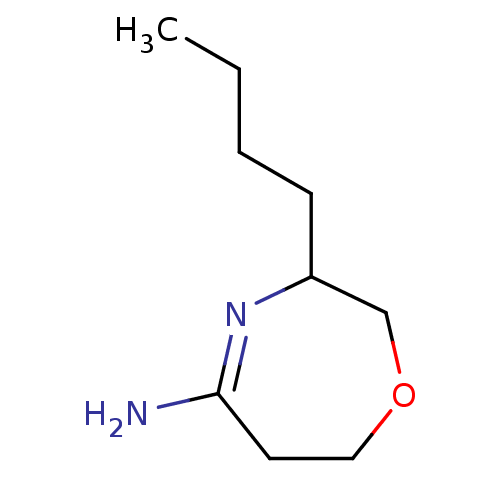
(3-Butyl-[1,4]oxazepan-(5Z)-ylideneamine | CHEMBL89...)Show InChI InChI=1S/C9H18N2O/c1-2-3-4-8-7-12-6-5-9(10)11-8/h8H,2-7H2,1H3,(H2,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104653
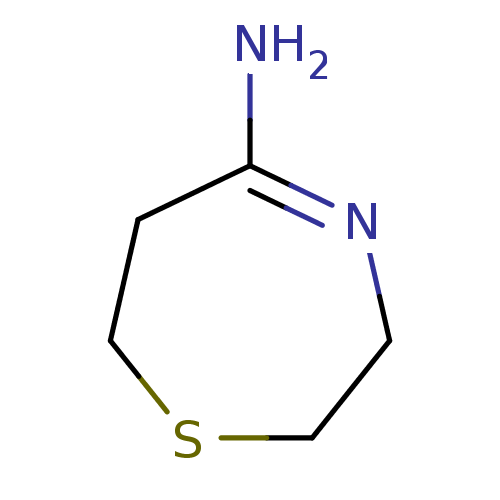
(CHEMBL88308 | [1,4]Thiazepan-(5E)-ylideneamine | [...)Show InChI InChI=1S/C5H10N2S/c6-5-1-3-8-4-2-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50104649

(7-(2-Nitro-ethyl)-azepan-(2Z)-ylideneamine | CHEMB...)Show InChI InChI=1S/C8H14N3O2/c9-8-4-2-1-3-7(10-8)5-6-11(12)13/h7H,1-6H2,(H-,9,10)/q-1/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of endothelial nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50104655

(((E)-7-But-2-enyl)-azepan-(2Z)-ylideneamine | CHEM...)Show InChI InChI=1S/C10H18N2/c1-2-3-6-9-7-4-5-8-10(11)12-9/h2-3,9H,4-8H2,1H3,(H2,11,12)/b3-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50049257

(Azepan-(2Z)-ylideneamine | CHEMBL315857 | CHEMBL54...)Show InChI InChI=1S/C6H12N2/c7-6-4-2-1-3-5-8-6/h1-5H2,(H2,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50005841

((exo) 4-Amino-5-chloro-N-(hexahydro-pyrrolizin-1-y...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC[C@H]1CCN2CCC[C@H]12 Show InChI InChI=1S/C16H22ClN3O2/c1-22-15-8-13(18)12(17)7-11(15)16(21)19-9-10-4-6-20-5-2-3-14(10)20/h7-8,10,14H,2-6,9,18H2,1H3,(H,19,21)/t10-,14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine receptor D2 |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50005836

(4-Amino-5-chloro-N-{1-[3-(4-fluoro-phenoxy)-propyl...)Show SMILES COC1CN(CCCOc2ccc(F)cc2)CCC1NC(=O)c1cc(Cl)c(N)cc1OC Show InChI InChI=1S/C23H29ClFN3O4/c1-30-21-13-19(26)18(24)12-17(21)23(29)27-20-8-10-28(14-22(20)31-2)9-3-11-32-16-6-4-15(25)5-7-16/h4-7,12-13,20,22H,3,8-11,14,26H2,1-2H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha-2 receptor |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50181838

(4-amino-5-chloro-2-ethoxy-N-[(1S,7aS)-hexahydro-1H...)Show SMILES CCOc1cc(N)c(Cl)cc1C(=O)NC[C@@H]1CCN2CCC[C@@H]12 Show InChI InChI=1S/C17H24ClN3O2/c1-2-23-16-9-14(19)13(18)8-12(16)17(22)20-10-11-5-7-21-6-3-4-15(11)21/h8-9,11,15H,2-7,10,19H2,1H3,(H,20,22)/t11-,15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine receptor D2 |
J Med Chem 49: 1125-39 (2006)
Article DOI: 10.1021/jm0509501
BindingDB Entry DOI: 10.7270/Q2W096Q0 |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50104648

(3-(2-Nitro-ethyl)-[1,4]oxazepan-(5Z)-ylideneamine ...)Show InChI InChI=1S/C7H12N3O3/c8-7-2-4-13-5-6(9-7)1-3-10(11)12/h6H,1-5H2,(H-,8,9)/q-1/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104651
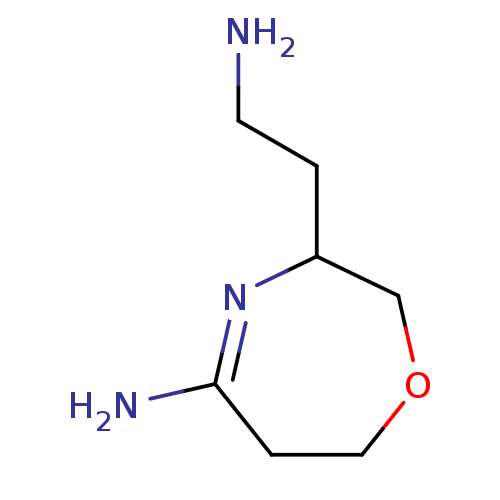
(3-(2-Amino-ethyl)-5-imino-[1,4]oxazepane | CHEMBL8...)Show InChI InChI=1S/C7H15N3O/c8-3-1-6-5-11-4-2-7(9)10-6/h6H,1-5,8H2,(H2,9,10) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 6.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50104653
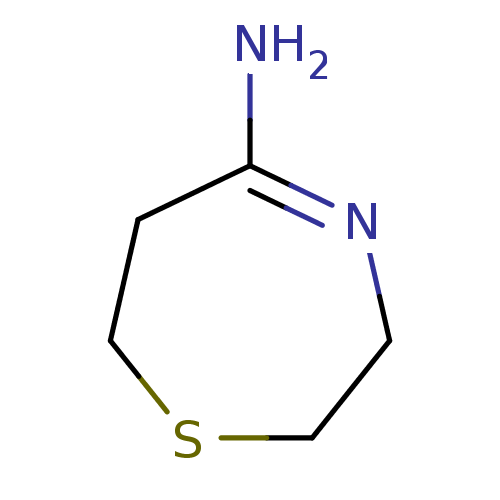
(CHEMBL88308 | [1,4]Thiazepan-(5E)-ylideneamine | [...)Show InChI InChI=1S/C5H10N2S/c6-5-1-3-8-4-2-7-5/h1-4H2,(H2,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, brain
(Homo sapiens (Human)) | BDBM50104651

(3-(2-Amino-ethyl)-5-imino-[1,4]oxazepane | CHEMBL8...)Show InChI InChI=1S/C7H15N3O/c8-3-1-6-5-11-4-2-7(9)10-6/h6H,1-5,8H2,(H2,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 8.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of Neuronal nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50104657
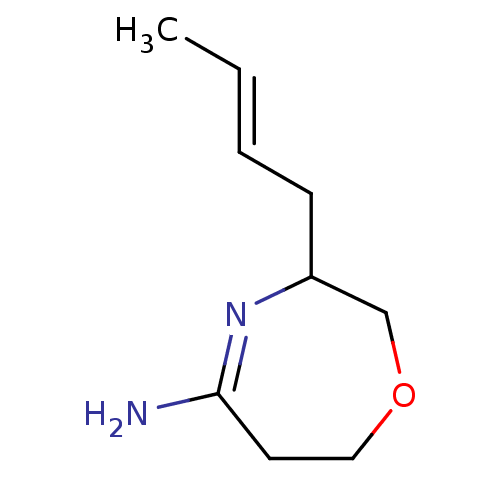
(((E)-3-But-2-enyl)-[1,4]oxazepan-(5Z)-ylideneamine...)Show InChI InChI=1S/C9H16N2O/c1-2-3-4-8-7-12-6-5-9(10)11-8/h2-3,8H,4-7H2,1H3,(H2,10,11)/b3-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50049249
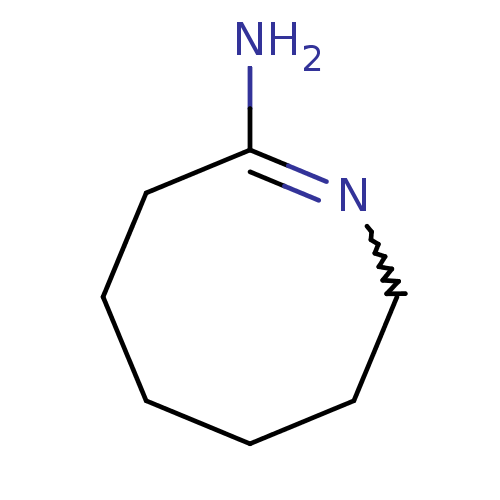
(Azocan-(2Z)-ylideneamine | CHEMBL329431)Show InChI InChI=1S/C7H14N2/c8-7-5-3-1-2-4-6-9-7/h1-6H2,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Inhibition of inducible nitric oxide synthase |
Bioorg Med Chem Lett 11: 2651-3 (2001)
BindingDB Entry DOI: 10.7270/Q24T6HPK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data