 Found 384 hits with Last Name = 'mortenson' and Initial = 'pn'
Found 384 hits with Last Name = 'mortenson' and Initial = 'pn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trans-sialidase
(Trypanosoma cruzi) | BDBM50118216
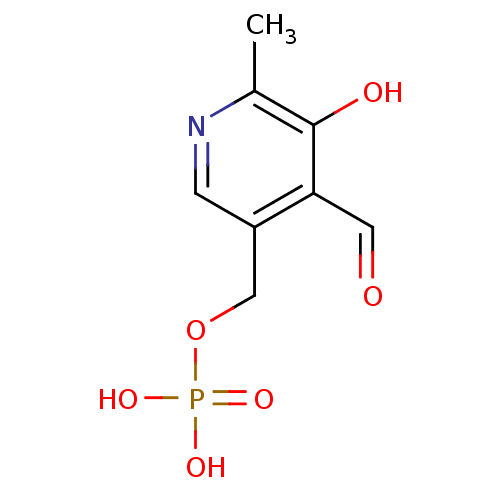
((4-formyl-5-hydroxy-6-methylpyridin-3-yl)methyl di...)Show InChI InChI=1S/C8H10NO6P/c1-5-8(11)7(3-10)6(2-9-5)4-15-16(12,13)14/h2-3,11H,4H2,1H3,(H2,12,13,14) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trans-Sialidase |
Bioorg Med Chem Lett 19: 589-96 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.065
BindingDB Entry DOI: 10.7270/Q2X92C7J |
More data for this
Ligand-Target Pair | |
Trans-sialidase
(Trypanosoma cruzi) | BDBM4706

((2R,3R,4S)-3-acetamido-4-hydroxy-2-[(1R,2R)-1,2,3-...)Show SMILES CC(=O)N[C@@H]1[C@@H](O)C=C(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r,c:7| Show InChI InChI=1S/C11H17NO8/c1-4(14)12-8-5(15)2-7(11(18)19)20-10(8)9(17)6(16)3-13/h2,5-6,8-10,13,15-17H,3H2,1H3,(H,12,14)(H,18,19)/t5-,6+,8+,9+,10+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.23E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of Trypanosoma cruzi trans-Sialidase |
Bioorg Med Chem Lett 19: 589-96 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.065
BindingDB Entry DOI: 10.7270/Q2X92C7J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50520331
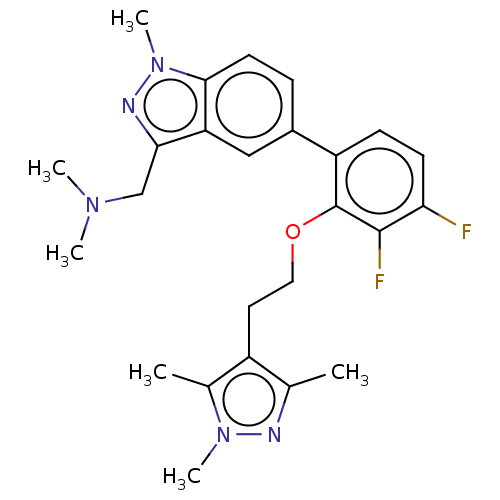
(CHEMBL4445137)Show SMILES CN(C)Cc1nn(C)c2ccc(cc12)-c1ccc(F)c(F)c1OCCc1c(C)nn(C)c1C Show InChI InChI=1S/C25H29F2N5O/c1-15-18(16(2)31(5)28-15)11-12-33-25-19(8-9-21(26)24(25)27)17-7-10-23-20(13-17)22(14-30(3)4)29-32(23)6/h7-10,13H,11-12,14H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Medicines
Curated by ChEMBL
| Assay Description
Inhibition of human N-myristoyltransferase assessed as reduction in CoASH production by fluorogenic detection based assay |
J Med Chem 63: 4430-4444 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01581
BindingDB Entry DOI: 10.7270/Q2ZG6WM7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50520335

(CHEMBL4465173)Show InChI InChI=1S/C18H15Cl2N3/c19-16-7-4-14(10-17(16)20)12-21-11-13-2-5-15(6-3-13)18-22-8-1-9-23-18/h1-10,21H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Medicines
Curated by ChEMBL
| Assay Description
Inhibition of beta2 adrenoreceptor (unknown origin) |
J Med Chem 63: 4430-4444 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01581
BindingDB Entry DOI: 10.7270/Q2ZG6WM7 |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50520336
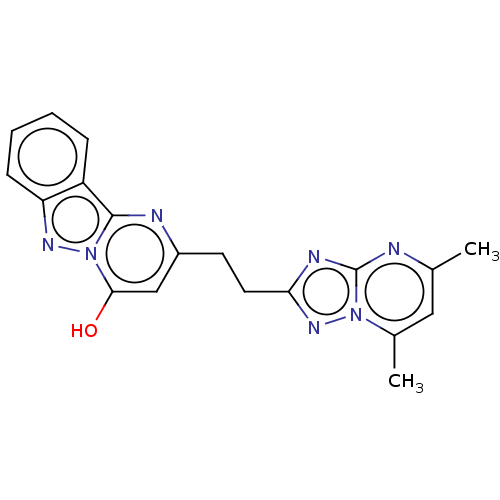
(CHEMBL4470042)Show SMILES Cc1cc(C)n2nc(CCc3cc(O)n4nc5ccccc5c4n3)nc2n1 Show InChI InChI=1S/C19H17N7O/c1-11-9-12(2)25-19(20-11)22-16(24-25)8-7-13-10-17(27)26-18(21-13)14-5-3-4-6-15(14)23-26/h3-6,9-10,27H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Medicines
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 using cAMP substrate by HTRF assay |
J Med Chem 63: 4430-4444 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01581
BindingDB Entry DOI: 10.7270/Q2ZG6WM7 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615811

(CHEMBL5272869)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(N3CCC3)c(Cl)cc2c1 |r,wU:7.10,wD:4.3,(8.38,4.12,;7.6,2.78,;6.83,4.12,;8.94,2.01,;6.27,2.01,;4.94,2.78,;3.6,2.01,;3.6,.47,;4.94,-.3,;6.27,.47,;2.27,-.3,;.94,.47,;.94,2.01,;-.4,-.3,;-.4,-1.84,;-1.73,-2.6,;-3.06,-1.83,;-4.38,-2.6,;-5.71,-1.84,;-7.05,-2.61,;-7.45,-4.12,;-8.94,-3.72,;-8.53,-2.21,;-5.71,-.31,;-7.05,.46,;-4.4,.46,;-3.06,-.3,;-1.74,.48,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Oryctolagus cuniculus (Rabbit)) | BDBM50420304
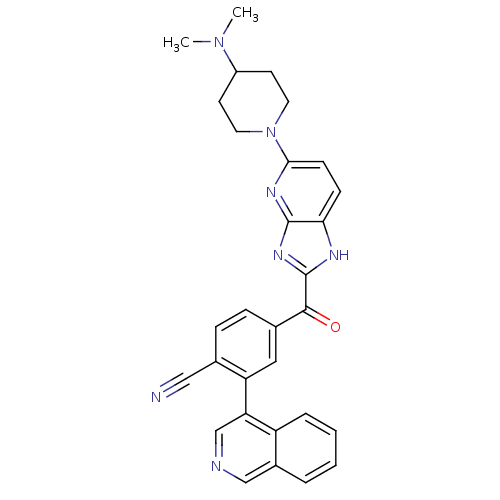
(CHEMBL2089065 | US8598217, 165)Show SMILES CN(C)C1CCN(CC1)c1ccc2[nH]c(nc2n1)C(=O)c1ccc(C#N)c(c1)-c1cncc2ccccc12 Show InChI InChI=1S/C30H27N7O/c1-36(2)22-11-13-37(14-12-22)27-10-9-26-29(34-27)35-30(33-26)28(38)19-7-8-20(16-31)24(15-19)25-18-32-17-21-5-3-4-6-23(21)25/h3-10,15,17-18,22H,11-14H2,1-2H3,(H,33,34,35) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG
US Patent
| Assay Description
A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... |
US Patent US8598217 (2013)
BindingDB Entry DOI: 10.7270/Q2BG2MN8 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615823

(CHEMBL5289520)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1C[C@@H](C1)C(C)(C)O |r,wU:19.21,wD:21.26,1.0,(-8.73,-.66,;-7.96,-1.99,;-8.37,-3.51,;-6.88,-3.91,;-6.48,-2.39,;-5.14,-1.62,;-3.81,-2.39,;-2.49,-1.62,;-1.16,-2.39,;.17,-1.62,;.17,-.08,;-1.17,.69,;-2.49,-.09,;-3.83,.68,;-5.14,-.1,;-6.48,.67,;1.51,.69,;1.51,2.23,;2.84,-.08,;4.17,.69,;4.58,2.2,;6.07,1.8,;5.66,.29,;7.4,2.57,;8.17,3.91,;6.63,3.91,;8.73,1.8,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112617
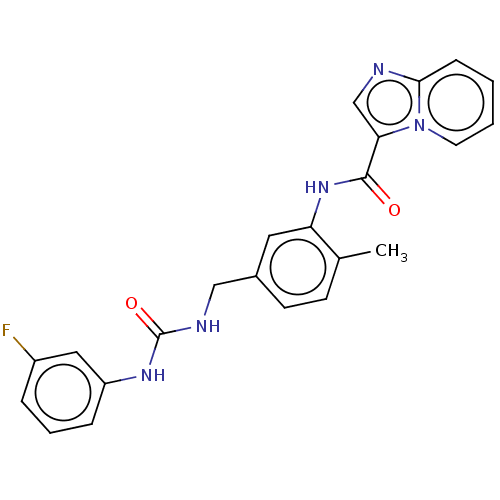
(CHEMBL3608786)Show SMILES Cc1ccc(CNC(=O)Nc2cccc(F)c2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H20FN5O2/c1-15-8-9-16(13-26-23(31)27-18-6-4-5-17(24)12-18)11-19(15)28-22(30)20-14-25-21-7-2-3-10-29(20)21/h2-12,14H,13H2,1H3,(H,28,30)(H2,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615812

(CHEMBL5289730)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:19.21,wD:1.0,22.28,(-9.12,-.87,;-8.35,-2.21,;-8.76,-3.72,;-7.27,-4.12,;-6.86,-2.61,;-5.53,-1.84,;-4.2,-2.6,;-2.88,-1.83,;-1.55,-2.6,;-.21,-1.84,;-.21,-.3,;-1.55,.48,;-2.88,-.3,;-4.22,.46,;-5.53,-.31,;-6.86,.46,;1.12,.47,;1.12,2.01,;2.45,-.3,;3.79,.47,;3.79,2.01,;5.12,2.78,;6.45,2.01,;6.45,.47,;5.12,-.3,;7.79,2.78,;8.56,4.12,;7.02,4.12,;9.12,2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615775

(CHEMBL5279860)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2cc(ncc2c1)N1CCC1 |r,wU:7.10,wD:4.3,(6.84,4.12,;7.61,2.78,;8.38,4.12,;8.94,2.01,;6.27,2.01,;4.94,2.78,;3.6,2.01,;3.6,.47,;4.94,-.3,;6.27,.47,;2.27,-.3,;.94,.47,;.94,2.01,;-.4,-.3,;-.4,-1.84,;-1.73,-2.6,;-3.06,-1.83,;-4.38,-2.6,;-5.71,-1.84,;-5.71,-.31,;-4.4,.46,;-3.06,-.3,;-1.74,.48,;-7.05,-2.61,;-8.53,-2.21,;-8.94,-3.72,;-7.45,-4.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615791

(CHEMBL5275536)Show SMILES CC(C)(O)[C@H]1CC2(C[C@@H](C2)NC(=O)c2cnc3nc(ccc3c2)C2CC2)C1 |r,wU:8.10,wD:4.3,(8.76,4.37,;7.99,3.04,;7.22,4.37,;9.32,2.27,;6.66,2.27,;5.17,2.67,;4.76,1.15,;3.27,1.55,;2.87,.04,;4.36,-.36,;1.54,-.73,;.2,.04,;.2,1.58,;-1.13,-.73,;-1.13,-2.27,;-2.46,-3.04,;-3.8,-2.27,;-5.12,-3.03,;-6.45,-2.27,;-6.45,-.75,;-5.13,.03,;-3.8,-.74,;-2.47,.04,;-7.78,-3.04,;-8.55,-4.37,;-9.32,-3.04,;6.25,.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615797

(CHEMBL5282861)Show SMILES CC(C)(O)[C@H]1C[C@@H](C1)NC(=O)c1cnc2nc(C3CC3)c(Cl)cc2c1 |r,wU:6.8,wD:4.3,(7.82,3.82,;7.05,2.48,;6.28,3.82,;8.39,1.71,;5.72,1.71,;4.23,2.11,;3.83,.6,;5.31,.2,;2.49,-.18,;1.16,.59,;1.16,2.13,;-.18,-.18,;-.18,-1.71,;-1.51,-2.48,;-2.84,-1.71,;-4.18,-2.48,;-5.51,-1.71,;-6.84,-2.48,;-7.62,-3.82,;-8.39,-2.48,;-5.51,-.18,;-6.84,.59,;-4.18,.59,;-2.84,-.18,;-1.51,.59,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Oryctolagus cuniculus (Rabbit)) | BDBM107751
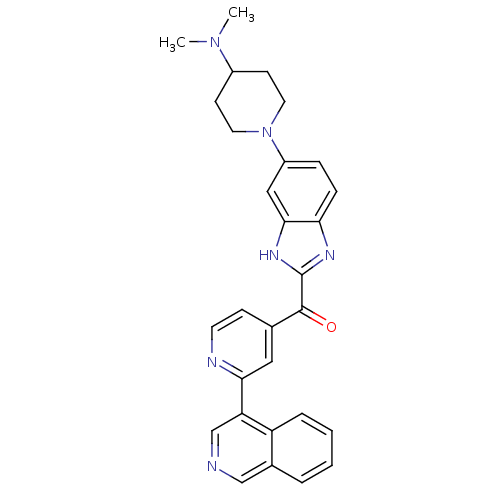
(CHEMBL2089063 | US8598217, 89)Show SMILES CN(C)C1CCN(CC1)c1ccc2nc([nH]c2c1)C(=O)c1ccnc(c1)-c1cncc2ccccc12 Show InChI InChI=1S/C29H28N6O/c1-34(2)21-10-13-35(14-11-21)22-7-8-25-27(16-22)33-29(32-25)28(36)19-9-12-31-26(15-19)24-18-30-17-20-5-3-4-6-23(20)24/h3-9,12,15-18,21H,10-11,13-14H2,1-2H3,(H,32,33) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG
US Patent
| Assay Description
A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... |
US Patent US8598217 (2013)
BindingDB Entry DOI: 10.7270/Q2BG2MN8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Oryctolagus cuniculus (Rabbit)) | BDBM107755
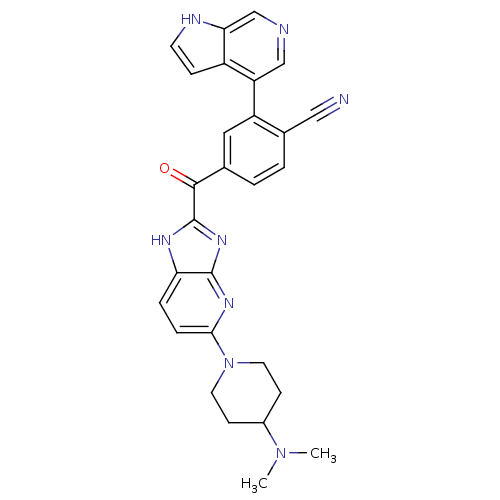
(US8598217, 140)Show SMILES CN(C)C1CCN(CC1)c1ccc2[nH]c(nc2n1)C(=O)c1ccc(C#N)c(c1)-c1cncc2[nH]ccc12 Show InChI InChI=1S/C28H26N8O/c1-35(2)19-8-11-36(12-9-19)25-6-5-23-27(33-25)34-28(32-23)26(37)17-3-4-18(14-29)21(13-17)22-15-30-16-24-20(22)7-10-31-24/h3-7,10,13,15-16,19,31H,8-9,11-12H2,1-2H3,(H,32,33,34) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG
US Patent
| Assay Description
A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... |
US Patent US8598217 (2013)
BindingDB Entry DOI: 10.7270/Q2BG2MN8 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615779
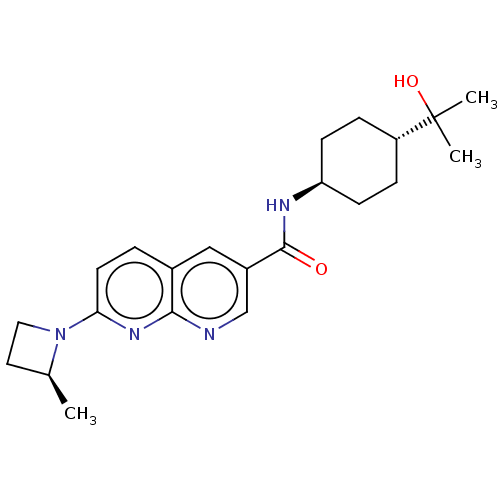
(CHEMBL5285125)Show SMILES C[C@H]1CCN1c1ccc2cc(cnc2n1)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:18.20,wD:1.0,21.27,(-9.12,-.87,;-8.35,-2.21,;-8.76,-3.72,;-7.27,-4.12,;-6.86,-2.61,;-5.53,-1.84,;-5.53,-.31,;-4.22,.46,;-2.88,-.3,;-1.55,.48,;-.21,-.3,;-.21,-1.84,;-1.54,-2.6,;-2.88,-1.83,;-4.2,-2.6,;1.12,.47,;1.12,2.01,;2.45,-.3,;3.79,.47,;3.79,2.01,;5.12,2.78,;6.45,2.01,;6.45,.47,;5.12,-.3,;7.79,2.78,;7.02,4.12,;8.56,4.12,;9.12,2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112628
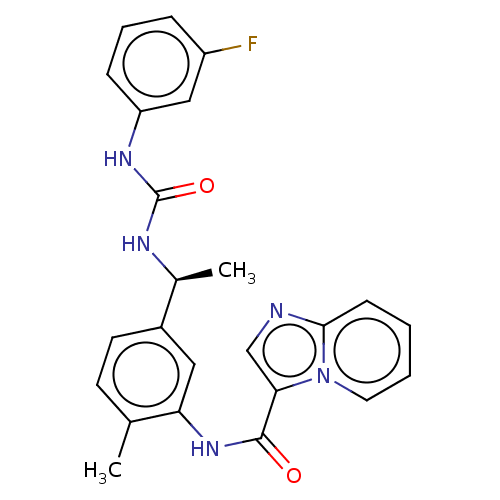
(CHEMBL3608789)Show SMILES C[C@H](NC(=O)Nc1cccc(F)c1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-9-10-17(16(2)27-24(32)28-19-7-5-6-18(25)13-19)12-20(15)29-23(31)21-14-26-22-8-3-4-11-30(21)22/h3-14,16H,1-2H3,(H,29,31)(H2,27,28,32)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615795

(CHEMBL5279371)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1CC[C@](C)(O)CC1 |r,wU:19.21,wD:22.26,1.0,(-8.57,-.49,;-7.8,-1.82,;-8.2,-3.33,;-6.72,-3.73,;-6.31,-2.22,;-4.98,-1.45,;-3.64,-2.22,;-2.31,-1.45,;-.98,-2.22,;.36,-1.45,;.36,.09,;-.98,.86,;-2.31,.09,;-3.64,.86,;-4.98,.09,;-6.31,.86,;1.69,.86,;1.69,2.4,;3.02,.09,;4.36,.86,;4.36,2.4,;5.69,3.17,;7.03,2.4,;8.57,2.4,;7.8,3.73,;7.03,.86,;5.69,.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615800
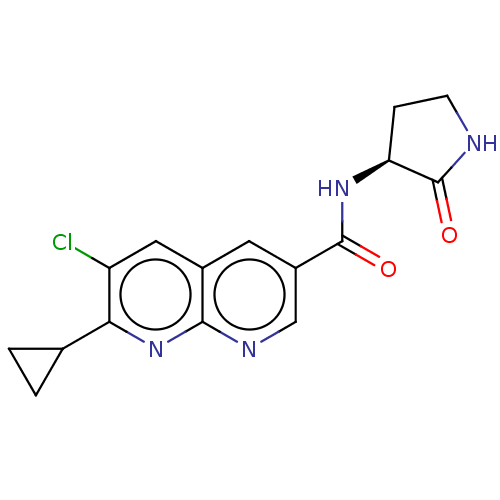
(CHEMBL5266142)Show SMILES Clc1cc2cc(cnc2nc1C1CC1)C(=O)N[C@H]1CCNC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615813
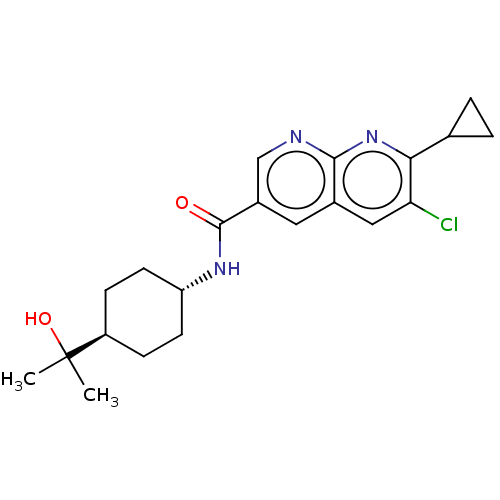
(CHEMBL5269778)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(C3CC3)c(Cl)cc2c1 |r,wU:7.10,wD:4.3,(8.2,4.03,;7.43,2.69,;6.66,4.03,;8.76,1.92,;6.1,1.92,;4.76,2.69,;3.43,1.92,;3.43,.38,;4.76,-.39,;6.1,.38,;2.1,-.39,;.76,.38,;.76,1.92,;-.57,-.39,;-.57,-1.93,;-1.9,-2.69,;-3.24,-1.92,;-4.56,-2.69,;-5.89,-1.93,;-7.22,-2.7,;-7.99,-4.03,;-8.76,-2.7,;-5.89,-.4,;-7.22,.37,;-4.57,.37,;-3.24,-.39,;-1.91,.39,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615802

(CHEMBL5269001)Show SMILES C[C@H]1CCN1c1cc2ncc(cc2cn1)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:18.20,wD:1.0,21.27,(-9.12,-.87,;-8.35,-2.21,;-8.76,-3.72,;-7.27,-4.12,;-6.86,-2.61,;-5.53,-1.84,;-4.2,-2.6,;-2.88,-1.83,;-1.55,-2.6,;-.21,-1.84,;-.21,-.3,;-1.55,.48,;-2.88,-.3,;-4.22,.46,;-5.53,-.31,;1.12,.47,;1.12,2.01,;2.45,-.3,;3.79,.47,;3.79,2.01,;5.12,2.78,;6.45,2.01,;6.45,.47,;5.12,-.3,;7.79,2.78,;8.56,4.12,;7.02,4.12,;9.12,2.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615792
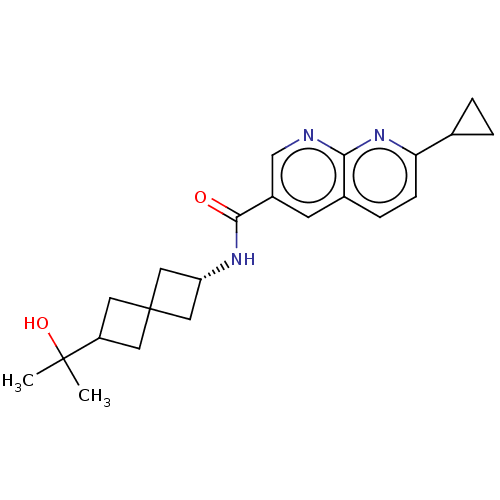
(CHEMBL5279720)Show SMILES CC(C)(O)C1CC2(C[C@@H](C2)NC(=O)c2cnc3nc(ccc3c2)C2CC2)C1 |r,wU:8.10,(7.22,4.37,;7.99,3.04,;8.76,4.37,;9.32,2.27,;6.66,2.27,;5.17,2.67,;4.76,1.15,;3.27,1.55,;2.87,.04,;4.36,-.36,;1.54,-.73,;.2,.04,;.2,1.58,;-1.13,-.73,;-1.13,-2.27,;-2.46,-3.04,;-3.8,-2.27,;-5.12,-3.03,;-6.45,-2.27,;-6.45,-.75,;-5.13,.03,;-3.8,-.74,;-2.47,.04,;-7.78,-3.04,;-8.55,-4.37,;-9.32,-3.04,;6.25,.75,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615824
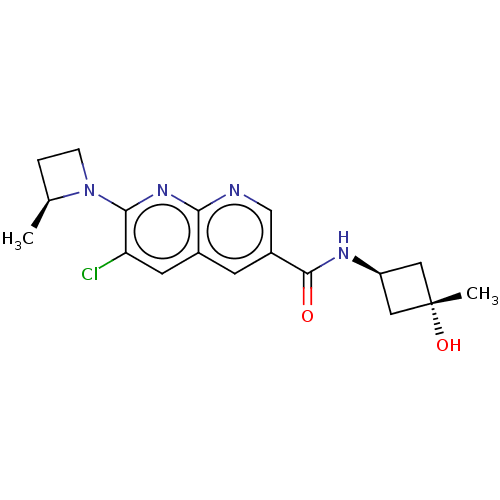
(CHEMBL5278680)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1C[C@](C)(O)C1 |r,wU:19.21,wD:21.25,1.0,(-8.17,-.27,;-7.4,-1.61,;-7.81,-3.12,;-6.32,-3.52,;-5.91,-2.01,;-4.58,-1.24,;-3.25,-2,;-1.93,-1.23,;-.6,-2,;.74,-1.24,;.74,.3,;-.6,1.08,;-1.93,.3,;-3.27,1.06,;-4.58,.29,;-5.91,1.06,;2.07,1.07,;2.07,2.61,;3.4,.3,;4.74,1.07,;5.14,2.58,;6.63,2.19,;8.17,2.19,;7.4,3.52,;6.22,.67,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615825

(CHEMBL5266618)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1C[C@@](C)(O)C1 |r,wU:19.21,21.25,wD:1.0,(-8.17,-.27,;-7.4,-1.61,;-7.81,-3.12,;-6.32,-3.52,;-5.91,-2.01,;-4.58,-1.24,;-3.25,-2,;-1.93,-1.23,;-.6,-2,;.74,-1.24,;.74,.3,;-.6,1.08,;-1.93,.3,;-3.27,1.06,;-4.58,.29,;-5.91,1.06,;2.07,1.07,;2.07,2.61,;3.4,.3,;4.74,1.07,;5.14,2.58,;6.63,2.19,;7.4,3.52,;8.17,2.19,;6.22,.67,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Oryctolagus cuniculus (Rabbit)) | BDBM107761
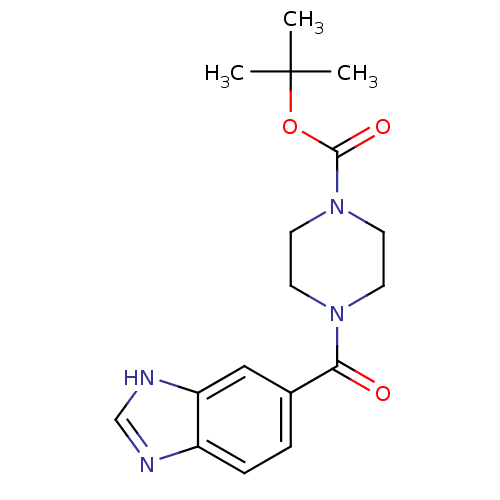
(US8598217, 174)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)C(=O)c1ccc2nc[nH]c2c1 Show InChI InChI=1S/C17H22N4O3/c1-17(2,3)24-16(23)21-8-6-20(7-9-21)15(22)12-4-5-13-14(10-12)19-11-18-13/h4-5,10-11H,6-9H2,1-3H3,(H,18,19) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG
US Patent
| Assay Description
A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... |
US Patent US8598217 (2013)
BindingDB Entry DOI: 10.7270/Q2BG2MN8 |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615796

(CHEMBL5267588)Show SMILES C[C@H]1CCN1c1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1CCNC1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
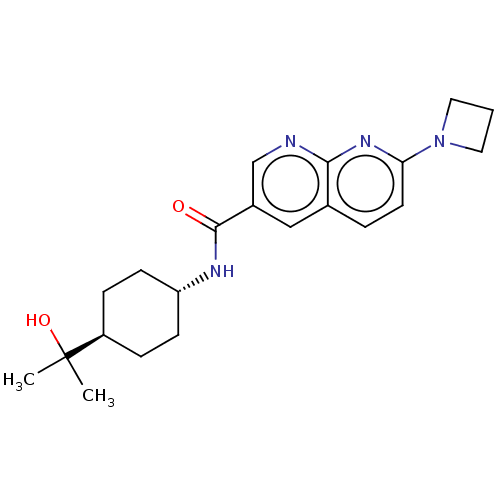
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615778
(CHEMBL5283521)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(ccc2c1)N1CCC1 |r,wU:7.10,wD:4.3,(6.84,4.12,;7.61,2.78,;8.38,4.12,;8.94,2.01,;6.27,2.01,;4.94,2.78,;3.6,2.01,;3.6,.47,;4.94,-.3,;6.27,.47,;2.27,-.3,;.94,.47,;.94,2.01,;-.4,-.3,;-.4,-1.84,;-1.73,-2.6,;-3.06,-1.83,;-4.38,-2.6,;-5.71,-1.84,;-5.71,-.31,;-4.4,.46,;-3.06,-.3,;-1.74,.48,;-7.05,-2.61,;-8.53,-2.21,;-8.94,-3.72,;-7.45,-4.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112631
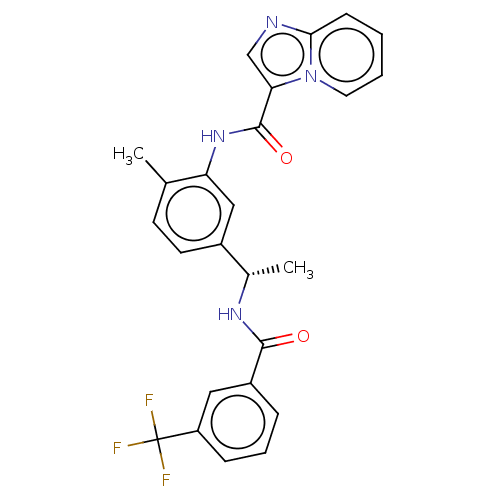
(CHEMBL3608790)Show SMILES C[C@H](NC(=O)c1cccc(c1)C(F)(F)F)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C25H21F3N4O2/c1-15-9-10-17(16(2)30-23(33)18-6-5-7-19(12-18)25(26,27)28)13-20(15)31-24(34)21-14-29-22-8-3-4-11-32(21)22/h3-14,16H,1-2H3,(H,30,33)(H,31,34)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576323
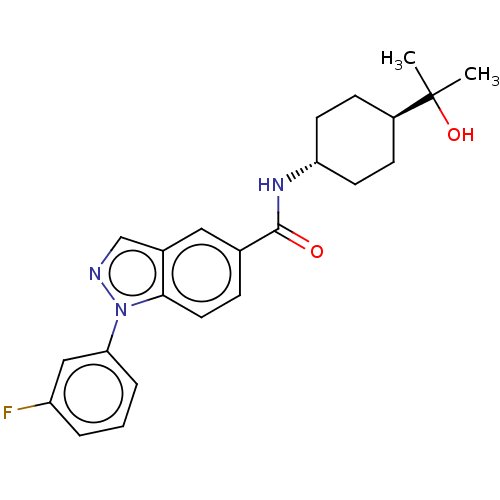
(CHEMBL4866146)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ncc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(17.72,-11.38,;16.96,-12.72,;18.5,-12.71,;16.96,-14.26,;15.63,-11.95,;15.63,-10.41,;14.29,-9.63,;12.97,-10.41,;12.97,-11.95,;14.29,-12.71,;11.64,-9.64,;10.3,-10.41,;8.97,-9.65,;10.31,-11.95,;11.65,-12.72,;11.65,-14.27,;10.31,-15.04,;10,-16.55,;8.46,-16.72,;7.83,-15.31,;8.97,-14.27,;8.98,-12.72,;11.03,-17.69,;10.55,-19.15,;11.59,-20.29,;13.09,-19.97,;13.56,-18.5,;15.07,-18.17,;12.53,-17.36,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50112618

(CHEMBL3608787)Show SMILES C[C@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
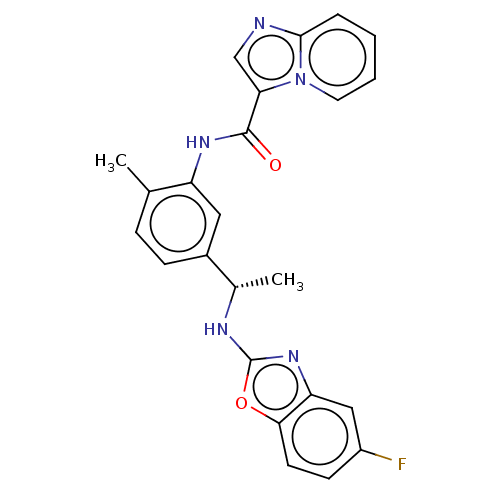
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112634
(CHEMBL3608791)Show SMILES C[C@H](Nc1nc2cc(F)ccc2o1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H20FN5O2/c1-14-6-7-16(15(2)27-24-29-19-12-17(25)8-9-21(19)32-24)11-18(14)28-23(31)20-13-26-22-5-3-4-10-30(20)22/h3-13,15H,1-2H3,(H,27,29)(H,28,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615798
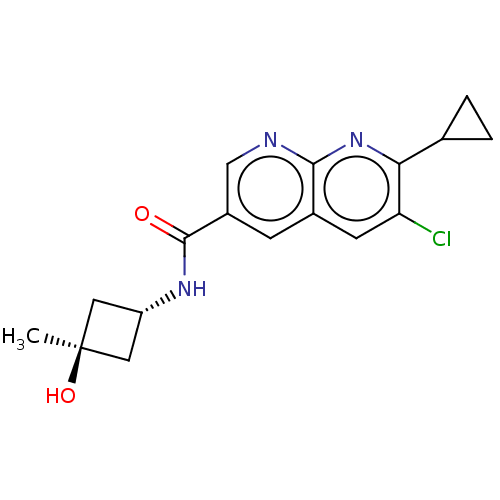
(CHEMBL5273656)Show SMILES C[C@]1(O)C[C@@H](C1)NC(=O)c1cnc2nc(C3CC3)c(Cl)cc2c1 |r,wU:4.6,wD:1.1,(7.82,2.1,;6.28,2.1,;7.05,3.43,;4.79,2.49,;4.39,.98,;5.88,.58,;3.05,.21,;1.72,.98,;1.72,2.52,;.39,.21,;.39,-1.33,;-.95,-2.1,;-2.28,-1.33,;-3.61,-2.1,;-4.95,-1.33,;-6.28,-2.1,;-7.05,-3.43,;-7.82,-2.1,;-4.95,.21,;-6.28,.98,;-3.61,.98,;-2.28,.21,;-.95,.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615782
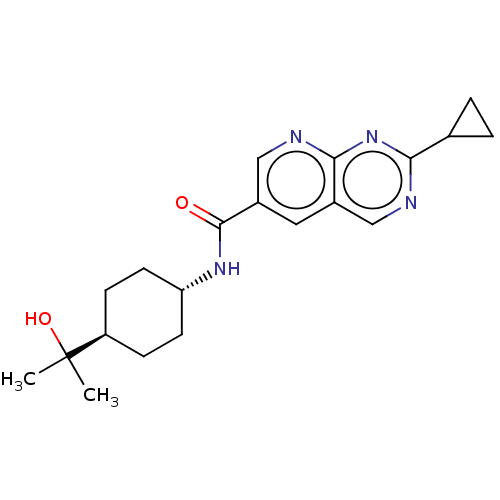
(CHEMBL5282228)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(ncc2c1)C1CC1 |r,wU:7.10,wD:4.3,(6.66,4.03,;7.43,2.69,;8.2,4.03,;8.76,1.92,;6.1,1.92,;4.76,2.69,;3.43,1.92,;3.43,.38,;4.76,-.39,;6.1,.38,;2.09,-.39,;.76,.38,;.76,1.92,;-.57,-.39,;-.57,-1.93,;-1.9,-2.69,;-3.24,-1.92,;-4.56,-2.69,;-5.89,-1.93,;-5.89,-.4,;-4.57,.37,;-3.24,-.39,;-1.91,.39,;-7.22,-2.7,;-8.76,-2.7,;-7.99,-4.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615810
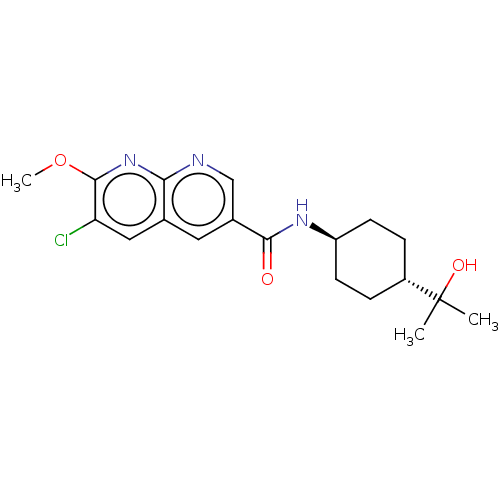
(CHEMBL5277213)Show SMILES COc1nc2ncc(cc2cc1Cl)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:16.17,wD:19.24,(-8.66,-2.59,;-7.33,-3.36,;-5.99,-2.59,;-4.66,-3.36,;-3.34,-2.59,;-2.01,-3.36,;-.68,-2.59,;-.68,-1.05,;-2.02,-.28,;-3.34,-1.06,;-4.68,-.29,;-5.99,-1.07,;-7.33,-.3,;.66,-.28,;.66,1.26,;1.99,-1.05,;3.32,-.28,;3.32,1.26,;4.66,2.03,;5.99,1.26,;5.99,-.28,;4.66,-1.05,;7.33,2.03,;8.1,3.36,;6.56,3.36,;8.66,1.26,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615799
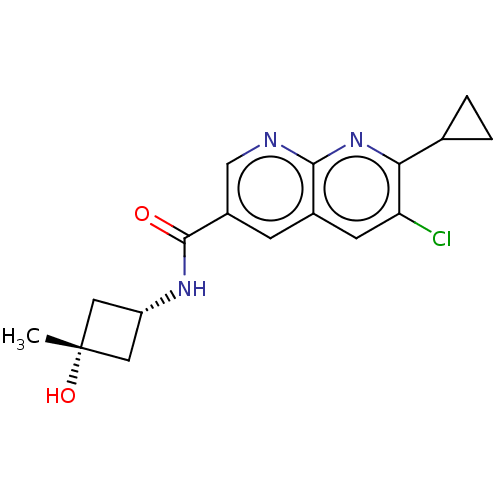
(CHEMBL5268479)Show SMILES C[C@@]1(O)C[C@@H](C1)NC(=O)c1cnc2nc(C3CC3)c(Cl)cc2c1 |r,wU:1.1,4.6,(7.05,3.43,;6.28,2.1,;7.82,2.1,;4.79,2.5,;4.39,.98,;5.88,.58,;3.05,.21,;1.72,.98,;1.72,2.52,;.39,.21,;.39,-1.33,;-.95,-2.1,;-2.28,-1.33,;-3.61,-2.1,;-4.95,-1.33,;-6.28,-2.1,;-7.05,-3.43,;-7.82,-2.1,;-4.95,.21,;-6.28,.98,;-3.61,.98,;-2.28,.21,;-.95,.98,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576316
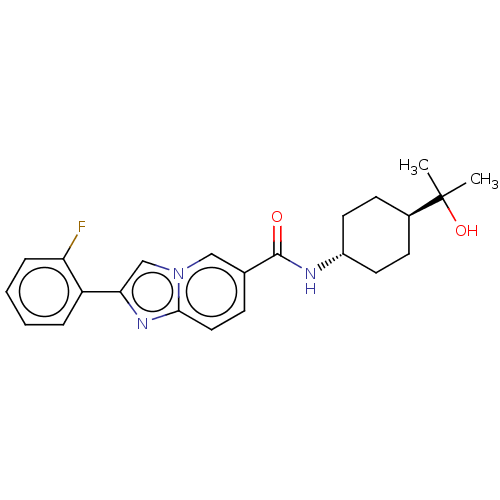
(CHEMBL4870703)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1F |r,wU:7.10,wD:4.3,(2.16,-11.57,;2.98,-12.88,;3.7,-11.51,;1.68,-13.7,;4.35,-13.61,;4.4,-15.15,;5.77,-15.87,;7.07,-15.05,;7.01,-13.51,;5.65,-12.79,;8.44,-15.77,;9.74,-14.95,;9.68,-13.41,;11.1,-15.66,;11.16,-17.2,;12.51,-17.92,;13.81,-17.1,;15.29,-17.52,;16.15,-16.25,;15.2,-15.04,;13.76,-15.57,;12.4,-14.84,;17.68,-16.19,;18.5,-17.5,;20.03,-17.44,;20.76,-16.08,;19.93,-14.77,;18.39,-14.83,;17.57,-13.53,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Oryctolagus cuniculus (Rabbit)) | BDBM107765

(US8598217, 166)Show SMILES O=C(c1nc2nc(ccc2[nH]1)N1CCNCC1)c1ccc(C#N)c(c1)-c1cncc2ccccc12 Show InChI InChI=1S/C27H21N7O/c28-14-18-6-5-17(13-21(18)22-16-30-15-19-3-1-2-4-20(19)22)25(35)27-31-23-7-8-24(32-26(23)33-27)34-11-9-29-10-12-34/h1-8,13,15-16,29H,9-12H2,(H,31,32,33) | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG
US Patent
| Assay Description
A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... |
US Patent US8598217 (2013)
BindingDB Entry DOI: 10.7270/Q2BG2MN8 |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112618

(CHEMBL3608787)Show SMILES C[C@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526494
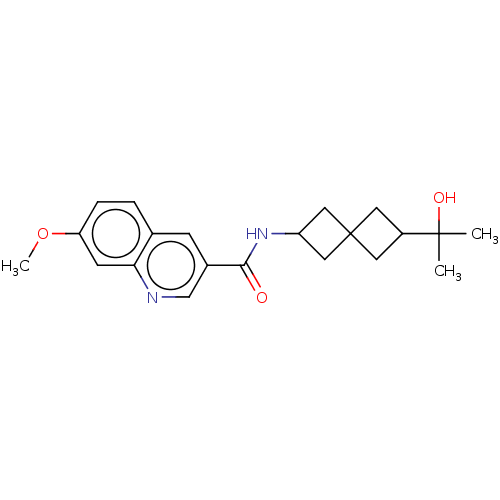
(CHEMBL4439454)Show SMILES COc1ccc2cc(cnc2c1)C(=O)NC1CC2(C1)CC(C2)C(C)(C)O |(1.86,-17.99,;3.19,-18.77,;4.53,-18,;4.53,-16.45,;5.86,-15.68,;7.2,-16.44,;8.52,-15.67,;9.86,-16.43,;9.87,-17.98,;8.53,-18.76,;7.2,-17.99,;5.86,-18.77,;11.19,-15.65,;11.17,-14.11,;12.53,-16.41,;13.86,-15.63,;15.34,-16.02,;15.74,-14.52,;14.24,-14.14,;17.22,-14.92,;17.62,-13.43,;16.13,-13.03,;18.95,-12.66,;18.17,-11.32,;19.72,-11.32,;20.29,-13.43,)| Show InChI InChI=1S/C21H26N2O3/c1-20(2,25)15-8-21(9-15)10-16(11-21)23-19(24)14-6-13-4-5-17(26-3)7-18(13)22-12-14/h4-7,12,15-16,25H,8-11H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112615

(CHEMBL3608785)Show SMILES Cc1ccc(CNC(=O)Nc2ccccc2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H21N5O2/c1-16-10-11-17(14-25-23(30)26-18-7-3-2-4-8-18)13-19(16)27-22(29)20-15-24-21-9-5-6-12-28(20)21/h2-13,15H,14H2,1H3,(H,27,29)(H2,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615808

(CHEMBL5289474)Show SMILES CC(C)c1ccc2cc(cnc2n1)C(=O)N[C@H]1CC[C@@H](CC1)C(C)(C)O |r,wU:16.17,wD:19.24,(-7.33,-4.13,;-7.33,-2.59,;-8.66,-1.82,;-5.99,-1.82,;-5.99,-.3,;-4.68,.48,;-3.34,-.29,;-2.02,.49,;-.68,-.28,;-.68,-1.82,;-2.01,-2.59,;-3.34,-1.82,;-4.66,-2.59,;.66,.49,;.66,2.03,;1.99,-.28,;3.32,.49,;3.32,2.03,;4.66,2.8,;5.99,2.03,;5.99,.49,;4.66,-.28,;7.33,2.8,;8.1,4.13,;6.56,4.13,;8.66,2.03,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576314
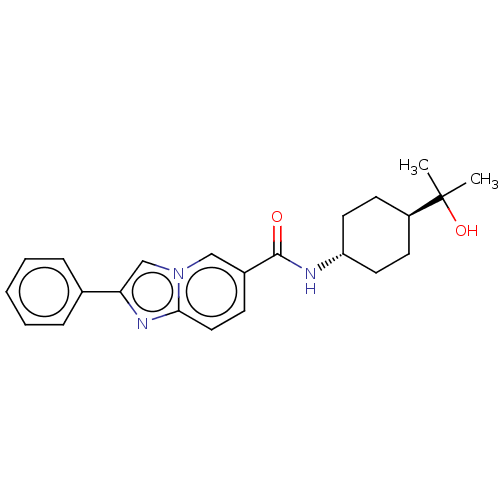
(CHEMBL4864805)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2nc(cn2c1)-c1ccccc1 |r,wU:7.10,wD:4.3,(2.48,-1.86,;3.3,-3.17,;4.02,-1.8,;2,-3.99,;4.67,-3.9,;4.72,-5.44,;6.09,-6.16,;7.39,-5.34,;7.33,-3.8,;5.97,-3.08,;8.76,-6.06,;10.06,-5.24,;10,-3.7,;11.42,-5.96,;11.48,-7.5,;12.83,-8.21,;14.13,-7.39,;15.61,-7.81,;16.46,-6.54,;15.52,-5.33,;14.08,-5.86,;12.72,-5.13,;18,-6.48,;18.81,-7.79,;20.35,-7.73,;21.08,-6.37,;20.25,-5.06,;18.71,-5.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615780
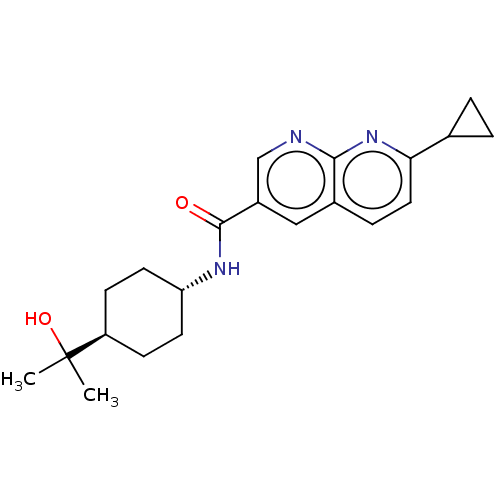
(CHEMBL5285108)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(ccc2c1)C1CC1 |r,wU:7.10,wD:4.3,(6.66,4.03,;7.43,2.69,;8.2,4.03,;8.76,1.92,;6.1,1.92,;4.76,2.69,;3.43,1.92,;3.43,.38,;4.76,-.39,;6.1,.38,;2.1,-.39,;.76,.38,;.76,1.92,;-.57,-.39,;-.57,-1.93,;-1.9,-2.69,;-3.24,-1.92,;-4.56,-2.69,;-5.89,-1.93,;-5.89,-.4,;-4.57,.37,;-3.24,-.39,;-1.91,.39,;-7.22,-2.7,;-7.99,-4.03,;-8.76,-2.7,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526495
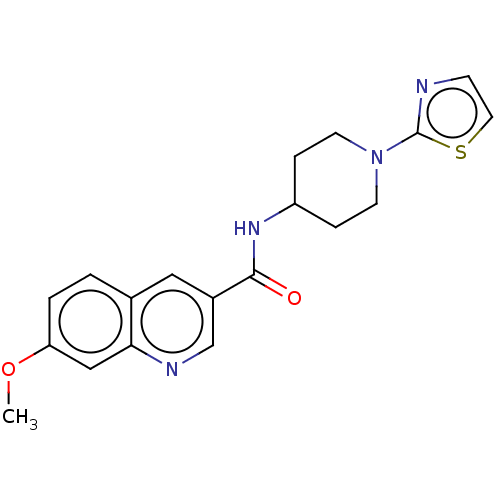
(CHEMBL4452633)Show InChI InChI=1S/C19H20N4O2S/c1-25-16-3-2-13-10-14(12-21-17(13)11-16)18(24)22-15-4-7-23(8-5-15)19-20-6-9-26-19/h2-3,6,9-12,15H,4-5,7-8H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112615

(CHEMBL3608785)Show SMILES Cc1ccc(CNC(=O)Nc2ccccc2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H21N5O2/c1-16-10-11-17(14-25-23(30)26-18-7-3-2-4-8-18)13-19(16)27-22(29)20-15-24-21-9-5-6-12-28(20)21/h2-13,15H,14H2,1H3,(H,27,29)(H2,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526485
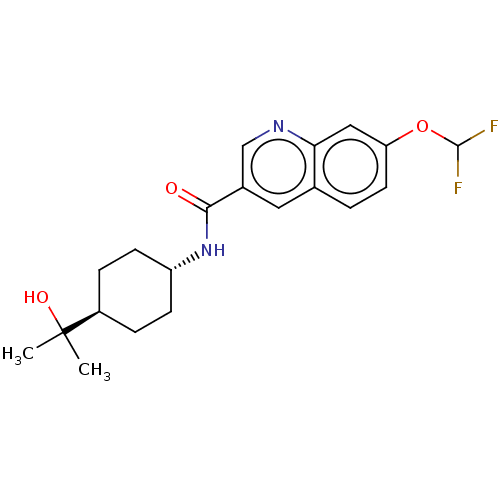
(CHEMBL4473072)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2cc(OC(F)F)ccc2c1 |r,wU:7.10,wD:4.3,(19.66,-11.66,;20.44,-13,;21.21,-11.66,;21.78,-13.76,;19.12,-13.78,;17.78,-13.01,;16.44,-13.79,;16.46,-15.32,;17.8,-16.09,;19.12,-15.32,;15.13,-16.1,;13.79,-15.34,;13.78,-13.8,;12.47,-16.12,;12.48,-17.67,;11.14,-18.44,;9.81,-17.68,;8.47,-18.45,;7.14,-17.68,;5.81,-18.45,;4.47,-17.68,;3.14,-18.45,;4.48,-16.14,;7.14,-16.14,;8.47,-15.37,;9.81,-16.13,;11.13,-15.36,)| Show InChI InChI=1S/C20H24F2N2O3/c1-20(2,26)14-4-6-15(7-5-14)24-18(25)13-9-12-3-8-16(27-19(21)22)10-17(12)23-11-13/h3,8-11,14-15,19,26H,4-7H2,1-2H3,(H,24,25)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50526485

(CHEMBL4473072)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2cc(OC(F)F)ccc2c1 |r,wU:7.10,wD:4.3,(19.66,-11.66,;20.44,-13,;21.21,-11.66,;21.78,-13.76,;19.12,-13.78,;17.78,-13.01,;16.44,-13.79,;16.46,-15.32,;17.8,-16.09,;19.12,-15.32,;15.13,-16.1,;13.79,-15.34,;13.78,-13.8,;12.47,-16.12,;12.48,-17.67,;11.14,-18.44,;9.81,-17.68,;8.47,-18.45,;7.14,-17.68,;5.81,-18.45,;4.47,-17.68,;3.14,-18.45,;4.48,-16.14,;7.14,-16.14,;8.47,-15.37,;9.81,-16.13,;11.13,-15.36,)| Show InChI InChI=1S/C20H24F2N2O3/c1-20(2,26)14-4-6-15(7-5-14)24-18(25)13-9-12-3-8-16(27-19(21)22)10-17(12)23-11-13/h3,8-11,14-15,19,26H,4-7H2,1-2H3,(H,24,25)/t14-,15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of full-length human His6-tagged HPGDS expressed in Escherichia coli BL21 (DE3) using PGH2 as substrate measured after 90 to 120 secs by R... |
Bioorg Med Chem 27: 1456-1478 (2019)
Article DOI: 10.1016/j.bmc.2019.02.017
BindingDB Entry DOI: 10.7270/Q2QV3R00 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50615809
(CHEMBL5288532)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1cnc2nc(ccc2c1)C1CCC1 |r,wU:7.10,wD:4.3,(8.38,4.12,;7.6,2.78,;6.83,4.12,;8.94,2.01,;6.27,2.01,;4.94,2.78,;3.6,2.01,;3.6,.47,;4.94,-.3,;6.27,.47,;2.27,-.3,;.94,.47,;.94,2.01,;-.4,-.3,;-.4,-1.84,;-1.73,-2.6,;-3.06,-1.83,;-4.38,-2.6,;-5.71,-1.84,;-5.71,-.31,;-4.4,.46,;-3.06,-.3,;-1.74,.48,;-7.04,-2.61,;-8.53,-2.21,;-8.94,-3.72,;-7.45,-4.12,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM50576324
(CHEMBL4868339)Show SMILES CC(C)(O)[C@H]1CC[C@@H](CC1)NC(=O)c1ccc2n(ccc2c1)-c1cccc(F)c1 |r,wU:7.10,wD:4.3,(32.28,-11.78,;31.52,-13.11,;33.06,-13.1,;31.53,-14.65,;30.2,-12.34,;30.2,-10.8,;28.86,-10.03,;27.54,-10.8,;27.53,-12.34,;28.86,-13.11,;26.2,-10.04,;24.87,-10.81,;23.54,-10.04,;24.88,-12.35,;26.21,-13.11,;26.22,-14.66,;24.88,-15.44,;24.56,-16.95,;23.03,-17.12,;22.39,-15.7,;23.54,-14.67,;23.55,-13.12,;25.6,-18.09,;25.12,-19.55,;26.15,-20.69,;27.66,-20.37,;28.13,-18.89,;29.63,-18.56,;27.09,-17.76,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HPGDS assessed as reduction in PGD2 formation using PGH2 as substrate by mass spectrometry |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128113
BindingDB Entry DOI: 10.7270/Q2B28047 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data