 Found 67 hits with Last Name = 'moss' and Initial = 'j'
Found 67 hits with Last Name = 'moss' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257517
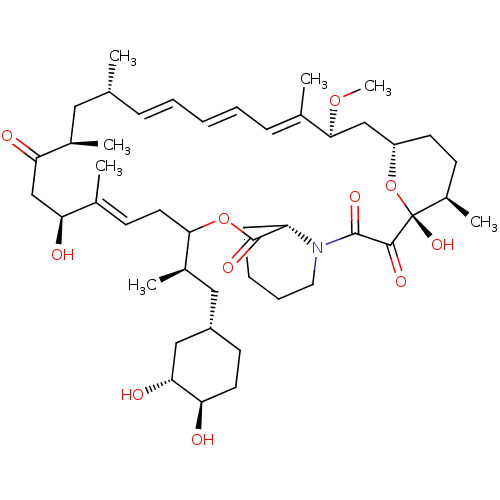
(US9505773, 10)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1 |r,c:29,t:42,44,46| Show InChI InChI=1S/C46H71NO11/c1-28-13-9-8-10-14-30(3)42(56-7)26-35-19-17-33(6)46(55,58-35)43(52)44(53)47-22-12-11-15-36(47)45(54)57-41(32(5)24-34-18-20-37(48)40(51)25-34)21-16-29(2)38(49)27-39(50)31(4)23-28/h8-10,13-14,16,28,31-38,40-42,48-49,51,55H,11-12,15,17-27H2,1-7H3/b10-8+,13-9+,29-16+,30-14+/t28-,31-,32-,33-,34+,35+,36+,37-,38+,40-,41?,42+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM92863
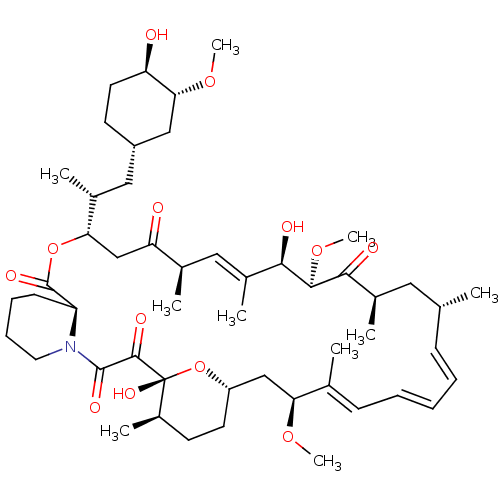
(US9505773, Rapamycin | mTOR Inhibitor, Rapamycin)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C/C=C/C=C(C)/[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,29,33,t:31| Show InChI InChI=1S/C51H79NO13/c1-30-16-12-11-13-17-31(2)42(61-8)28-38-21-19-36(7)51(60,65-38)48(57)49(58)52-23-15-14-18-39(52)50(59)64-43(33(4)26-37-20-22-40(53)44(27-37)62-9)29-41(54)32(3)25-35(6)46(56)47(63-10)45(55)34(5)24-30/h11-13,16-17,25,30,32-34,36-40,42-44,46-47,53,56,60H,14-15,18-24,26-29H2,1-10H3/b13-11+,16-12-,31-17+,35-25+/t30-,32-,33-,34-,36-,37+,38?,39+,40-,42+,43+,44-,46-,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257515
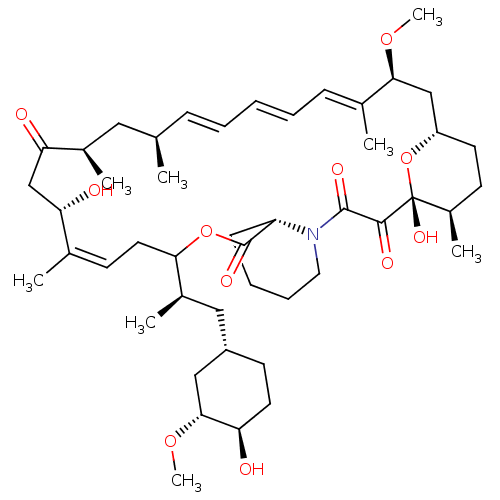
(US9505773, 8)Show SMILES CO[C@@H]1C[C@H](C[C@@H](C)C2C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@@H]3CC[C@@H](C)[C@@](O)(O3)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |r,c:10,27,t:23,25| Show InChI InChI=1S/C47H73NO11/c1-29-14-10-9-11-15-31(3)42(56-7)27-36-20-18-34(6)47(55,59-36)44(52)45(53)48-23-13-12-16-37(48)46(54)58-41(22-17-30(2)39(50)28-40(51)32(4)24-29)33(5)25-35-19-21-38(49)43(26-35)57-8/h9-11,14-15,17,29,32-39,41-43,49-50,55H,12-13,16,18-28H2,1-8H3/b11-9+,14-10+,30-17+,31-15+/t29-,32-,33-,34-,35+,36+,37+,38-,39+,41?,42+,43-,47-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257521

(US9505773, 14)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(O)CC(=O)[C@H](C)\C=C(C)\[C@@H](O)CC(=O)[C@@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:21.22,7.7,9.9,37.39,42.44,60.63,wD:4.11,45.47,25.56,54.57,57.59,32.34,2.1,c:36,t:49,51,53,(-4.64,-8.27,;-3.3,-7.52,;-3.27,-5.98,;-4.67,-6.54,;-6,-5.78,;-7.34,-6.54,;-8.67,-5.78,;-8.67,-4.23,;-10,-3.47,;-7.34,-3.47,;-8.67,-2.69,;-6,-4.23,;-7.34,-1.93,;-8.67,-1.15,;-6,-1.15,;-4.67,-1.93,;-6,.38,;-7.34,1.15,;-7.34,2.69,;-6,3.47,;-4.67,2.69,;-4.67,1.15,;-3.33,.38,;-3.33,-1.15,;-2,1.15,;-2,2.69,;-.67,3.47,;.67,2.69,;.67,1.15,;2,3.47,;3.33,2.69,;3.33,1.15,;4.67,3.47,;4.67,5,;6,2.69,;6,1.15,;4.67,.38,;7.34,.38,;8.67,1.15,;7.34,-1.15,;8.67,-1.93,;10,-1.15,;8.67,-3.47,;7.34,-4.23,;7.34,-5.78,;6,-6.54,;6,-8.08,;4.67,-5.78,;3.33,-6.54,;2,-5.78,;.67,-6.54,;-.67,-5.78,;-2,-6.54,;-2,-8.08,;-3.33,3.47,;-4.67,4.23,;-3.33,5,;-2,5.78,;-.67,5,;.67,5.78,;.67,7.31,;2,8.08,;-.67,8.08,;-2,7.31,)| Show InChI InChI=1S/C51H79NO12/c1-31-14-10-9-11-15-32(2)46(62-8)29-41-22-17-37(7)51(61,64-41)48(58)49(59)52-23-13-12-16-42(52)50(60)63-47(36(6)26-38-18-20-39(53)21-19-38)28-40(54)27-43(55)34(4)25-35(5)45(57)30-44(56)33(3)24-31/h9-11,14-15,25,31,33-34,36-42,45-47,53-54,57,61H,12-13,16-24,26-30H2,1-8H3/b11-9+,14-10+,32-15+,35-25+/t31-,33+,34-,36-,37-,38-,39-,40?,41+,42+,45+,46+,47+,51-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257524
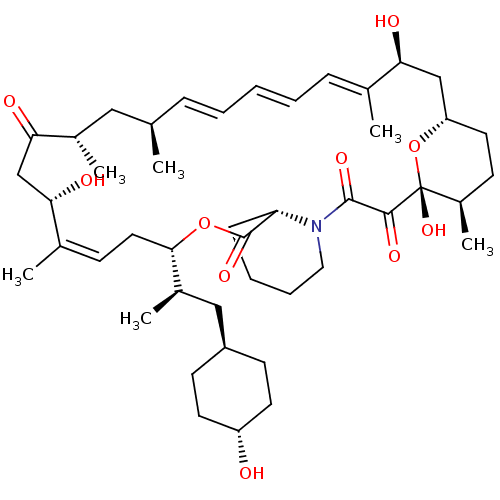
(US9505773, 19)Show SMILES C[C@H](C[C@H]1CC[C@H](O)CC1)[C@@H]1C\C=C(C)\[C@@H](O)CC(=O)[C@@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@@H](O)C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 |r,wU:52.54,40.41,38.39,6.6,20.21,15.16,wD:35.43,23.24,3.2,1.0,10.58,32.33,c:13,30,t:26,28,(-4.82,-16.51,;-3.33,-16.91,;-3.33,-15.37,;-2,-14.6,;-.67,-15.37,;.67,-14.6,;.67,-13.06,;2,-12.29,;-.67,-12.29,;-2,-13.06,;-2,-17.68,;-.67,-16.91,;6,-17.68,;6,-19.22,;4.67,-19.99,;7.34,-19.99,;8.67,-19.22,;7.34,-21.53,;8.67,-22.3,;10,-21.53,;8.67,-23.84,;7.18,-24.24,;8.05,-26.15,;6,-26.92,;6,-28.46,;4.67,-26.15,;3.33,-26.92,;2,-26.15,;.67,-26.92,;-.67,-26.15,;-2,-26.92,;-2,-28.46,;-3.33,-26.15,;-3.33,-24.61,;-4.67,-26.92,;-6,-26.15,;-7.34,-26.92,;-8.67,-26.15,;-8.67,-24.61,;-10,-23.84,;-7.34,-23.84,;-8.67,-23.07,;-6,-24.61,;-7.34,-22.3,;-8.67,-21.53,;-6,-21.53,;-4.67,-22.3,;-6,-19.99,;-7.34,-19.22,;-7.34,-17.68,;-6,-16.91,;-4.67,-17.68,;-4.67,-19.22,;-3.33,-19.99,;-3.33,-21.53,;-2,-19.22,)| Show InChI InChI=1S/C45H69NO10/c1-28-12-8-7-9-13-29(2)38(48)26-36-21-16-33(6)45(54,56-36)42(51)43(52)46-23-11-10-14-37(46)44(53)55-41(32(5)25-34-17-19-35(47)20-18-34)22-15-30(3)39(49)27-40(50)31(4)24-28/h7-9,12-13,15,28,31-39,41,47-49,54H,10-11,14,16-27H2,1-6H3/b9-7+,12-8+,29-13+,30-15+/t28-,31+,32-,33-,34-,35-,36+,37+,38+,39+,41+,45-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257513

(US9505773, 5)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:21.22,7.7,9.9,53.56,30.32,35.37,wD:4.11,38.40,50.52,47.50,2.1,c:29,t:42,44,46,(-3.31,-9.4,;-1.96,-8.65,;-1.94,-7.11,;-3.33,-7.67,;-4.67,-6.9,;-6,-7.67,;-7.34,-6.9,;-7.34,-5.36,;-8.67,-4.59,;-6,-4.59,;-7.34,-3.82,;-4.67,-5.36,;-6,-3.05,;-7.34,-2.28,;-4.67,-2.28,;-3.33,-3.05,;-4.67,-.74,;-6,.03,;-6,1.57,;-4.67,2.34,;-3.33,1.57,;-3.33,.03,;-2,-.74,;-2,-2.28,;-.67,.03,;-.67,1.57,;3.33,1.93,;4.67,1.15,;4.67,-.38,;3.33,-1.15,;6,-1.15,;7.34,-.38,;6,-2.69,;7.34,-3.47,;8.67,-2.69,;7.34,-5,;6,-5.78,;8.11,-6.34,;7.34,-7.67,;8.11,-9.01,;6,-6.9,;4.67,-7.67,;3.33,-6.9,;2,-7.67,;.67,-6.9,;-.67,-7.67,;-.67,-9.21,;-2,2.34,;-3.33,3.11,;-2,3.88,;-1.23,5.21,;.31,5.21,;1.08,6.54,;.31,7.88,;1.08,9.21,;-1.23,7.88,;-2,6.54,)| Show InChI InChI=1S/C46H71NO10/c1-29-13-9-8-10-14-31(3)42(55-7)27-37-22-17-34(6)46(54,57-37)43(51)44(52)47-24-12-11-15-38(47)45(53)56-41(33(5)26-35-18-20-36(48)21-19-35)23-16-30(2)39(49)28-40(50)32(4)25-29/h8-10,13-14,16,29,32-39,41-42,48-49,54H,11-12,15,17-28H2,1-7H3/b10-8+,13-9+,30-16+,31-14+/t29-,32-,33-,34-,35-,36-,37+,38+,39+,41?,42+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257519
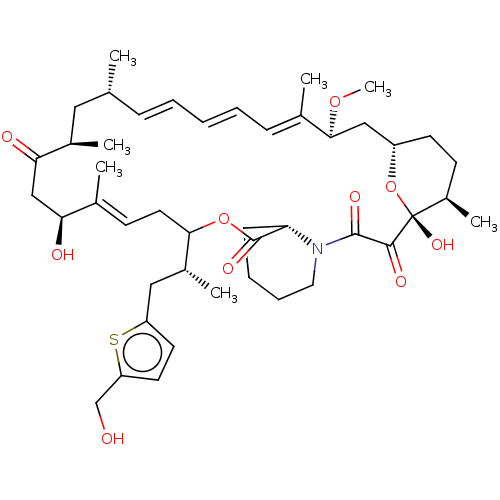
(US9505773, 12)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)Cc1ccc(CO)s1 |r,c:29,t:42,44,46| Show InChI InChI=1S/C45H65NO10S/c1-28-13-9-8-10-14-30(3)41(54-7)25-34-18-17-33(6)45(53,56-34)42(50)43(51)46-22-12-11-15-37(46)44(52)55-40(32(5)24-35-19-20-36(27-47)57-35)21-16-29(2)38(48)26-39(49)31(4)23-28/h8-10,13-14,16,19-20,28,31-34,37-38,40-41,47-48,53H,11-12,15,17-18,21-27H2,1-7H3/b10-8+,13-9+,29-16+,30-14+/t28-,31-,32-,33-,34+,37+,38+,40?,41+,45-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257529
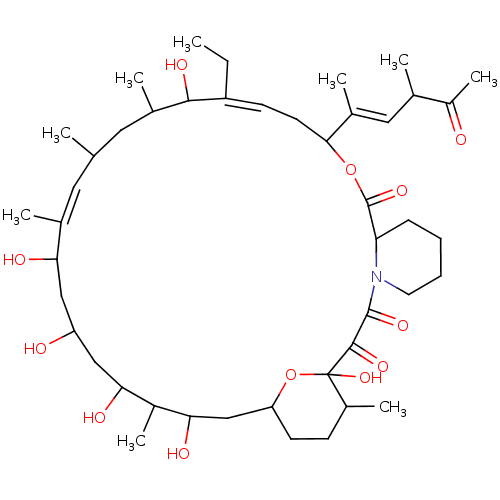
(US9505773, Meridamycin)Show SMILES [H]C12CCC(C)C(O)(O1)C(=O)C(=O)N1CCCCC1C(=O)OC(C\C=C(CC)/C(O)C(C)CC(C)\C=C(C)/C(O)CC(O)CC(O)C(C)C(O)C2)C(\C)=C\C(C)C(C)=O |t:26,37| Show InChI InChI=1S/C45H73NO12/c1-10-33-15-17-40(28(5)21-26(3)32(9)47)57-44(55)36-13-11-12-18-46(36)43(54)42(53)45(56)30(7)14-16-35(58-45)24-39(51)31(8)38(50)23-34(48)22-37(49)27(4)19-25(2)20-29(6)41(33)52/h15,19,21,25-26,29-31,34-41,48-52,56H,10-14,16-18,20,22-24H2,1-9H3/b27-19-,28-21+,33-15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257528

(US9505773, FK506)Show SMILES CO[C@@H]1C[C@@H](CC[C@H]1O)\C=C(/C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H]([C@H](C[C@H]2C)OC)[C@H](C[C@@H](C)C\C(C)=C/[C@@H](CC=C)C(=O)C[C@H](O)[C@H]1C)OC |c:45| Show InChI InChI=1S/C44H69NO12/c1-10-13-31-19-25(2)18-26(3)20-37(54-8)40-38(55-9)22-28(5)44(52,57-40)41(49)42(50)45-17-12-11-14-32(45)43(51)56-39(29(6)34(47)24-35(31)48)27(4)21-30-15-16-33(46)36(23-30)53-7/h10,19,21,26,28-34,36-40,46-47,52H,1,11-18,20,22-24H2,2-9H3/b25-19-,27-21+/t26-,28+,29+,30-,31+,32-,33+,34-,36+,37-,38-,39+,40+,44+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257527
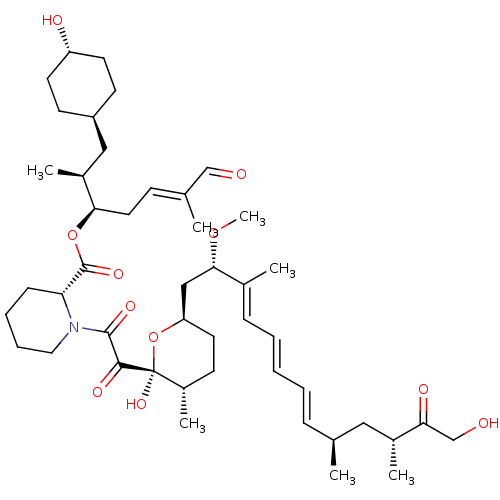
(US9505773, 22)Show SMILES CO[C@@H](C[C@H]1CC[C@H](C)[C@](O)(O1)C(=O)C(=O)N1CCCC[C@@H]1C(=O)O[C@H](C\C=C(/C)C=O)[C@@H](C)C[C@H]1CC[C@H](O)CC1)C(\C)=C\C=C\C=C\[C@H](C)C[C@@H](C)C(=O)CO |r,wU:21.23,9.9,7.7,52.55,38.40,wD:9.12,4.3,49.52,35.36,32.34,25.26,2.1,(-4.06,6.54,;-4.06,5,;-2.73,4.23,;-2.73,2.69,;-4.06,1.92,;-5.4,2.69,;-6.73,1.92,;-6.73,.38,;-8.06,-.39,;-5.4,-.39,;-4.41,-1.57,;-4.06,.38,;-6.38,-1.57,;-7.9,-1.3,;-5.86,-3.02,;-4.34,-3.29,;-6.85,-4.2,;-8.36,-3.93,;-9.35,-5.11,;-8.83,-6.56,;-7.31,-6.82,;-6.32,-5.64,;-4.8,-5.91,;-3.81,-4.73,;-4.28,-7.36,;-2.76,-7.63,;-1.77,-6.45,;-.25,-6.71,;.73,-5.53,;.21,-4.09,;2.25,-5.8,;3.24,-4.62,;-2.23,-9.07,;-3.22,-10.25,;-.72,-9.34,;-.19,-10.79,;-1.18,-11.97,;-.65,-13.42,;.86,-13.68,;1.39,-15.13,;1.85,-12.5,;1.33,-11.06,;-1.39,5,;-1.39,6.54,;-.06,4.23,;1.27,5,;2.61,4.23,;3.94,5,;5.27,4.23,;6.61,5,;6.61,6.54,;7.94,4.23,;9.28,5,;9.28,6.54,;10.61,4.23,;10.61,2.69,;11.94,5,;13.28,4.23,)| Show InChI InChI=1S/C46H71NO11/c1-30(25-33(4)40(51)29-49)13-9-8-10-14-32(3)42(56-7)27-38-22-17-35(6)46(55,58-38)43(52)44(53)47-24-12-11-15-39(47)45(54)57-41(23-16-31(2)28-48)34(5)26-36-18-20-37(50)21-19-36/h8-10,13-14,16,28,30,33-39,41-42,49-50,55H,11-12,15,17-27,29H2,1-7H3/b10-8+,13-9+,31-16+,32-14+/t30-,33+,34-,35-,36-,37-,38+,39+,41+,42-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257520

(US9505773, 13)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)Cc1ccncc1 |r,c:29,t:42,44,46| Show InChI InChI=1S/C45H64N2O9/c1-29-13-9-8-10-14-31(3)41(54-7)27-36-18-17-34(6)45(53,56-36)42(50)43(51)47-24-12-11-15-37(47)44(52)55-40(33(5)26-35-20-22-46-23-21-35)19-16-30(2)38(48)28-39(49)32(4)25-29/h8-10,13-14,16,20-23,29,32-34,36-38,40-41,48,53H,11-12,15,17-19,24-28H2,1-7H3/b10-8+,13-9+,30-16+,31-14+/t29-,32-,33-,34-,36+,37+,38+,40?,41+,45-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257525
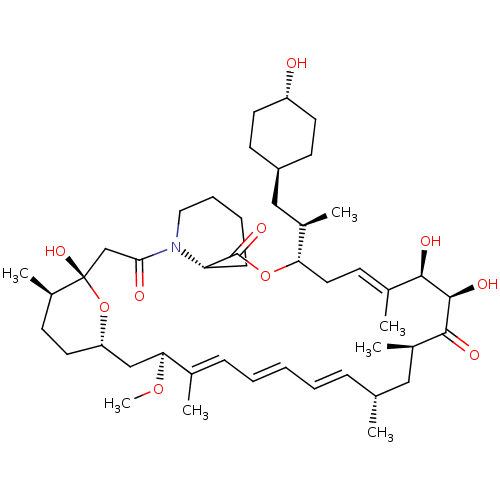
(US9505773, 20)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@](O)(CC(=O)N3CCCC[C@H]3C(=O)O[C@@H](C\C=C(C)\[C@@H](O)[C@@H](O)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@H]1CC[C@H](O)CC1)O2 |r,wU:19.19,9.9,7.7,34.35,28.29,52.54,wD:4.59,37.38,49.50,46.48,23.23,2.1,30.31,c:26,t:40,42,44,(-1.01,-24.29,;-2.35,-25.06,;-2.35,-26.6,;-3.68,-27.37,;-5.01,-26.6,;-6.35,-27.37,;-7.68,-26.6,;-7.68,-25.06,;-9.01,-24.29,;-6.35,-24.29,;-7.68,-23.52,;-6.35,-22.75,;-5.01,-21.98,;-3.68,-22.75,;-5.01,-20.44,;-6.35,-19.67,;-6.35,-18.13,;-5.01,-17.36,;-3.68,-18.13,;-3.68,-19.67,;-2.35,-20.44,;-2.35,-21.98,;-1.01,-19.67,;-1.01,-18.13,;.32,-17.36,;5.01,-18.63,;5.01,-20.17,;3.68,-20.94,;6.35,-20.94,;7.68,-20.17,;6.35,-22.48,;5.01,-23.25,;7.68,-23.25,;9.01,-22.48,;7.68,-24.79,;6.19,-25.19,;8.08,-26.28,;6.99,-27.37,;7.4,-28.89,;5.66,-26.6,;4.32,-27.37,;2.99,-26.6,;1.66,-27.37,;.32,-26.6,;-1.01,-27.37,;-1.01,-28.91,;-2.35,-17.36,;-3.68,-16.59,;-2.35,-15.82,;-1.26,-14.73,;.23,-15.13,;1.32,-14.04,;.92,-12.55,;2.01,-11.47,;-.57,-12.16,;-1.66,-13.24,;-5.01,-25.06,)| Show InChI InChI=1S/C46H73NO10/c1-29-13-9-8-10-14-30(2)40(55-7)27-37-22-17-34(6)46(54,57-37)28-41(49)47-24-12-11-15-38(47)45(53)56-39(32(4)26-35-18-20-36(48)21-19-35)23-16-31(3)42(50)44(52)43(51)33(5)25-29/h8-10,13-14,16,29,32-40,42,44,48,50,52,54H,11-12,15,17-28H2,1-7H3/b10-8+,13-9+,30-14+,31-16+/t29-,32-,33-,34-,35-,36-,37+,38+,39+,40+,42-,44-,46+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257523
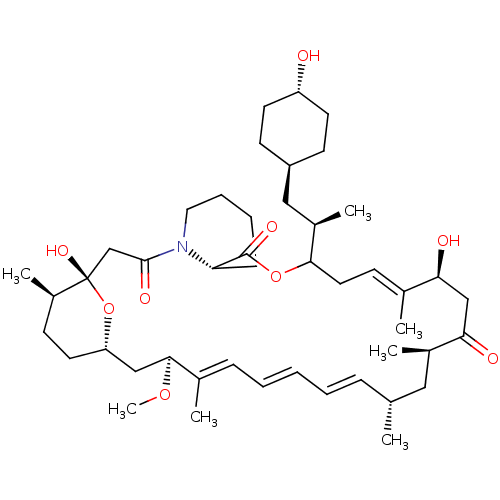
(US9505773, 18)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@](O)(CC(=O)N3CCCC[C@H]3C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@H]1CC[C@H](O)CC1)O2 |r,wU:19.19,9.9,7.7,51.53,33.34,28.29,wD:4.58,36.37,48.49,45.47,2.1,c:26,t:39,41,43,(-.77,-3.25,;-2.1,-4.02,;-2.1,-5.56,;-3.44,-6.33,;-4.77,-5.56,;-6.1,-6.33,;-7.44,-5.56,;-7.44,-4.02,;-8.77,-3.25,;-6.1,-3.25,;-7.44,-2.48,;-6.1,-1.71,;-4.77,-.94,;-3.44,-1.71,;-4.77,.6,;-6.1,1.37,;-6.1,2.91,;-4.77,3.68,;-3.44,2.91,;-3.44,1.37,;-2.1,.6,;-2.1,-.94,;-.77,1.37,;-.77,2.91,;3.92,3.18,;5.26,2.41,;5.26,.87,;3.92,.1,;6.59,.1,;7.92,.87,;6.59,-1.44,;7.92,-2.21,;9.26,-1.44,;7.92,-3.75,;6.44,-4.15,;8.32,-5.24,;7.23,-6.33,;7.64,-7.84,;5.9,-5.56,;4.57,-6.33,;3.23,-5.56,;1.9,-6.33,;.56,-5.56,;-.77,-6.33,;-.77,-7.87,;-2.1,3.68,;-3.59,4.08,;-2.1,5.22,;-1.33,6.55,;.21,6.55,;.98,7.89,;.21,9.22,;.98,10.56,;-1.33,9.22,;-2.1,7.89,;-4.77,-4.02,)| Show InChI InChI=1S/C46H73NO9/c1-30-13-9-8-10-14-32(3)43(54-7)27-38-22-17-35(6)46(53,56-38)29-44(51)47-24-12-11-15-39(47)45(52)55-42(34(5)26-36-18-20-37(48)21-19-36)23-16-31(2)40(49)28-41(50)33(4)25-30/h8-10,13-14,16,30,33-40,42-43,48-49,53H,11-12,15,17-29H2,1-7H3/b10-8+,13-9+,31-16+,32-14+/t30-,33-,34-,35-,36-,37-,38+,39+,40+,42?,43+,46+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257526
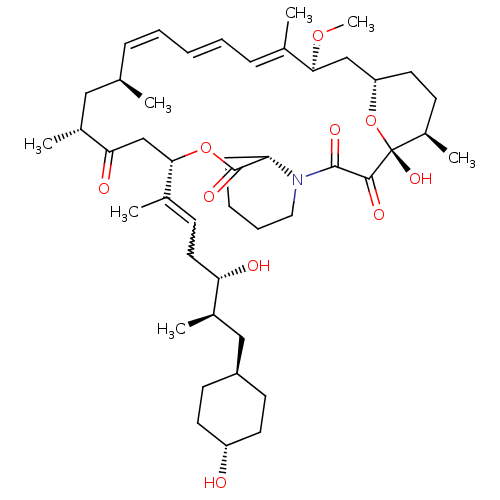
(US9505773, 21)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@@](O)(O2)C(=O)C(=O)N2CCCC[C@H]2C(=O)O[C@@H](CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)C(C)=CC[C@H](O)[C@H](C)C[C@H]1CC[C@H](O)CC1 |r,wU:21.22,9.9,7.7,29.31,53.56,wD:4.11,32.34,50.52,47.50,2.1,25.43,45.48,t:36,38,40,(-.62,-3.93,;-1.95,-4.7,;-1.95,-6.24,;-3.29,-7.01,;-4.62,-6.24,;-5.95,-7.01,;-7.29,-6.24,;-7.29,-4.7,;-8.62,-3.93,;-5.95,-3.93,;-7.29,-3.16,;-4.62,-4.7,;-5.95,-2.39,;-7.29,-1.62,;-4.62,-1.62,;-3.29,-2.39,;-4.62,-.08,;-5.95,.69,;-5.95,2.23,;-4.62,3,;-3.29,2.23,;-3.29,.69,;-1.95,-.08,;-1.95,-1.62,;4.07,-.58,;5.41,.19,;5.41,-1.35,;8.07,-2.89,;9.41,-2.12,;8.07,-4.43,;6.59,-4.83,;8.47,-5.92,;7.38,-7.01,;7.79,-8.52,;6.05,-6.24,;4.72,-7.01,;3.38,-6.24,;2.05,-7.01,;.72,-6.24,;-.62,-7.01,;-.62,-8.55,;1.96,1.38,;4.48,2.15,;1.96,2.92,;.63,3.69,;-.46,2.6,;.63,1.51,;-1.95,3,;-3.29,3.77,;-1.95,4.54,;-.86,5.63,;.62,5.23,;1.71,6.32,;1.32,7.81,;2.4,8.9,;-.17,8.21,;-1.26,7.12,)| Show InChI InChI=1S/C46H71NO10/c1-29-13-9-8-10-14-30(2)41(55-7)27-37-22-17-34(6)46(54,57-37)43(51)44(52)47-24-12-11-15-38(47)45(53)56-42(28-40(50)32(4)25-29)31(3)16-23-39(49)33(5)26-35-18-20-36(48)21-19-35/h8-10,13-14,16,29,32-39,41-42,48-49,54H,11-12,15,17-28H2,1-7H3/b10-8+,13-9+,30-14+,31-16-/t29-,32-,33-,34-,35-,36-,37+,38+,39+,41+,42+,46-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257516
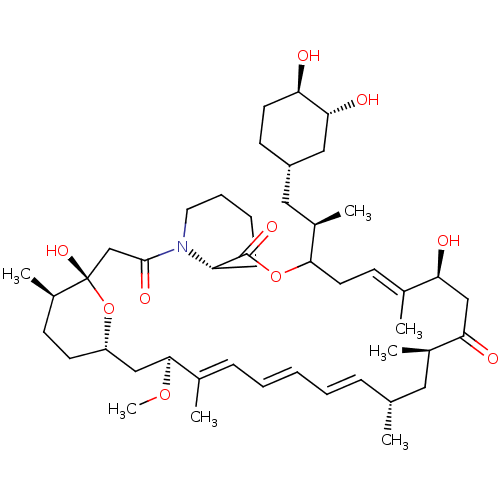
(US9505773, 9)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@](O)(CC(=O)N3CCCC[C@H]3C(=O)OC(C\C=C(C)\[C@@H](O)CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1)O2 |r,c:26,t:39,41,43| Show InChI InChI=1S/C46H73NO10/c1-29-13-9-8-10-14-31(3)43(55-7)26-36-19-17-34(6)46(54,57-36)28-44(52)47-22-12-11-15-37(47)45(53)56-42(33(5)24-35-18-20-38(48)41(51)25-35)21-16-30(2)39(49)27-40(50)32(4)23-29/h8-10,13-14,16,29,32-39,41-43,48-49,51,54H,11-12,15,17-28H2,1-7H3/b10-8+,13-9+,30-16+,31-14+/t29-,32-,33-,34-,35+,36+,37+,38-,39+,41-,42?,43+,46+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257514
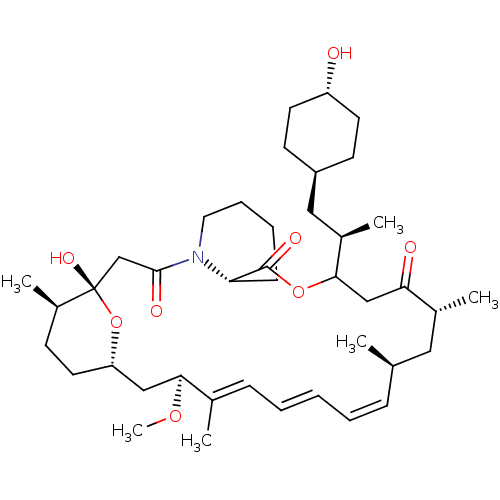
(US9505773, 6)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@](O)(CC(=O)N3CCCC[C@H]3C(=O)OC(CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)[C@H](C)C[C@H]1CC[C@H](O)CC1)O2 |r,wU:19.19,7.7,9.9,45.47,27.28,wD:4.52,30.31,42.43,39.41,2.1,t:33,35,37,(-3.31,-9.4,;-1.96,-8.65,;-1.94,-7.11,;-3.33,-7.67,;-4.67,-6.9,;-6,-7.67,;-7.34,-6.9,;-7.34,-5.36,;-8.67,-4.59,;-6,-4.59,;-7.34,-3.82,;-6,-3.05,;-4.67,-2.28,;-3.33,-3.05,;-4.67,-.74,;-6,.03,;-6,1.57,;-4.67,2.34,;-3.33,1.57,;-3.33,.03,;-2,-.74,;-2,-2.28,;-.67,.03,;-.67,1.57,;.67,2.34,;7.34,-3.47,;8.67,-2.69,;7.34,-5,;6,-5.78,;8.11,-6.34,;7.34,-7.67,;8.11,-9.01,;6,-6.9,;4.67,-7.67,;3.33,-6.9,;2,-7.67,;.67,-6.9,;-.67,-7.67,;-.67,-9.21,;-2,2.34,;-3.33,3.11,;-2,3.88,;-1.23,5.21,;.31,5.21,;1.08,6.54,;.31,7.88,;1.08,9.21,;-1.23,7.88,;-2,6.54,;-4.67,-5.36,)| Show InChI InChI=1S/C41H65NO8/c1-27-12-8-7-9-13-28(2)37(48-6)24-34-20-15-31(5)41(47,50-34)26-39(45)42-21-11-10-14-35(42)40(46)49-38(25-36(44)29(3)22-27)30(4)23-32-16-18-33(43)19-17-32/h7-9,12-13,27,29-35,37-38,43,47H,10-11,14-26H2,1-6H3/b9-7+,12-8+,28-13+/t27-,29-,30-,31-,32-,33-,34+,35+,37+,38?,41+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257518

(US9505773, 11)Show SMILES CO[C@H]1C[C@@H]2CC[C@@H](C)[C@](O)(CC(=O)N3CCCC[C@H]3C(=O)O[C@@H](CC(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C1/C)C(\C)=C\CC(O)[C@H](C)C[C@@H]1CC[C@@H](O)[C@H](O)C1)O2 |r,t:33,35,37| Show InChI InChI=1S/C46H73NO10/c1-29-13-9-8-10-14-30(2)42(55-7)26-36-19-17-34(6)46(54,57-36)28-44(52)47-22-12-11-15-37(47)45(53)56-43(27-40(50)32(4)23-29)31(3)16-20-38(48)33(5)24-35-18-21-39(49)41(51)25-35/h8-10,13-14,16,29,32-39,41-43,48-49,51,54H,11-12,15,17-28H2,1-7H3/b10-8+,13-9+,30-14+,31-16+/t29-,32-,33-,34-,35+,36+,37+,38?,39-,41-,42+,43+,46+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM257522
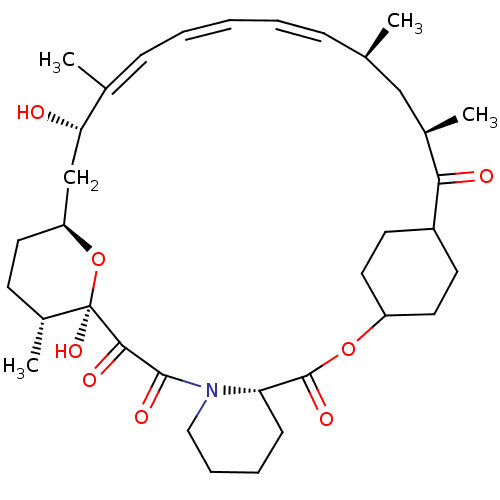
(US9505773, 16)Show SMILES C[C@@H]1CC[C@H]2C[C@H](O)\C(C)=C\C=C\C=C\[C@@H](C)C[C@@H](C)C(=O)C3CCC(CC3)OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@]1(O)O2 |r,wU:31.37,18.18,1.0,41.44,wD:4.46,6.6,15.15,t:9,11,13,(-8.73,-1.15,;-7.4,-1.93,;-7.4,-3.47,;-6.07,-4.23,;-4.73,-3.47,;-3.4,-4.23,;-2.07,-3.47,;-2.07,-1.93,;-.73,-4.23,;-.73,-5.78,;.6,-3.47,;1.93,-4.23,;3.27,-3.47,;4.6,-4.23,;5.94,-3.47,;7.4,-3.94,;8.17,-5.27,;8.31,-2.69,;7.4,-1.45,;5.94,-1.93,;7.4,.09,;8.73,.86,;4.63,2.18,;3.86,.84,;2.32,.84,;1.55,2.18,;2.32,3.51,;3.86,3.51,;-.73,3.47,;-2.07,2.69,;-2.07,1.15,;-3.4,3.47,;-3.4,5,;-4.73,5.78,;-6.07,5,;-6.07,3.47,;-4.73,2.69,;-4.73,1.15,;-3.4,.38,;-6.07,.38,;-7.4,1.15,;-6.07,-1.15,;-7.4,-.38,;-4.73,-1.93,)| Show InChI InChI=1S/C35H51NO8/c1-22-10-6-5-7-11-23(2)30(37)21-28-16-13-25(4)35(42,44-28)32(39)33(40)36-19-9-8-12-29(36)34(41)43-27-17-14-26(15-18-27)31(38)24(3)20-22/h5-7,10-11,22,24-30,37,42H,8-9,12-21H2,1-4H3/b7-5+,10-6+,23-11+/t22-,24-,25-,26?,27?,28+,29+,30+,35-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Isomerase Therapeutics Ltd.
US Patent
| Assay Description
The assay was conducted at 10° C. in 50 mM Tris buffer at pH8.0, 50 μM DTT, 100 mM NaCl, 0.005% NP40 with 6 nM FKBP12 and 60 μM substrate (... |
US Patent US9505773 (2016)
BindingDB Entry DOI: 10.7270/Q2P849TK |
More data for this
Ligand-Target Pair | |
Adenosine kinase
(Homo sapiens (Human)) | BDBM50100587
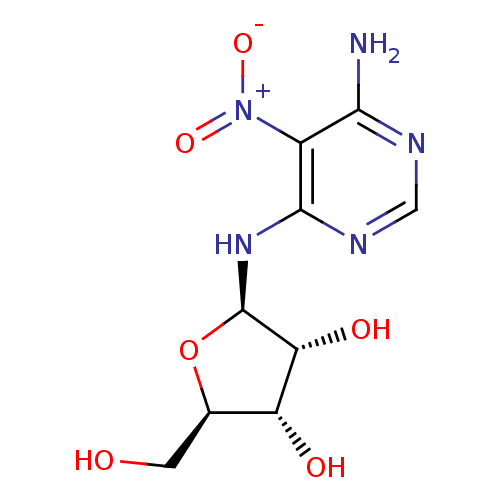
((2R,3R,4S,5R)-2-(6-Amino-5-nitro-pyrimidin-4-ylami...)Show SMILES Nc1ncnc(N[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O)c1[N+]([O-])=O Show InChI InChI=1S/C9H13N5O6/c10-7-4(14(18)19)8(12-2-11-7)13-9-6(17)5(16)3(1-15)20-9/h2-3,5-6,9,15-17H,1H2,(H3,10,11,12,13)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nucleic Acid Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of adenosine kinase from undialyzed W1-L2 lysate (0-50 uM) |
J Med Chem 31: 786-90 (1988)
Checked by Author
BindingDB Entry DOI: 10.7270/Q20R9PZF |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM172717
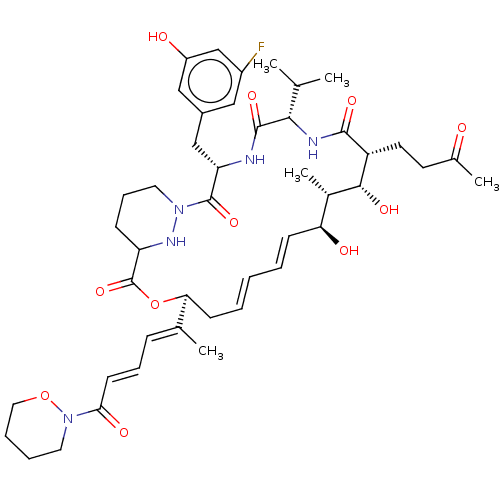
(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
The PPlase activity of recombinant CypA or D, produced by thrombin cleavage of GST-CypA or D, was determined by following the rate of hydrolysis of N... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM172715
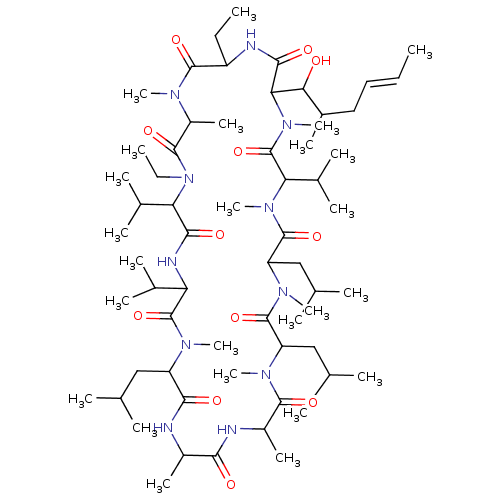
(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
The PPlase activity of recombinant CypA or D, produced by thrombin cleavage of GST-CypA or D, was determined by following the rate of hydrolysis of N... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM172716
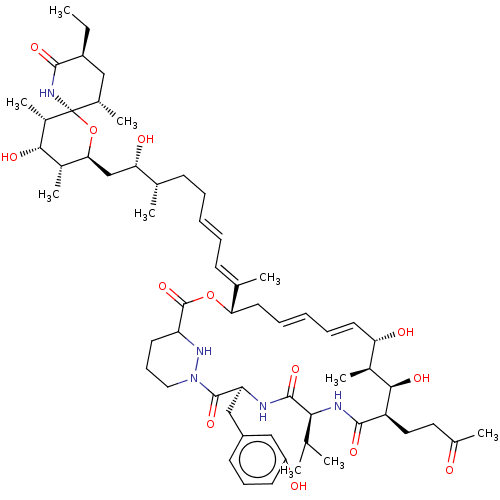
(US9090657, Sanglifehrin A, 5)Show SMILES CC[C@H]1C[C@H](C)[C@@]2(NC1=O)O[C@@H](C[C@H](O)[C@@H](C)CC\C=C\C=C(/C)[C@@H]1C\C=C\C=C\[C@H](O)[C@H](C)[C@@H](O)[C@@H](CCC(C)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc3cccc(O)c3)C(=O)N3CCCC(N3)C(=O)O1)[C@H](C)[C@H](O)[C@@H]2C |r,t:27,29| Show InChI InChI=1S/C60H91N5O13/c1-11-43-30-37(6)60(63-55(43)72)41(10)53(70)40(9)51(78-60)33-49(69)35(4)20-14-12-15-21-36(5)50-26-17-13-16-25-48(68)39(8)54(71)45(28-27-38(7)66)56(73)62-52(34(2)3)57(74)61-47(32-42-22-18-23-44(67)31-42)58(75)65-29-19-24-46(64-65)59(76)77-50/h12-13,15-18,21-23,25,31,34-35,37,39-41,43,45-54,64,67-71H,11,14,19-20,24,26-30,32-33H2,1-10H3,(H,61,74)(H,62,73)(H,63,72)/b15-12+,17-13+,25-16+,36-21+/t35-,37-,39-,40-,41-,43-,45+,46?,47-,48-,49-,50-,51-,52-,53-,54+,60+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
The PPlase activity of recombinant CypA or D, produced by thrombin cleavage of GST-CypA or D, was determined by following the rate of hydrolysis of N... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase A
(Homo sapiens (Human)) | BDBM172718
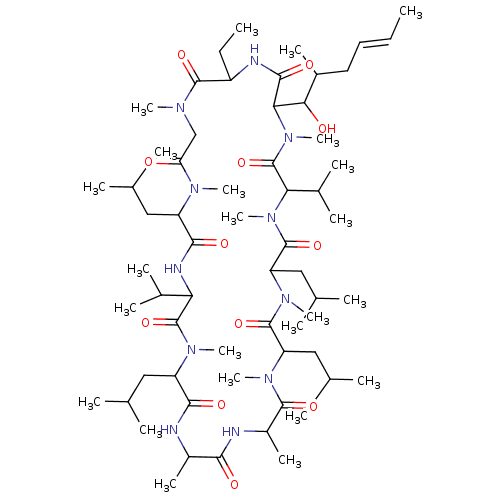
(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
The PPlase activity of recombinant CypA or D, produced by thrombin cleavage of GST-CypA or D, was determined by following the rate of hydrolysis of N... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM172718

(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.3 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the OAT1B1 and OAT1B3 uptake transporters, an in vitro uptake transporter assay from Solvo Biotechnology Inc. was used. U... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM172715

(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM172715

(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.3 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the OAT1B1 and OAT1B3 uptake transporters, an in vitro uptake transporter assay from Solvo Biotechnology Inc. was used. U... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM172715

(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | 7.3 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the OAT1B1 and OAT1B3 uptake transporters, an in vitro uptake transporter assay from Solvo Biotechnology Inc. was used. U... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM172718

(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM172715

(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.4 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the P-glycoprotein (Pgp/MDR1) transporter, an in vitro ATPase assay from Cyprotex was used. MDR1MDCK cells obtained from ... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM172718

(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | 7.4 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the P-glycoprotein (Pgp/MDR1) transporter, an in vitro ATPase assay from Cyprotex was used. MDR1MDCK cells obtained from ... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM172718

(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | 7.3 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the OAT1B1 and OAT1B3 uptake transporters, an in vitro uptake transporter assay from Solvo Biotechnology Inc. was used. U... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B3
(Homo sapiens (Human)) | BDBM172717

(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 7.3 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the OAT1B1 and OAT1B3 uptake transporters, an in vitro uptake transporter assay from Solvo Biotechnology Inc. was used. U... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM172718

(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM172718

(US11802108, Compound CSA | US9090657, Cyclosporine...)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(CC(C)C)N(C)C(=O)CN(C)C1=O)C(C)C Show InChI InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 1B1
(Homo sapiens (Human)) | BDBM172717

(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 7.3 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the OAT1B1 and OAT1B3 uptake transporters, an in vitro uptake transporter assay from Solvo Biotechnology Inc. was used. U... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Bile salt export pump
(Homo sapiens (Human)) | BDBM172717

(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM172715

(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM172717

(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM172715

(US9090657, DEBIO-025, 2)Show SMILES CCC1NC(=O)C(C(O)C(C)C\C=C\C)N(C)C(=O)C(C(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(CC(C)C)N(C)C(=O)C(C)NC(=O)C(C)NC(=O)C(CC(C)C)N(C)C(=O)C(NC(=O)C(C(C)C)N(CC)C(=O)C(C)N(C)C1=O)C(C)C Show InChI InChI=1S/C63H113N11O12/c1-26-29-30-40(16)52(75)51-56(79)66-44(27-2)59(82)68(20)43(19)58(81)74(28-3)49(38(12)13)55(78)67-48(37(10)11)62(85)69(21)45(31-34(4)5)54(77)64-41(17)53(76)65-42(18)57(80)70(22)46(32-35(6)7)60(83)71(23)47(33-36(8)9)61(84)72(24)50(39(14)15)63(86)73(51)25/h26,29,34-52,75H,27-28,30-33H2,1-25H3,(H,64,77)(H,65,76)(H,66,79)(H,67,78)/b29-26+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-binding cassette sub-family C member 2
(Homo sapiens (Human)) | BDBM172717

(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the MRP2, MRP3 and BSEP efflux transporters, an in vitro vesicular transporter assay from Solvo Biotechnology Inc. was us... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
ATP-dependent translocase ABCB1
(Homo sapiens (Human)) | BDBM172717

(US9090657, 24)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCC(C)=O)[C@H](O)[C@@H](C)[C@@H](O)\C=C\C=C\C[C@H](OC(=O)C2CCCN(N2)C(=O)[C@H](Cc2cc(O)cc(F)c2)NC1=O)C(\C)=C\C=C\C(=O)N1CCCCO1 |r,t:19,21| Show InChI InChI=1S/C45H62FN5O11/c1-27(2)40-43(58)47-36(25-31-23-32(46)26-33(53)24-31)44(59)50-20-12-14-35(49-50)45(60)62-38(28(3)13-11-17-39(55)51-21-9-10-22-61-51)16-8-6-7-15-37(54)30(5)41(56)34(42(57)48-40)19-18-29(4)52/h6-8,11,13,15,17,23-24,26-27,30,34-38,40-41,49,53-54,56H,9-10,12,14,16,18-22,25H2,1-5H3,(H,47,58)(H,48,57)/b8-6+,15-7+,17-11+,28-13+/t30-,34+,35?,36-,37-,38-,40-,41+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
NEUROVIVE PHARMACEUTICAL AB
US Patent
| Assay Description
To assess the inhibition of the P-glycoprotein (Pgp/MDR1) transporter, an in vitro ATPase assay from Cyprotex was used. MDR1MDCK cells obtained from ... |
US Patent US9090657 (2015)
BindingDB Entry DOI: 10.7270/Q22Z148H |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50167696
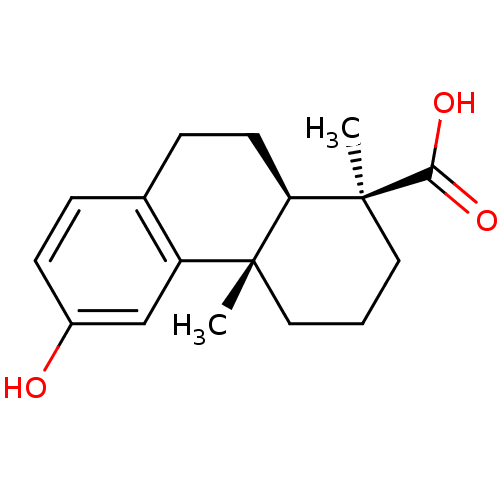
((1S,4aS,10aR)-1,2,3,4,4a,9,10,10a-octahydro-6-hydr...)Show SMILES C[C@@]1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(O)=O |r| Show InChI InChI=1S/C17H22O3/c1-16-8-3-9-17(2,15(19)20)14(16)7-5-11-4-6-12(18)10-13(11)16/h4,6,10,14,18H,3,5,7-9H2,1-2H3,(H,19,20)/t14-,16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50172185

((1S,4aS,10aR)-6-Hydroxy-1,4a-dimethyl-1,2,3,4,4a,9...)Show SMILES C[C@@]1(CCC[C@@]2(C)[C@H]1CCc1ccc(O)cc21)C(=O)NCC12CC3CC(CC(C3)C1)C2 |r,THB:22:23:26:30.21.29,22:21:24.23.28:26,29:21:24:28.27.26,29:27:24:30.22.21| Show InChI InChI=1S/C28H39NO2/c1-26-8-3-9-27(2,24(26)7-5-21-4-6-22(30)13-23(21)26)25(31)29-17-28-14-18-10-19(15-28)12-20(11-18)16-28/h4,6,13,18-20,24,30H,3,5,7-12,14-17H2,1-2H3,(H,29,31)/t18?,19?,20?,24-,26-,27+,28?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50241904
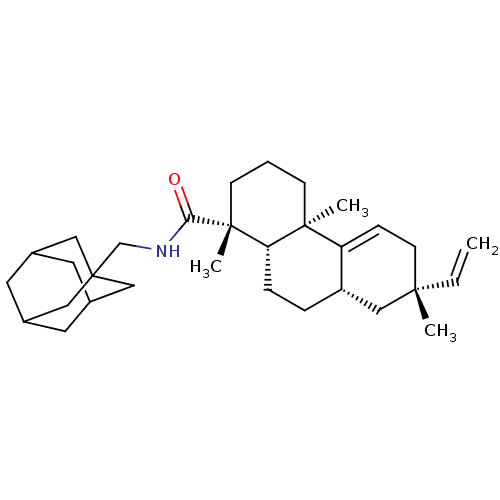
((1R,4aR,7S,8aS,10aS)-1,4a,7-Trimethyl-7-vinyl-1,2,...)Show SMILES C[C@@]1(CC=C2[C@@H](CC[C@@H]3[C@@](C)(CCC[C@@]23C)C(=O)NCC23CC4CC(CC(C4)C2)C3)C1)C=C |r,c:3,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20| Show InChI InChI=1S/C31H47NO/c1-5-28(2)12-9-25-24(19-28)7-8-26-29(25,3)10-6-11-30(26,4)27(33)32-20-31-16-21-13-22(17-31)15-23(14-21)18-31/h5,9,21-24,26H,1,6-8,10-20H2,2-4H3,(H,32,33)/t21?,22?,23?,24-,26-,28-,29-,30+,31?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM20177
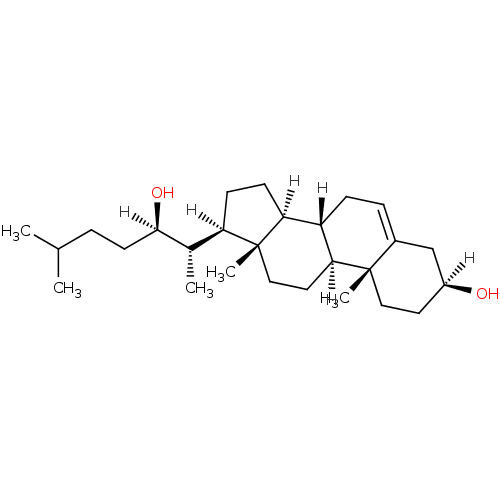
((1S,2R,5S,10S,11S,14R,15S)-14-[(2S,3R)-3-hydroxy-6...)Show SMILES [H][C@@](O)(CCC(C)C)[C@@H](C)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CC=C4C[C@@]([H])(O)CC[C@]4(C)[C@@]3([H])CC[C@]12C |r,t:19| Show InChI InChI=1S/C27H46O2/c1-17(2)6-11-25(29)18(3)22-9-10-23-21-8-7-19-16-20(28)12-14-26(19,4)24(21)13-15-27(22,23)5/h7,17-18,20-25,28-29H,6,8-16H2,1-5H3/t18-,20-,21-,22+,23-,24-,25+,26-,27+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.50E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50241903
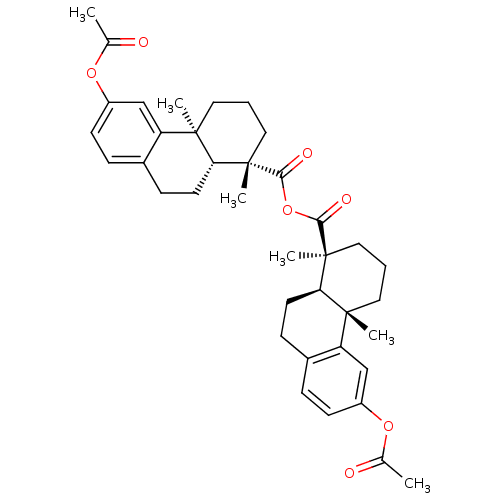
(CHEMBL506838 | acetyl Podocarpic acid anhydride)Show SMILES CC(=O)Oc1ccc2CC[C@H]3[C@](C)(CCC[C@]3(C)c2c1)C(=O)OC(=O)[C@@]1(C)CCC[C@@]2(C)[C@H]1CCc1ccc(OC(C)=O)cc21 |r| Show InChI InChI=1S/C38H46O7/c1-23(39)43-27-13-9-25-11-15-31-35(3,29(25)21-27)17-7-19-37(31,5)33(41)45-34(42)38(6)20-8-18-36(4)30-22-28(44-24(2)40)14-10-26(30)12-16-32(36)38/h9-10,13-14,21-22,31-32H,7-8,11-12,15-20H2,1-6H3/t31-,32-,35-,36-,37+,38+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50126018
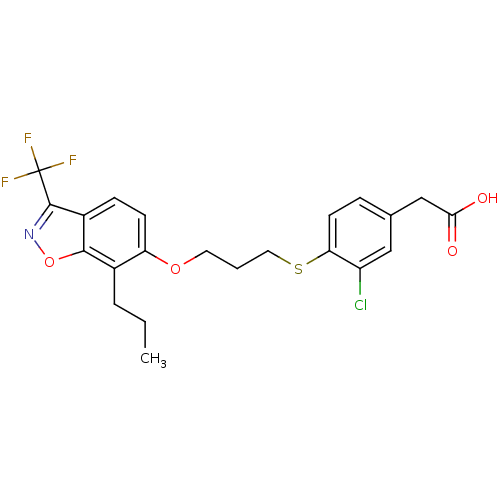
(2-(3-chloro-4-(3-(7-propyl-3-(trifluoromethyl)benz...)Show SMILES CCCc1c(OCCCSc2ccc(CC(O)=O)cc2Cl)ccc2c(noc12)C(F)(F)F Show InChI InChI=1S/C22H21ClF3NO4S/c1-2-4-14-17(7-6-15-20(14)31-27-21(15)22(24,25)26)30-9-3-10-32-18-8-5-13(11-16(18)23)12-19(28)29/h5-8,11H,2-4,9-10,12H2,1H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50241902
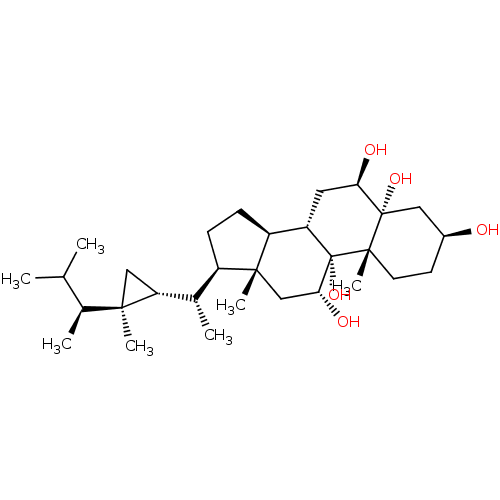
(CHEMBL486198 | Gorgost-5-ene-3beta,9alpha,11alpha-...)Show SMILES CC(C)[C@H](C)[C@@]1(C)C[C@@H]1[C@@H](C)[C@H]1CC[C@H]2[C@@H]3C[C@@H](O)[C@@]4(O)C[C@@H](O)CC[C@]4(C)[C@@]3(O)[C@H](O)C[C@]12C |r| Show InChI InChI=1S/C30H52O5/c1-16(2)18(4)26(5)14-23(26)17(3)20-8-9-21-22-12-24(32)29(34)13-19(31)10-11-28(29,7)30(22,35)25(33)15-27(20,21)6/h16-25,31-35H,8-15H2,1-7H3/t17-,18-,19-,20+,21-,22-,23+,24+,25+,26+,27+,28-,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50241894
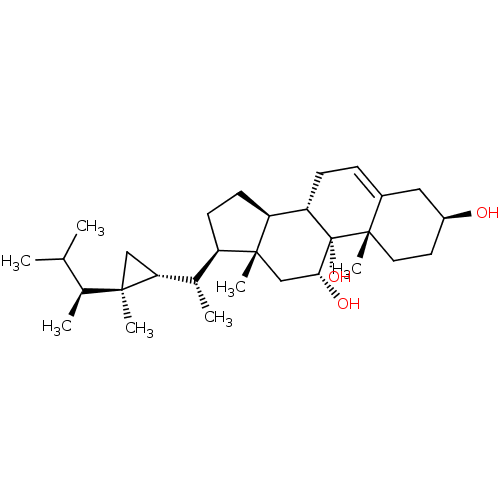
(CHEMBL486197 | gorgostane-3-beta,9-alpha,5-alpha,6...)Show SMILES CC(C)[C@H](C)[C@@]1(C)C[C@@H]1[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3(O)[C@H](O)C[C@]12C |r,t:18| Show InChI InChI=1S/C30H50O3/c1-17(2)19(4)27(5)15-25(27)18(3)22-10-11-23-24-9-8-20-14-21(31)12-13-29(20,7)30(24,33)26(32)16-28(22,23)6/h8,17-19,21-26,31-33H,9-16H2,1-7H3/t18-,19-,21-,22+,23-,24-,25+,26+,27+,28+,29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50241893
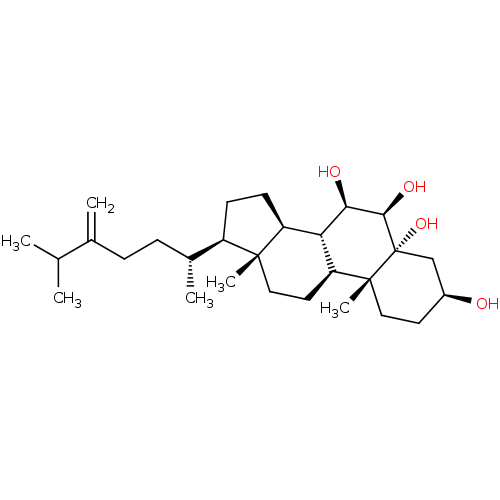
(CHEMBL520370 | ergost-24(28)-ene-3-beta,5-alpha,6-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3[C@@H](O)[C@@H](O)[C@@]4(O)C[C@@H](O)CC[C@]4(C)[C@H]3CC[C@]12C |r| Show InChI InChI=1S/C28H48O4/c1-16(2)17(3)7-8-18(4)20-9-10-21-23-22(12-13-26(20,21)5)27(6)14-11-19(29)15-28(27,32)25(31)24(23)30/h16,18-25,29-32H,3,7-15H2,1-2,4-6H3/t18-,19+,20-,21+,22+,23+,24-,25-,26-,27-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at human recombinant LXRalpha expressed in Escherichia coli BL21 cells assessed as association of recombinant SRC1 to LXRalpha ligan... |
J Nat Prod 68: 1247-52 (2005)
Article DOI: 10.1021/np050182g
BindingDB Entry DOI: 10.7270/Q2GH9HQ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































