 Found 10374 hits with Last Name = 'mu' and Initial = 'y'
Found 10374 hits with Last Name = 'mu' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423789
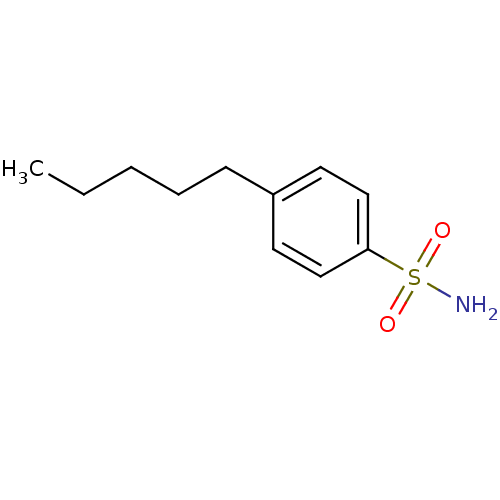
(4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...)Show InChI InChI=1S/C11H17NO2S/c1-2-3-4-5-10-6-8-11(9-7-10)15(12,13)14/h6-9H,2-5H2,1H3,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423789

(4-Pentyl-Benzenesulfonamide | 4-Pentylbenzenesulfo...)Show InChI InChI=1S/C11H17NO2S/c1-2-3-4-5-10-6-8-11(9-7-10)15(12,13)14/h6-9H,2-5H2,1H3,(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.000800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM719
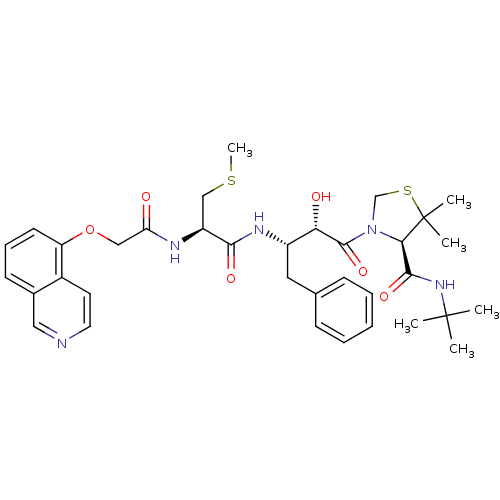
((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...)Show SMILES CSC[C@H](NC(=O)COc1cccc2cnccc12)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C35H45N5O6S2/c1-34(2,3)39-32(44)30-35(4,5)48-21-40(30)33(45)29(42)25(17-22-11-8-7-9-12-22)38-31(43)26(20-47-6)37-28(41)19-46-27-14-10-13-23-18-36-16-15-24(23)27/h7-16,18,25-26,29-30,42H,17,19-21H2,1-6H3,(H,37,41)(H,38,43)(H,39,44)/t25-,26-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00230 | -69.1 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute
| Assay Description
Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... |
Antimicrob Agents Chemother 37: 810-7 (1993)
Article DOI: 10.1128/aac.37.4.810
BindingDB Entry DOI: 10.7270/Q2KH0KHT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423788
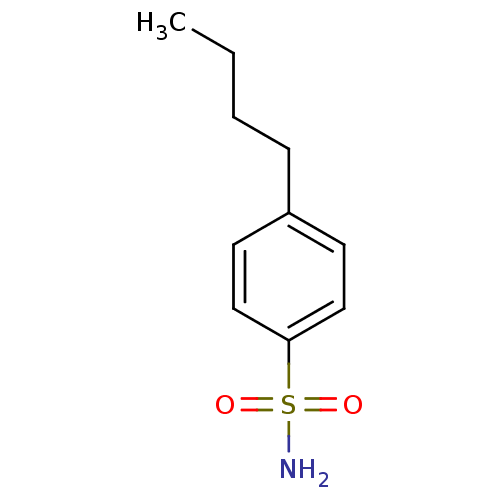
(4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...)Show InChI InChI=1S/C10H15NO2S/c1-2-3-4-9-5-7-10(8-6-9)14(11,12)13/h5-8H,2-4H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423788

(4-Butyl-Benzenesulfonamide | 4-Butylbenzenesulfona...)Show InChI InChI=1S/C10H15NO2S/c1-2-3-4-9-5-7-10(8-6-9)14(11,12)13/h5-8H,2-4H2,1H3,(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM579

((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...)Show SMILES CSC[C@H](NC(=O)COc1cccc2cnccc12)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C33H41N5O6S2/c1-33(2,3)37-31(42)26-19-46-20-38(26)32(43)29(40)24(15-21-9-6-5-7-10-21)36-30(41)25(18-45-4)35-28(39)17-44-27-12-8-11-22-16-34-14-13-23(22)27/h5-14,16,24-26,29,40H,15,17-20H2,1-4H3,(H,35,39)(H,36,41)(H,37,42)/t24-,25-,26-,29-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.00550 | -66.9 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute
| Assay Description
Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... |
Antimicrob Agents Chemother 37: 810-7 (1993)
Article DOI: 10.1128/aac.37.4.810
BindingDB Entry DOI: 10.7270/Q2KH0KHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM793
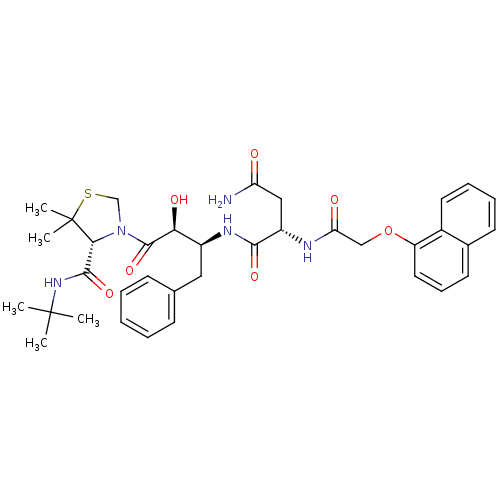
((2S)-N-[(2S,3S)-4-[(4R)-4-(tert-butylcarbamoyl)-5,...)Show SMILES CC(C)(C)NC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)COc1cccc2ccccc12 |r| Show InChI InChI=1S/C36H45N5O7S/c1-35(2,3)40-33(46)31-36(4,5)49-21-41(31)34(47)30(44)25(18-22-12-7-6-8-13-22)39-32(45)26(19-28(37)42)38-29(43)20-48-27-17-11-15-23-14-9-10-16-24(23)27/h6-17,25-26,30-31,44H,18-21H2,1-5H3,(H2,37,42)(H,38,43)(H,39,45)(H,40,46)/t25-,26-,30-,31+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.00680 | -66.3 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
National Cancer Institute
| Assay Description
Inhibition constants were determined by a fluorometric assay with the fluorogenic substrate Arg-Glu(EDANS)-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Lys(DABCYL)-Ar... |
Antimicrob Agents Chemother 37: 810-7 (1993)
Article DOI: 10.1128/aac.37.4.810
BindingDB Entry DOI: 10.7270/Q2KH0KHT |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423787
(4-Propyl-Benzenesulfonamide | 4-Propylbenzenesulfo...)Show InChI InChI=1S/C9H13NO2S/c1-2-3-8-4-6-9(7-5-8)13(10,11)12/h4-7H,2-3H2,1H3,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50423787

(4-Propyl-Benzenesulfonamide | 4-Propylbenzenesulfo...)Show InChI InChI=1S/C9H13NO2S/c1-2-3-8-4-6-9(7-5-8)13(10,11)12/h4-7H,2-3H2,1H3,(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50415863
(CHEMBL358263)Show InChI InChI=1S/C8H11NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h3-6H,2H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50415863

(CHEMBL358263)Show InChI InChI=1S/C8H11NO2S/c1-2-7-3-5-8(6-4-7)12(9,10)11/h3-6H,2H2,1H3,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255850
(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3R)-3...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C26H40N4/c1-26(15-7-3-2-4-8-16-26)29-18-13-22(14-19-29)30-24-12-6-5-11-23(24)28-25(30)21-10-9-17-27-20-21/h5-6,11-12,21-22,27H,2-4,7-10,13-20H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0858 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400876
(CHEMBL2204347)Show SMILES CNC(=O)[C@H](CN1CCC2(CC1)OCCc1cc(F)sc21)Cc1ccccc1Cl |r| Show InChI InChI=1S/C22H26ClFN2O2S/c1-25-21(27)17(12-15-4-2-3-5-18(15)23)14-26-9-7-22(8-10-26)20-16(6-11-28-22)13-19(24)29-20/h2-5,13,17H,6-12,14H2,1H3,(H,25,27)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255899

(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3S)-3...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)[C@H]1CCCNC1 |r| Show InChI InChI=1S/C26H40N4/c1-26(15-7-3-2-4-8-16-26)29-18-13-22(14-19-29)30-24-12-6-5-11-23(24)28-25(30)21-10-9-17-27-20-21/h5-6,11-12,21-22,27H,2-4,7-10,13-20H2,1H3/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400875
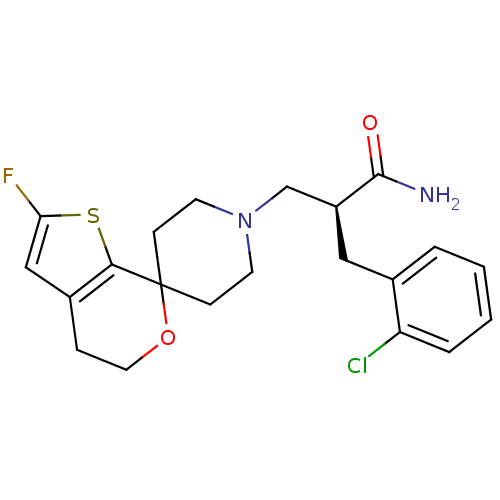
(CHEMBL2204353)Show SMILES NC(=O)[C@H](CN1CCC2(CC1)OCCc1cc(F)sc21)Cc1ccccc1Cl |r| Show InChI InChI=1S/C21H24ClFN2O2S/c22-17-4-2-1-3-14(17)11-16(20(24)26)13-25-8-6-21(7-9-25)19-15(5-10-27-21)12-18(23)28-19/h1-4,12,16H,5-11,13H2,(H2,24,26)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400874

(CHEMBL2086410)Show SMILES NC(=O)[C@H](CN1CCC2(CC1)OCCc1cc(F)sc21)Cc1ccccc1F |r| Show InChI InChI=1S/C21H24F2N2O2S/c22-17-4-2-1-3-14(17)11-16(20(24)26)13-25-8-6-21(7-9-25)19-15(5-10-27-21)12-18(23)28-19/h1-4,12,16H,5-11,13H2,(H2,24,26)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400873
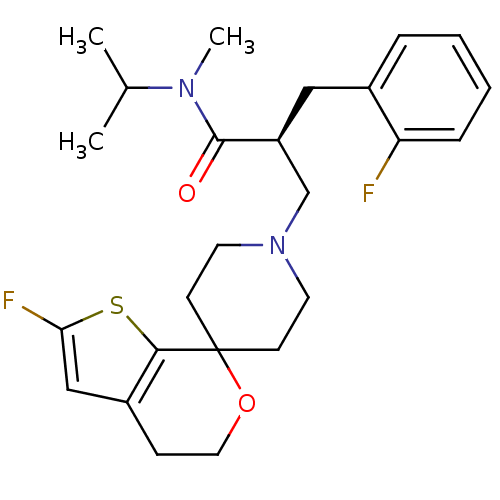
(CHEMBL2088029)Show SMILES CC(C)N(C)C(=O)[C@H](CN1CCC2(CC1)OCCc1cc(F)sc21)Cc1ccccc1F |r| Show InChI InChI=1S/C25H32F2N2O2S/c1-17(2)28(3)24(30)20(14-18-6-4-5-7-21(18)26)16-29-11-9-25(10-12-29)23-19(8-13-31-25)15-22(27)32-23/h4-7,15,17,20H,8-14,16H2,1-3H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255901
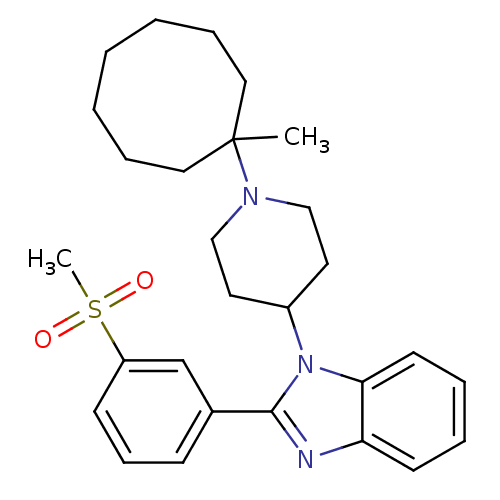
(1-[1-(1-Methylcyclooctyl)piperidin-4-yl]-2-[3-(met...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1cccc(c1)S(C)(=O)=O Show InChI InChI=1S/C28H37N3O2S/c1-28(17-8-4-3-5-9-18-28)30-19-15-23(16-20-30)31-26-14-7-6-13-25(26)29-27(31)22-11-10-12-24(21-22)34(2,32)33/h6-7,10-14,21,23H,3-5,8-9,15-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289410
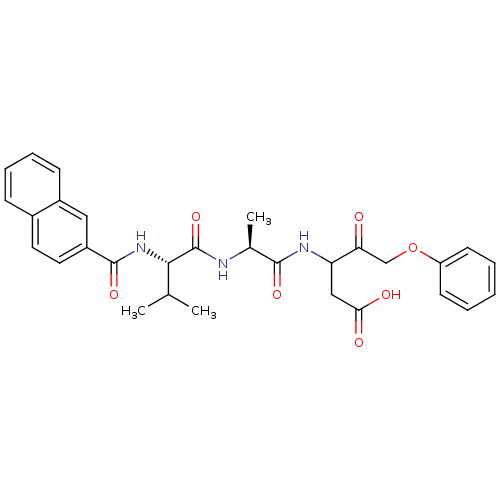
(CHEMBL26544 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C30H33N3O7/c1-18(2)27(33-29(38)22-14-13-20-9-7-8-10-21(20)15-22)30(39)31-19(3)28(37)32-24(16-26(35)36)25(34)17-40-23-11-5-4-6-12-23/h4-15,18-19,24,27H,16-17H2,1-3H3,(H,31,39)(H,32,37)(H,33,38)(H,35,36)/t19-,24?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289397
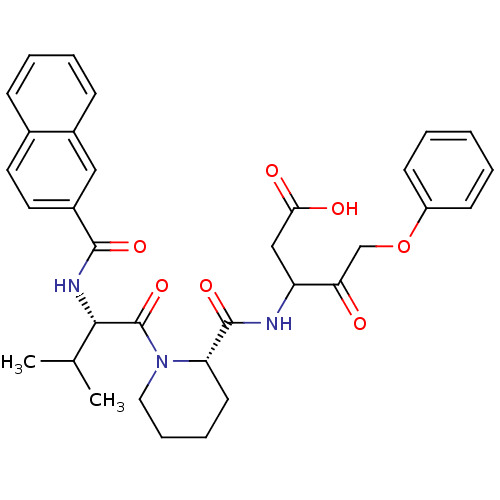
(3-[(1-{3-Methyl-2-[(naphthalene-2-carbonyl)-amino]...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N1CCCC[C@H]1C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C33H37N3O7/c1-21(2)30(35-31(40)24-16-15-22-10-6-7-11-23(22)18-24)33(42)36-17-9-8-14-27(36)32(41)34-26(19-29(38)39)28(37)20-43-25-12-4-3-5-13-25/h3-7,10-13,15-16,18,21,26-27,30H,8-9,14,17,19-20H2,1-2H3,(H,34,41)(H,35,40)(H,38,39)/t26?,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400872
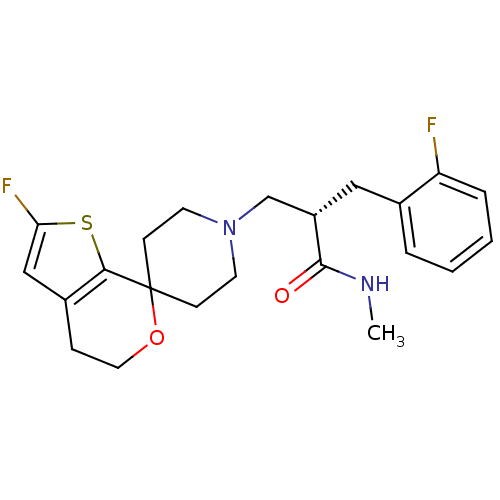
(CHEMBL2088033)Show SMILES CNC(=O)[C@H](CN1CCC2(CC1)OCCc1cc(F)sc21)Cc1ccccc1F |r| Show InChI InChI=1S/C22H26F2N2O2S/c1-25-21(27)17(12-15-4-2-3-5-18(15)23)14-26-9-7-22(8-10-26)20-16(6-11-28-22)13-19(24)29-20/h2-5,13,17H,6-12,14H2,1H3,(H,25,27)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255135
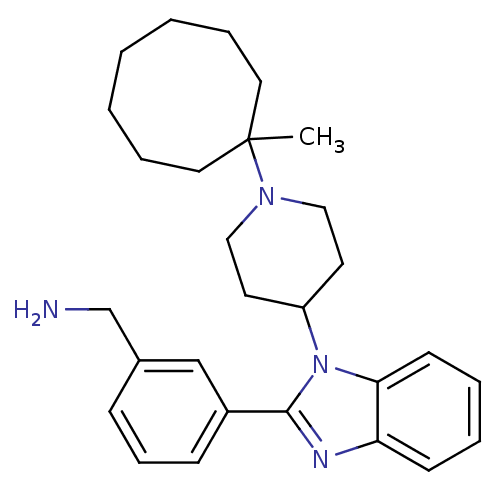
(1-(3-{1-[1-(1-Methylcyclooctyl)piperidin-4-yl]-1H-...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1cccc(CN)c1 Show InChI InChI=1S/C28H38N4/c1-28(16-7-3-2-4-8-17-28)31-18-14-24(15-19-31)32-26-13-6-5-12-25(26)30-27(32)23-11-9-10-22(20-23)21-29/h5-6,9-13,20,24H,2-4,7-8,14-19,21,29H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50375451

(CHEMBL408579)Show InChI InChI=1S/C13H15N3O/c1-8(14)7-16-13-10(6-15-16)3-4-12-11(13)5-9(2)17-12/h3-6,8H,7,14H2,1-2H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50262566
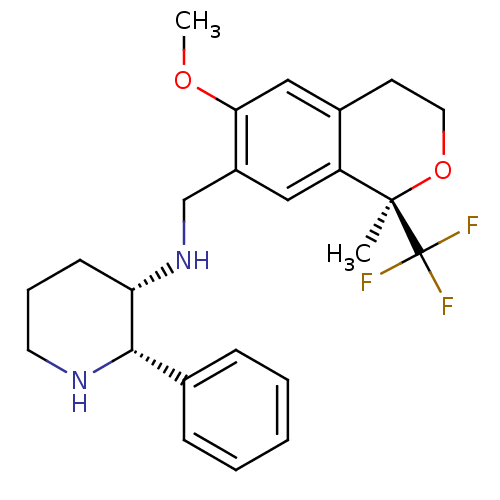
((2S,3S)-3-[(1R)-6-Methoxy-1-methyl-1-trifluorometh...)Show SMILES COc1cc2CCO[C@](C)(c2cc1CN[C@H]1CCCN[C@H]1c1ccccc1)C(F)(F)F |r| Show InChI InChI=1S/C24H29F3N2O2/c1-23(24(25,26)27)19-13-18(21(30-2)14-17(19)10-12-31-23)15-29-20-9-6-11-28-22(20)16-7-4-3-5-8-16/h3-5,7-8,13-14,20,22,28-29H,6,9-12,15H2,1-2H3/t20-,22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from NK1 receptor in human IM9 cells |
Bioorg Med Chem 16: 7193-205 (2008)
Article DOI: 10.1016/j.bmc.2008.06.047
BindingDB Entry DOI: 10.7270/Q2RV0NHN |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50289398

(CHEMBL553107 | Peptidic phenyl ketoether analogue)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2c1)C(=O)N1CCC[C@H]1C(=O)NC(CC(O)=O)C(=O)COc1ccccc1 Show InChI InChI=1S/C32H35N3O7/c1-20(2)29(34-30(39)23-15-14-21-9-6-7-10-22(21)17-23)32(41)35-16-8-13-26(35)31(40)33-25(18-28(37)38)27(36)19-42-24-11-4-3-5-12-24/h3-7,9-12,14-15,17,20,25-26,29H,8,13,16,18-19H2,1-2H3,(H,33,40)(H,34,39)(H,37,38)/t25?,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible inhibition of recombinant human IL-1 beta converting enzyme. |
Bioorg Med Chem Lett 7: 1337-1342 (1997)
Article DOI: 10.1016/S0960-894X(97)00220-5
BindingDB Entry DOI: 10.7270/Q2DN452C |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50375457
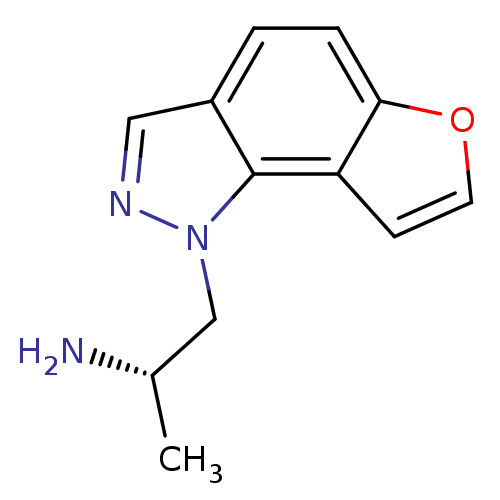
(CHEMBL261476)Show InChI InChI=1S/C12H13N3O/c1-8(13)7-15-12-9(6-14-15)2-3-11-10(12)4-5-16-11/h2-6,8H,7,13H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255898

(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3R)-1...)Show SMILES CN1CCC[C@H](C1)c1nc2ccccc2n1C1CCN(CC1)C1(C)CCCCCCC1 |r| Show InChI InChI=1S/C27H42N4/c1-27(16-8-4-3-5-9-17-27)30-19-14-23(15-20-30)31-25-13-7-6-12-24(25)28-26(31)22-11-10-18-29(2)21-22/h6-7,12-13,22-23H,3-5,8-11,14-21H2,1-2H3/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226128
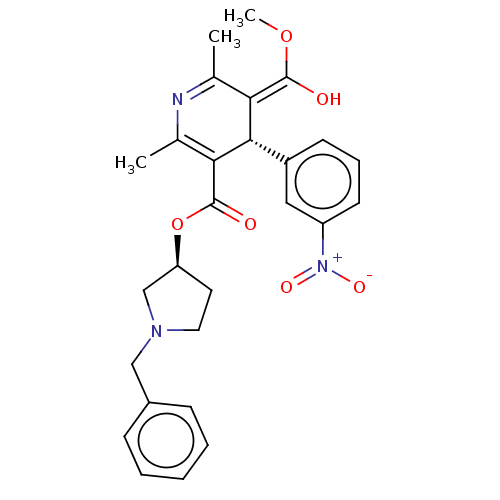
(CHEMBL2093893)Show SMILES Cl.[H][C@@]1(CCN(Cc2ccccc2)C1)OC(=O)C1=C(C)N=C(C)\C(=C(\O)OC)[C@]1([H])c1cccc(c1)[N+]([O-])=O |r,c:18,t:21| Show InChI InChI=1S/C27H29N3O6.ClH/c1-17-23(26(31)35-3)25(20-10-7-11-21(14-20)30(33)34)24(18(2)28-17)27(32)36-22-12-13-29(16-22)15-19-8-5-4-6-9-19;/h4-11,14,22,25,31H,12-13,15-16H2,1-3H3;1H/b26-23+;/t22-,25-;/m0./s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50160668
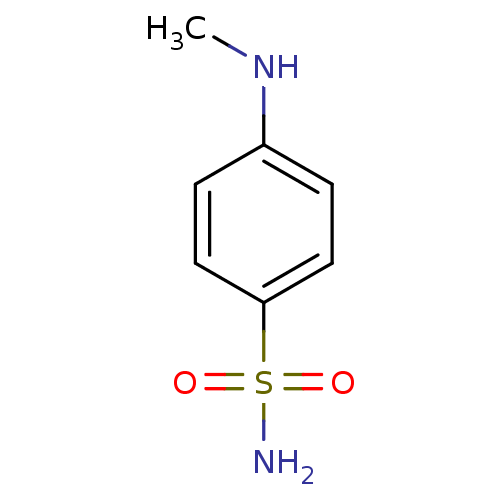
(4-Methylamino-benzenesulfonamide | CHEMBL174847)Show InChI InChI=1S/C7H10N2O2S/c1-9-6-2-4-7(5-3-6)12(8,10)11/h2-5,9H,1H3,(H2,8,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50160668

(4-Methylamino-benzenesulfonamide | CHEMBL174847)Show InChI InChI=1S/C7H10N2O2S/c1-9-6-2-4-7(5-3-6)12(8,10)11/h2-5,9H,1H3,(H2,8,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Graduate School
Curated by ChEMBL
| Assay Description
Binding affinity to human carbonic anhydrase 2 |
Bioorg Med Chem Lett 21: 141-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.050
BindingDB Entry DOI: 10.7270/Q2K938T6 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255953
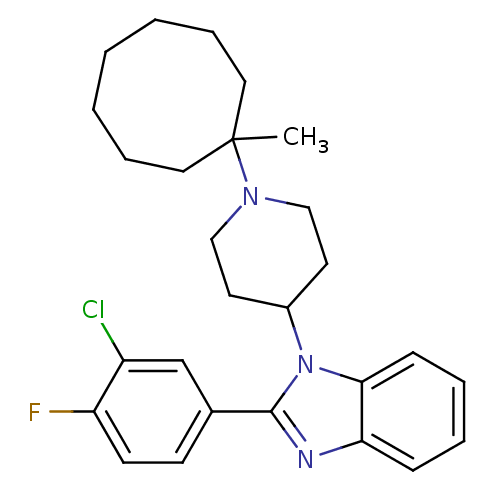
(2-(3-Chloro-4-fluorophenyl)-1-[1-(1-methylcyclooct...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C27H33ClFN3/c1-27(15-7-3-2-4-8-16-27)31-17-13-21(14-18-31)32-25-10-6-5-9-24(25)30-26(32)20-11-12-23(29)22(28)19-20/h5-6,9-12,19,21H,2-4,7-8,13-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(RAT) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400871
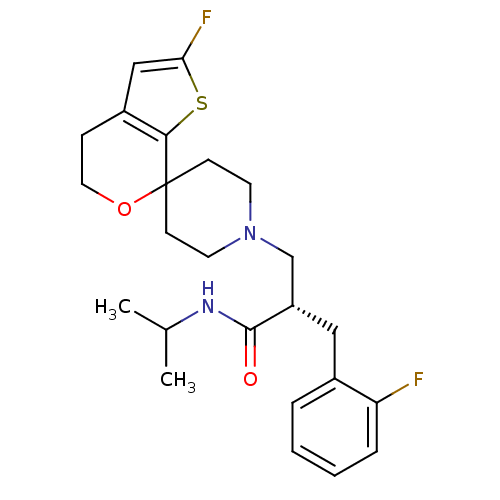
(CHEMBL2088030)Show SMILES CC(C)NC(=O)[C@H](CN1CCC2(CC1)OCCc1cc(F)sc21)Cc1ccccc1F |r| Show InChI InChI=1S/C24H30F2N2O2S/c1-16(2)27-23(29)19(13-17-5-3-4-6-20(17)25)15-28-10-8-24(9-11-28)22-18(7-12-30-24)14-21(26)31-22/h3-6,14,16,19H,7-13,15H2,1-2H3,(H,27,29)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.231 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255900
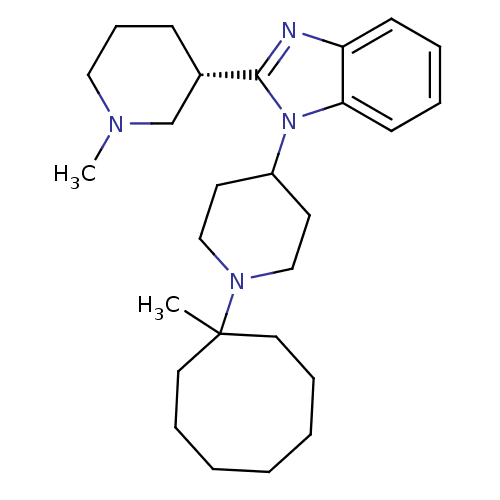
(1-[1-(1-Methylcyclooctyl)-4-piperidinyl]-2-[(3S)-1...)Show SMILES CN1CCC[C@@H](C1)c1nc2ccccc2n1C1CCN(CC1)C1(C)CCCCCCC1 |r| Show InChI InChI=1S/C27H42N4/c1-27(16-8-4-3-5-9-17-27)30-19-14-23(15-20-30)31-25-13-7-6-12-24(25)28-26(31)22-11-10-18-29(2)21-22/h6-7,12-13,22-23H,3-5,8-11,14-21H2,1-2H3/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095
(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50400870

(CHEMBL559569 | SB-612111)Show SMILES Cc1cccc2C(O)CC(CN3CCC(CC3)c3c(Cl)cccc3Cl)CCc12 Show InChI InChI=1S/C24H29Cl2NO/c1-16-4-2-5-20-19(16)9-8-17(14-23(20)28)15-27-12-10-18(11-13-27)24-21(25)6-3-7-22(24)26/h2-7,17-18,23,28H,8-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.253 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]nociceptin from human recombinant NOP receptor expressed in CHO cells after 60 mins by microbeta scintillation counting |
J Med Chem 54: 2687-700 (2011)
Article DOI: 10.1021/jm101487v
BindingDB Entry DOI: 10.7270/Q20V8DXB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50255134
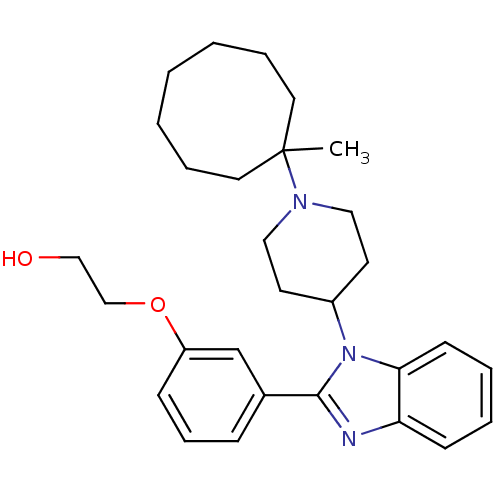
(2-(3-{1-[1-(1-Methylcyclooctyl)piperidin-4-yl]-1H-...)Show SMILES CC1(CCCCCCC1)N1CCC(CC1)n1c(nc2ccccc12)-c1cccc(OCCO)c1 Show InChI InChI=1S/C29H39N3O2/c1-29(16-7-3-2-4-8-17-29)31-18-14-24(15-19-31)32-27-13-6-5-12-26(27)30-28(32)23-10-9-11-25(22-23)34-21-20-33/h5-6,9-13,22,24,33H,2-4,7-8,14-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85096
(CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP)Show SMILES [#6]-[#8]-c1ccc(-[#6]-[#6]-2-[#7]-[#6](=O)-[#6]C3([#6]-[#6]-[#6]-[#6]-[#6]3)[#16]-[#16]-[#6]-[#6](-[#7]-[#6](=O)-[#6](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6](-[#6]-c3ccccc3)-[#7]-[#6]-2=O)-[#6](=O)-[#7]-2-[#6]-[#6]-[#6]-[#6]-2-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O)cc1 Show InChI InChI=1S/C52H74N14O12S2/c1-78-32-16-14-31(15-17-32)25-35-46(73)63-36(24-30-10-4-2-5-11-30)47(74)61-34(18-19-40(53)67)45(72)64-37(26-41(54)68)48(75)65-38(29-79-80-52(27-43(70)60-35)20-6-3-7-21-52)50(77)66-23-9-13-39(66)49(76)62-33(12-8-22-58-51(56)57)44(71)59-28-42(55)69/h2,4-5,10-11,14-17,33-39H,3,6-9,12-13,18-29H2,1H3,(H2,53,67)(H2,54,68)(H2,55,69)(H,59,71)(H,60,70)(H,61,74)(H,62,76)(H,63,73)(H,64,72)(H,65,75)(H4,56,57,58) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102398
(US8536192, I-39)Show SMILES CN(C)c1ccc(NC(=O)C2C[C@@]3(O)[C@H]4Cc5ccc(O)c6O[C@@H](C2=O)[C@]3(CCN4CC2CC2)c56)cc1 |r,TLB:11:12:16.34.15:29.28.27,THB:13:12:16.34.15:29.28.27| Show InChI InChI=1S/C29H33N3O5/c1-31(2)19-8-6-18(7-9-19)30-27(35)20-14-29(36)22-13-17-5-10-21(33)25-23(17)28(29,26(37-25)24(20)34)11-12-32(22)15-16-3-4-16/h5-10,16,20,22,26,33,36H,3-4,11-15H2,1-2H3,(H,30,35)/t20?,22-,26+,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50097750
(Butyric acid (1aR,1bS,4aS,7aR,7bR,8R,9R,9aS)-9-but...)Show SMILES CCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@H]3CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(=O)CCC |c:22,t:12| Show InChI InChI=1S/C28H40O7/c1-7-9-21(30)34-25-16(4)27(33)19-11-15(3)23(32)18(19)12-17(14-29)13-20(27)24-26(5,6)28(24,25)35-22(31)10-8-2/h11,13,16,18-20,24-25,29,33H,7-10,12,14H2,1-6H3/t16-,18+,19-,20+,24?,25-,27+,28-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by ChEMBL
| Assay Description
Displacement of 3[H]PDBu from Protein kinase C eta C1b domain |
Bioorg Med Chem Lett 11: 719-22 (2001)
BindingDB Entry DOI: 10.7270/Q2VM4BHZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50068972
((S)-3-(2-{3-[(Naphthalene-2-carbonyl)-amino]-2-oxo...)Show SMILES CCC(C(=O)N[C@@H](CC(O)=O)C(=O)CNCCc1ccccc1)n1cccc(NC(=O)c2ccc3ccccc3c2)c1=O Show InChI InChI=1S/C33H34N4O6/c1-2-28(32(42)36-27(20-30(39)40)29(38)21-34-17-16-22-9-4-3-5-10-22)37-18-8-13-26(33(37)43)35-31(41)25-15-14-23-11-6-7-12-24(23)19-25/h3-15,18-19,27-28,34H,2,16-17,20-21H2,1H3,(H,35,41)(H,36,42)(H,39,40)/t27-,28?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ferring Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IL-1 beta converting enzyme |
Bioorg Med Chem Lett 8: 959-64 (1999)
BindingDB Entry DOI: 10.7270/Q2ZP458C |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50004178
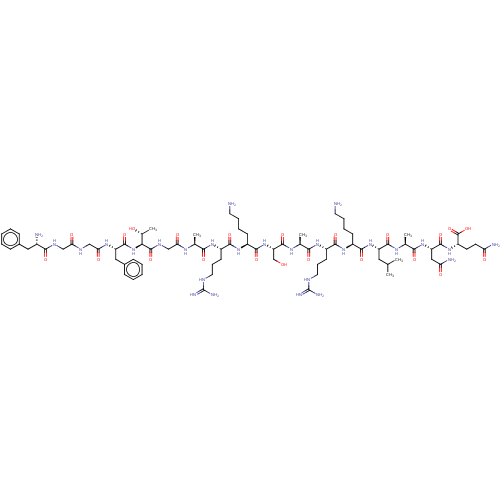
(Nociceptin | Nociceptin/orphanin FQ | ORPHANIN FQ)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccccc1)[C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O |r| Show InChI InChI=1S/C79H129N27O22/c1-41(2)33-54(72(122)95-44(5)66(116)103-56(36-59(84)110)73(123)102-53(77(127)128)27-28-58(83)109)104-70(120)49(23-13-15-29-80)100-69(119)52(26-18-32-90-79(87)88)99-65(115)43(4)96-75(125)57(40-107)105-71(121)50(24-14-16-30-81)101-68(118)51(25-17-31-89-78(85)86)98-64(114)42(3)94-61(112)39-93-76(126)63(45(6)108)106-74(124)55(35-47-21-11-8-12-22-47)97-62(113)38-91-60(111)37-92-67(117)48(82)34-46-19-9-7-10-20-46/h7-12,19-22,41-45,48-57,63,107-108H,13-18,23-40,80-82H2,1-6H3,(H2,83,109)(H2,84,110)(H,91,111)(H,92,117)(H,93,126)(H,94,112)(H,95,122)(H,96,125)(H,97,113)(H,98,114)(H,99,115)(H,100,119)(H,101,118)(H,102,123)(H,103,116)(H,104,120)(H,105,121)(H,106,124)(H,127,128)(H4,85,86,89)(H4,87,88,90)/t42-,43-,44-,45+,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]N/OFQ from human NOP receptor expressed in HEK293 cells |
J Med Chem 52: 610-25 (2009)
Article DOI: 10.1021/jm7012979
BindingDB Entry DOI: 10.7270/Q29K4C5K |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% |
J Med Chem 43: 4388-97 (2000)
BindingDB Entry DOI: 10.7270/Q2PG1R04 |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM35723

(CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...)Show SMILES Cc1ccccc1C(=O)Nc1ccc(C(=O)N2CCCC(O)c3cc(Cl)ccc23)c(C)c1 Show InChI InChI=1S/C26H25ClN2O3/c1-16-6-3-4-7-20(16)25(31)28-19-10-11-21(17(2)14-19)26(32)29-13-5-8-24(30)22-15-18(27)9-12-23(22)29/h3-4,6-7,9-12,14-15,24,30H,5,8,13H2,1-2H3,(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Second Tokushima Institute of New Drug Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 287: 860-7 (1998)
BindingDB Entry DOI: 10.7270/Q2S46QGC |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Rattus norvegicus (Rat)) | BDBM35667

(AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...)Show SMILES [#7]-[#6@H]-1-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6]-1=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-[#6](-[#7])=O Show InChI InChI=1S/C46H65N15O12S2/c47-27-22-74-75-23-33(45(73)61-17-5-9-34(61)44(72)56-28(8-4-16-53-46(51)52)39(67)54-21-37(50)65)60-43(71)32(20-36(49)64)59-40(68)29(14-15-35(48)63)55-41(69)31(18-24-6-2-1-3-7-24)58-42(70)30(57-38(27)66)19-25-10-12-26(62)13-11-25/h1-3,6-7,10-13,27-34,62H,4-5,8-9,14-23,47H2,(H2,48,63)(H2,49,64)(H2,50,65)(H,54,67)(H,55,69)(H,56,72)(H,57,66)(H,58,70)(H,59,68)(H,60,71)(H4,51,52,53)/t27-,28-,29-,30-,31-,32-,33-,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM102422
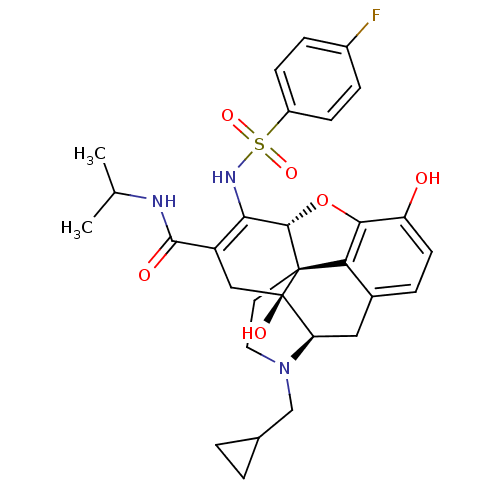
(US8536192, I-289)Show SMILES CC(C)NC(=O)C1=C(NS(=O)(=O)c2ccc(F)cc2)[C@@H]2Oc3c4c(C[C@H]5N(CC6CC6)CC[C@@]24[C@@]5(O)C1)ccc3O |r,c:6,TLB:35:34:22.23.24:32.26.31,THB:36:34:22.23.24:32.26.31| Show InChI InChI=1S/C30H34FN3O6S/c1-16(2)32-28(36)21-14-30(37)23-13-18-5-10-22(35)26-24(18)29(30,11-12-34(23)15-17-3-4-17)27(40-26)25(21)33-41(38,39)20-8-6-19(31)7-9-20/h5-10,16-17,23,27,33,35,37H,3-4,11-15H2,1-2H3,(H,32,36)/t23-,27+,29+,30-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.470 | -53.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Shionogi & Co. Ltd.
US Patent
| Assay Description
Binding assay of opioid receptor. |
US Patent US8536192 (2013)
BindingDB Entry DOI: 10.7270/Q24M935Z |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(RAT) | BDBM85095

(CAS_151171 | CONIVAPTAN | NSC_151171 | YM087)Show SMILES Cc1nc-2c(CCN(C(=O)c3ccc(NC(=O)c4ccccc4-c4ccccc4)cc3)c3ccccc-23)[nH]1 Show InChI InChI=1S/C32H26N4O2/c1-21-33-28-19-20-36(29-14-8-7-13-27(29)30(28)34-21)32(38)23-15-17-24(18-16-23)35-31(37)26-12-6-5-11-25(26)22-9-3-2-4-10-22/h2-18H,19-20H2,1H3,(H,33,34)(H,35,37) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 282: 301-8 (1997)
BindingDB Entry DOI: 10.7270/Q25T3J1N |
More data for this
Ligand-Target Pair | |
Voltage-dependent L-type calcium channel subunit alpha-1S
(Rattus norvegicus) | BDBM50226129
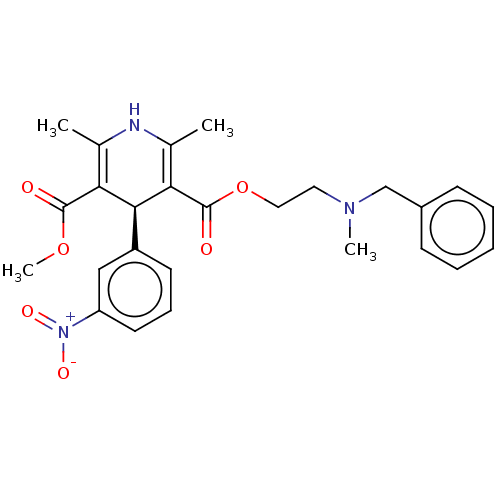
(CHEMBL1314450)Show SMILES COC(=O)C1=C(C)NC(C)=C([C@H]1c1cccc(c1)[N+]([O-])=O)C(=O)OCCN(C)Cc1ccccc1 |c:4,9| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,24,27H,13-14,16H2,1-4H3/t24-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate |
J Med Chem 29: 2504-11 (1986)
BindingDB Entry DOI: 10.7270/Q2QF8W3R |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50375452
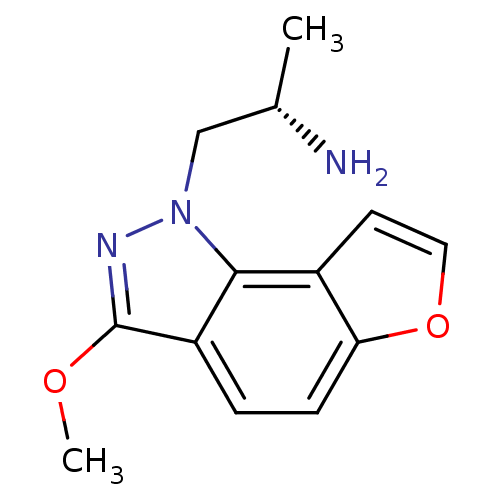
(CHEMBL262092)Show InChI InChI=1S/C13H15N3O2/c1-8(14)7-16-12-9-5-6-18-11(9)4-3-10(12)13(15-16)17-2/h3-6,8H,7,14H2,1-2H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]5-HT from human 5HT2C receptor expressed in CHO cells |
Bioorg Med Chem 16: 1966-82 (2008)
Article DOI: 10.1016/j.bmc.2007.10.100
BindingDB Entry DOI: 10.7270/Q2TH8NKR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data