

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxoacyl-[acyl-carrier-protein] synthase 1 [C171Q] (Mycobacterium tuberculosis) | BDBM214777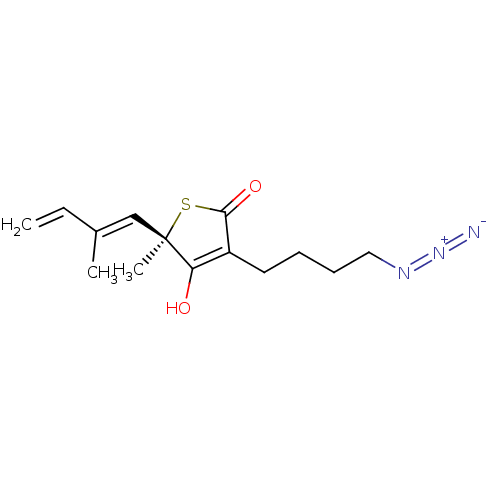 (TLM18) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 3.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
University of Wuerzburg | Assay Description Binding of TLM18 to wild-type and mutant KasA was quantified by monitoring changes in the intrinsic tryptophan fluorescence of the enzyme at waveleng... | J Biol Chem 288: 34190-204 (2013) Article DOI: 10.1074/jbc.M113.511436 BindingDB Entry DOI: 10.7270/Q22F7M8D | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3014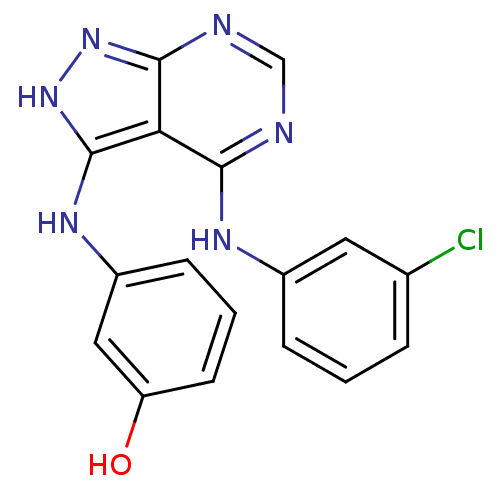 (3-((3-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3023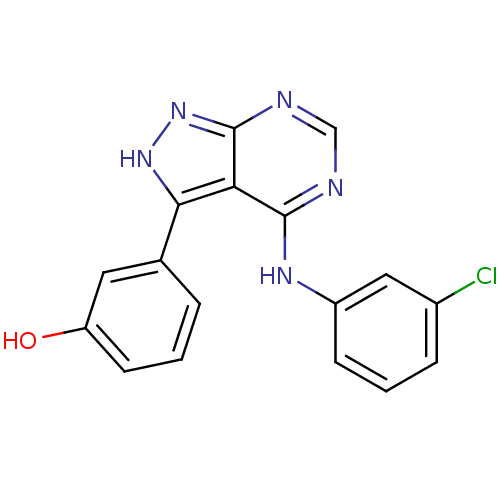 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3029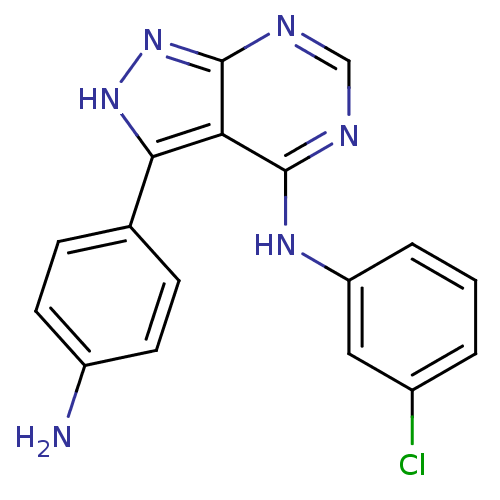 (3-(4-Aminophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3027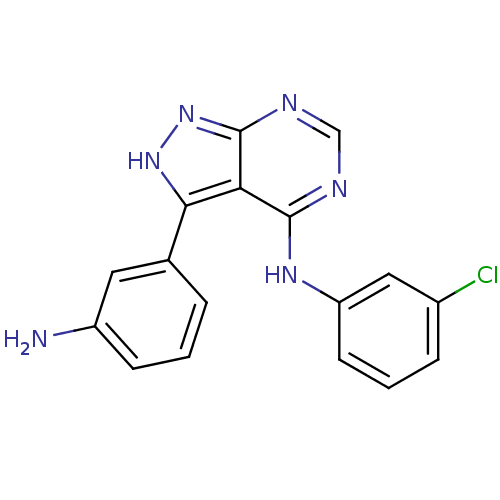 (3-(3-Aminophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3016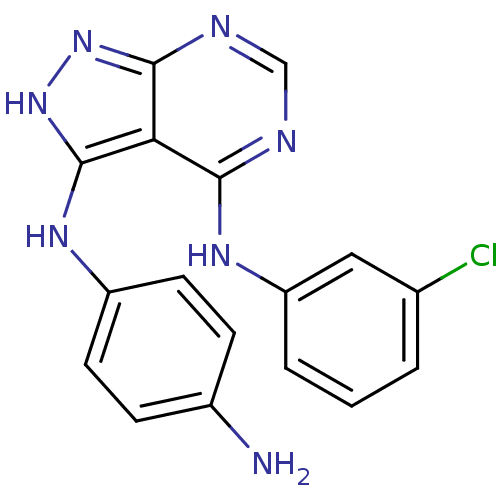 (3-((4-Aminophenyl)amino)-4-((3-chlorophenyl)amino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3025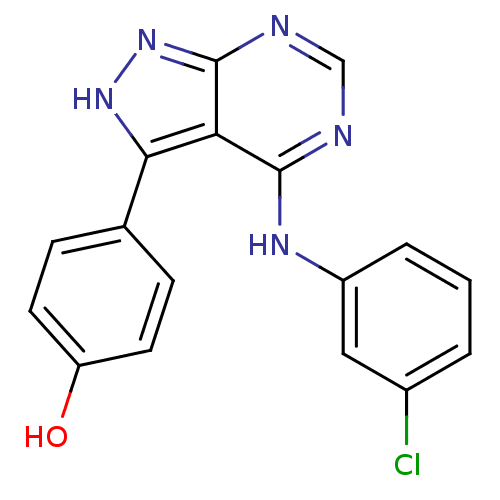 (3-(4-Hydroxyphenyl)-4-((3-chlorophenyl)amino)pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3021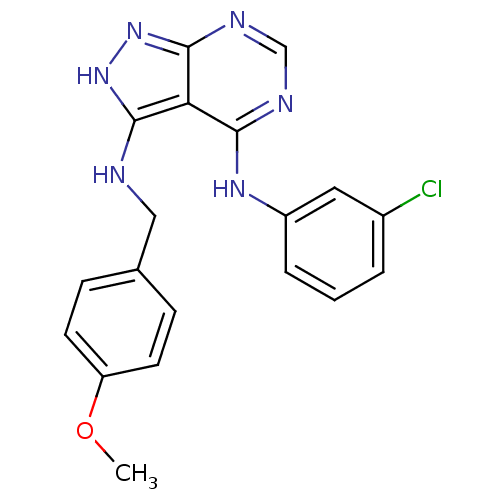 (3-((4-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3018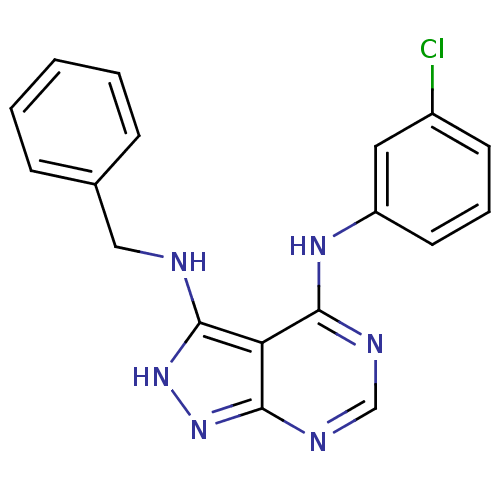 (3-(Benzylamino)-4-((3-chlorophenyl)amino)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3012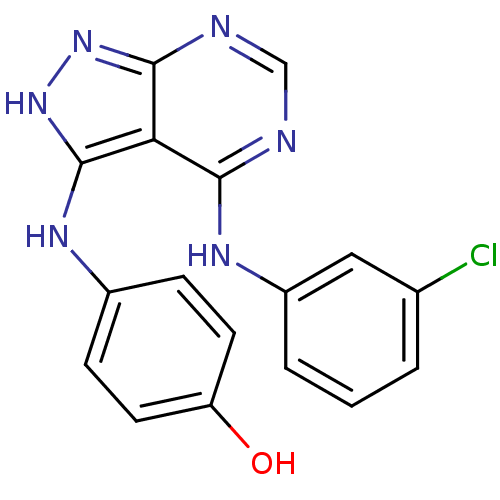 (3-((4-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3013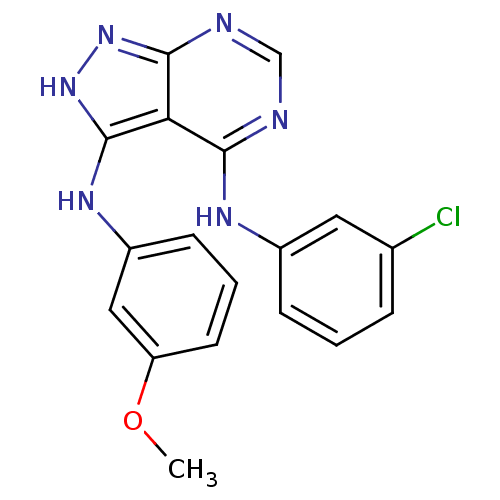 (3-((3-Methoxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3020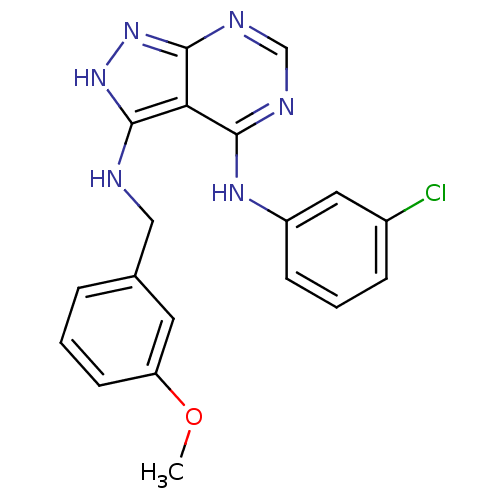 (3-((3-Methoxybenzyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3022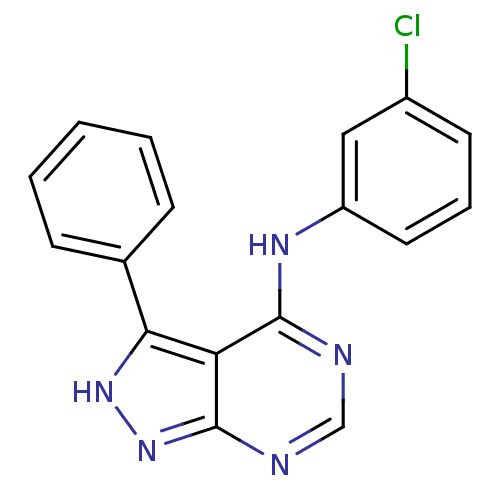 (4-(Phenylamino)pyrazolo[3,4-d]pyrimidine deriv. 23...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3023 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3019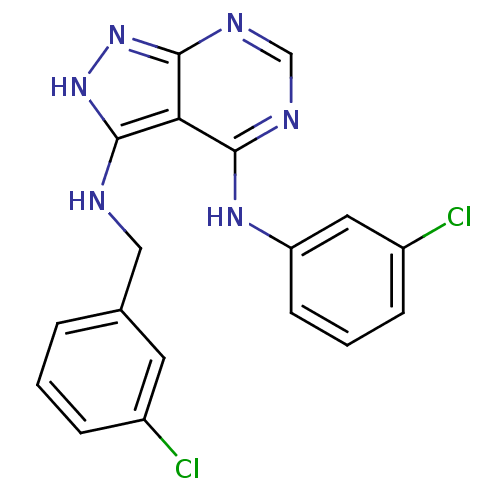 (3-((3-Chlorobenzyl)amino)-4-((3-chlorophenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3017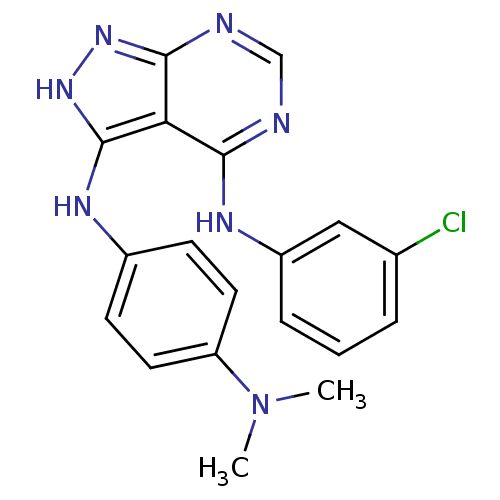 (3-((4-(Dimethylamino)phenyl)amino)-4-((3-chlorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3011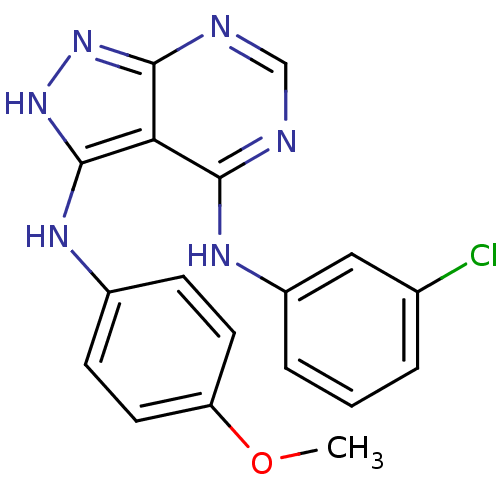 (3-((4-Methoxyphenyl)-amino)-4-((3-chlorophenyl)ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97610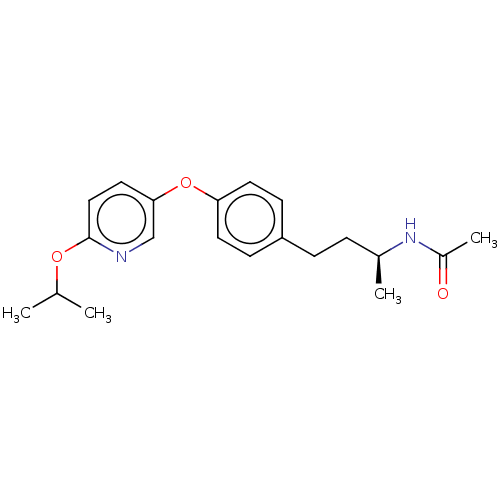 (CHEMBL1630715 | US8470841, 35) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3008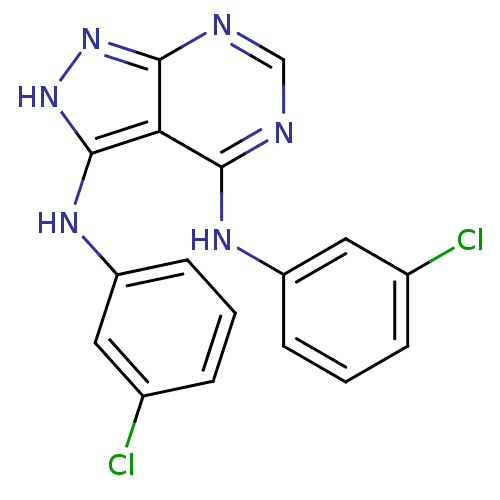 (3,4-Bis((3-chlorophenyl)amino)-1H-pyrazolo[3,4-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3026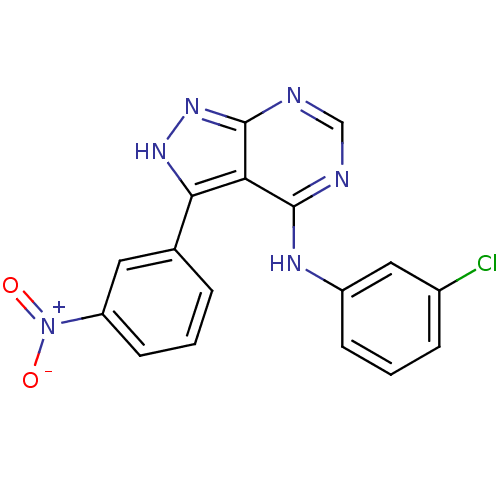 (3-(3-Nitrophenyl)-4-((3-chlorophenyl)amino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97601 (US8470841, 17) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97600 (US8470841, 12) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3006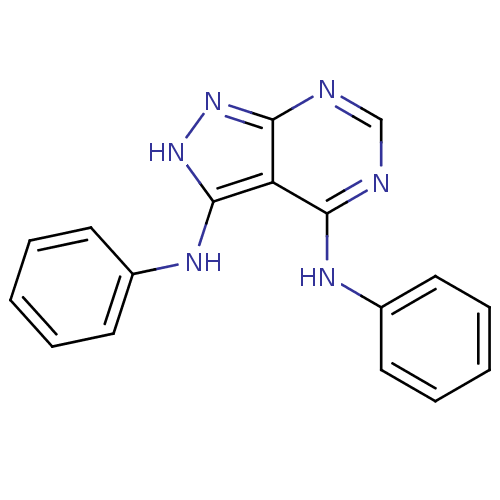 (3,4-Di-(phenylamino)-1H-pyrazolo[3,4-d]pyrimidine ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97609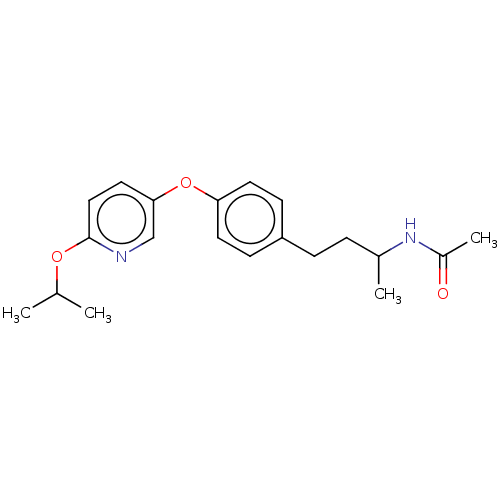 (US8470841, 33) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97611 (CHEMBL1630712 | US8470841, 36) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97623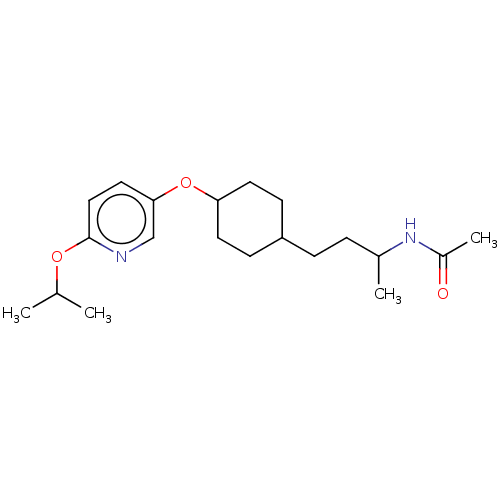 (US8470841, 52) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97622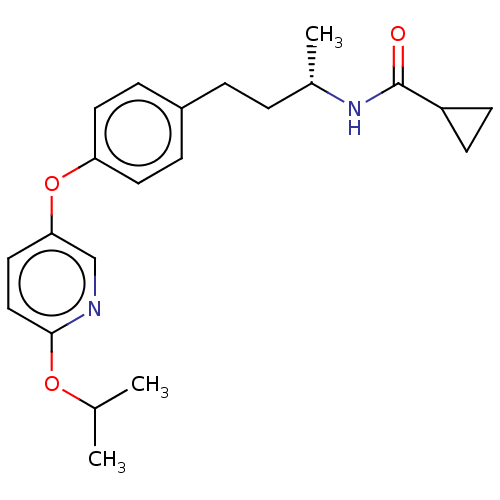 (CHEMBL1630701 | US8470841, 51) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3007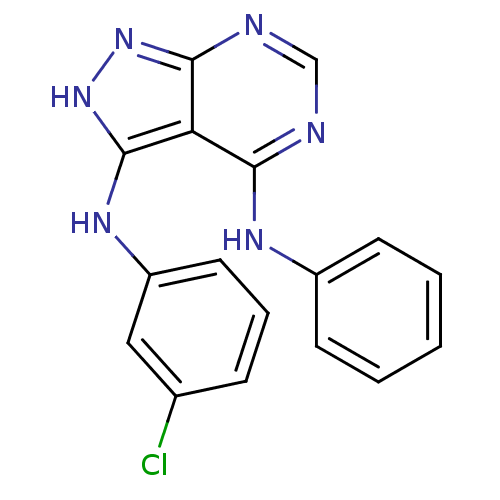 (3-((3-Chlorophenyl)amino)-4-(phenylamino)-1H-pyraz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97615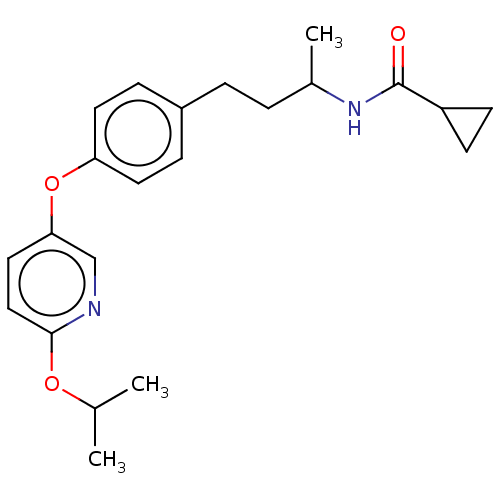 (US8470841, 44) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3024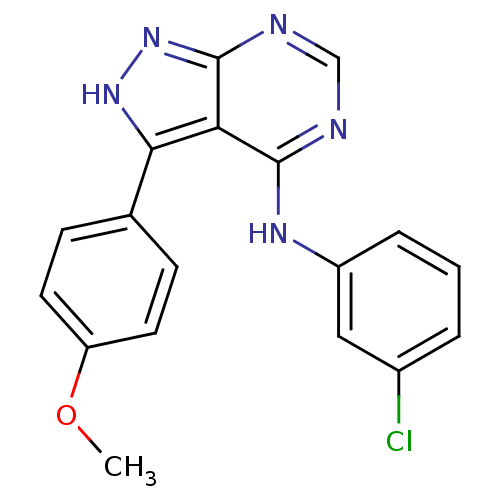 (3-(4-Methoxyphenyl)-4-((3-chlorophenyl)amino)-1H-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM64583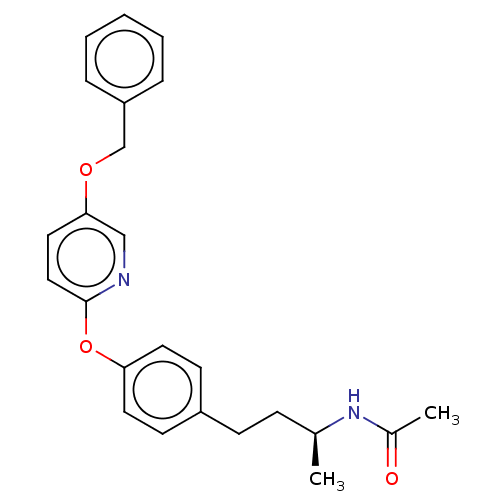 (BDBM50332551 | US8470841, 19) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97624 (US8470841, 59) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3009 (3-((3-Chlorophenyl)amino)-4-((3-bromophenyl)amino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3010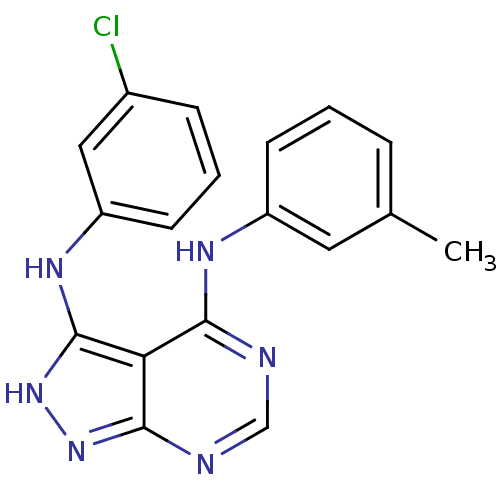 (3-((3-Chlorophenyl)amino)-4-((3-methylphenyl)amino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332552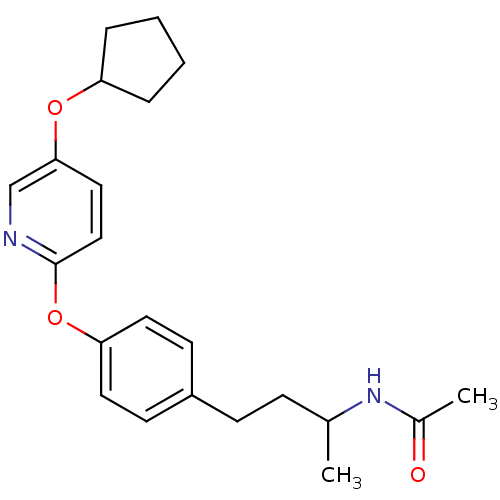 (CHEMBL1630708 | N-(4-(4-(5-(cyclopentyloxy)pyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97599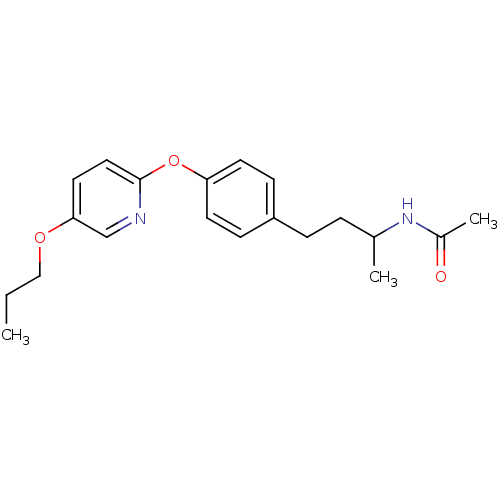 (US8470841, 11) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97597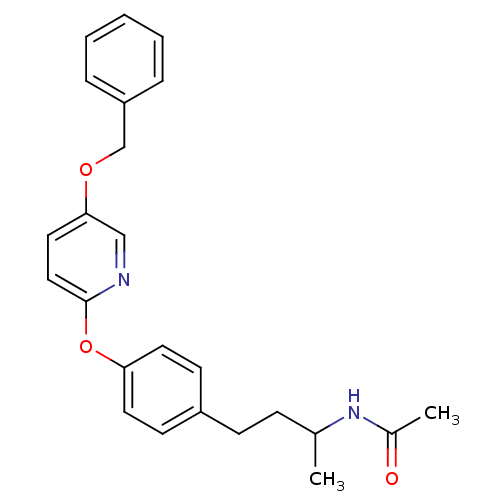 (US8470841, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97618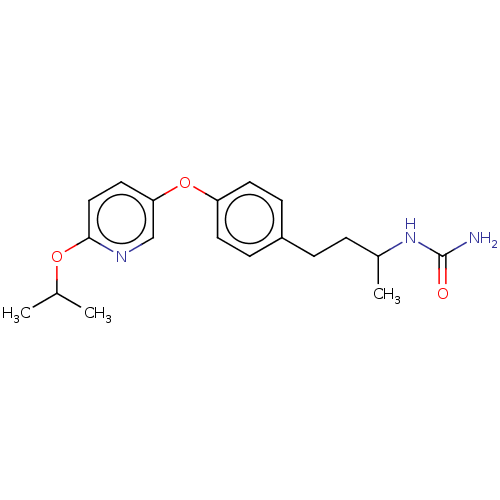 (CHEMBL1630702 | US8470841, 47) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3012 (3-((4-Hydroxyphenyl)amino)-4-((3-chlorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97598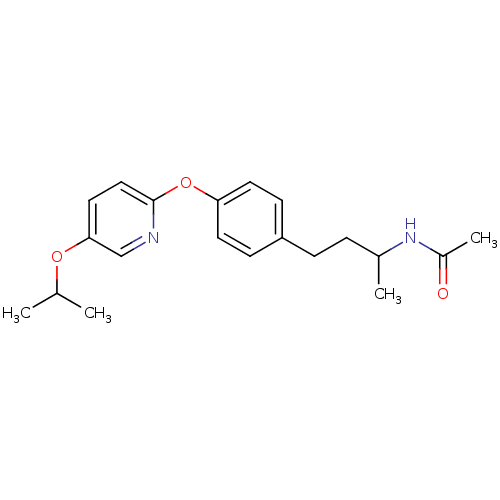 (US8470841, 10) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3005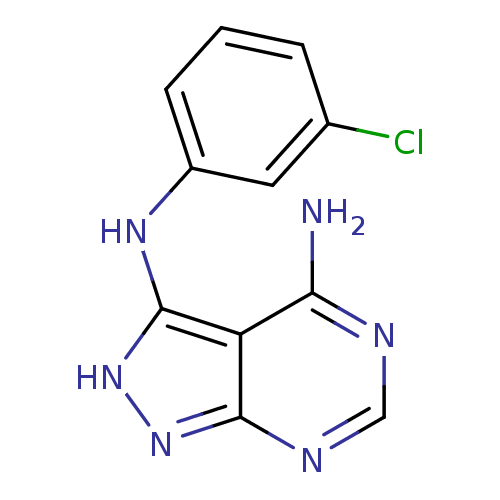 (3-((3-Chlorophenyl)amino)-4-amino-1H-pyrazolo[3,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | 20 |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM97621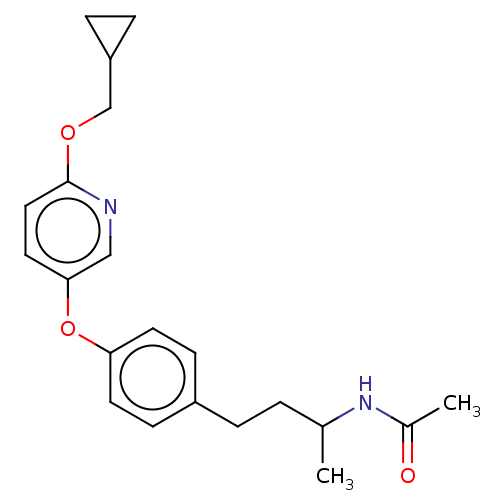 (US8470841, 50) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase transforming protein Abl (Abelson murine leukemia virus) | BDBM3023 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM97610 (CHEMBL1630715 | US8470841, 35) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332549 ((S)-N-(4-(4-(5-isopropoxypyridin-2-yloxy)phenyl)bu...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 [151-2458] (Homo sapiens (Human)) | BDBM50332555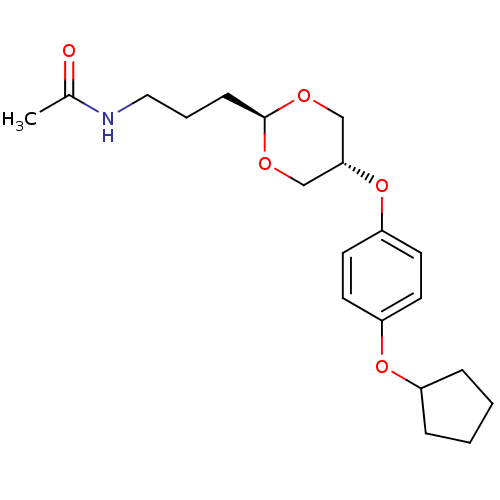 (CHEMBL1630705 | US8470841, 6 | trans-N-(3-(5-(4-(c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Sanofi US Patent | Assay Description the enzymatic activity of ACC and its inhibition by test substances were determined by a luminometric assay. | US Patent US8470841 (2013) BindingDB Entry DOI: 10.7270/Q2183537 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM3023 (3-{4-[(3-chlorophenyl)amino]-1H-pyrazolo[3,4-d]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3601-16 (1997) Article DOI: 10.1021/jm970124v BindingDB Entry DOI: 10.7270/Q2H70D0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 182 total ) | Next | Last >> |