 Found 458 hits with Last Name = 'nagumo' and Initial = 'y'
Found 458 hits with Last Name = 'nagumo' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50325534
(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596292

(CHEMBL5185211)Show SMILES [H][C@]12Oc3c4c(C[C@]5([H])C(CC[C@H]1N(C)C(=O)\C=C\c1ccoc1)[C@]24CCN5CC1CC1)ccc3O |r,THB:28:27:9:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50596293
(CHEMBL5188658)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14C5=CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)ccc3O |r,t:21,THB:10:9:17:4.5.6| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0356 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118178
(CHEMBL3613172)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)CCc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C32H40N2O2.ClH/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;/h2-6,10,12,17,23,25,28-29,35H,7-9,11,13-16,18-21H2,1H3;1H/t25-,28-,29+,31-,32-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118191

(CHEMBL3613173)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)Cc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C31H38N2O2.ClH/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;/h2-6,9,11,16,22,24,27-28,34H,7-8,10,12-15,17-20H2,1H3;1H/t24-,27-,28+,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50596292

(CHEMBL5185211)Show SMILES [H][C@]12Oc3c4c(C[C@]5([H])C(CC[C@H]1N(C)C(=O)\C=C\c1ccoc1)[C@]24CCN5CC1CC1)ccc3O |r,THB:28:27:9:4.5.6| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50274347
((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.25.8:18.20.19,THB:6:5:9.25.8:18.20.19| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.178 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Delta-type opioid receptor
(MOUSE) | BDBM50118177
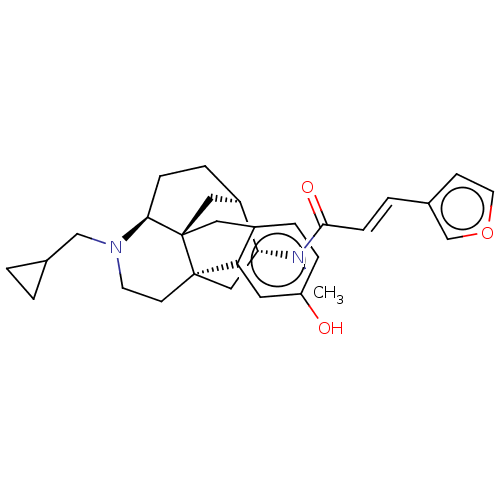
(CHEMBL3613171)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)\C=C\c1ccoc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C30H36N2O3.ClH/c1-31(28(34)9-4-21-10-13-35-19-21)26-17-29-11-12-32(18-20-2-3-20)27-8-6-23(26)16-30(27,29)15-22-5-7-24(33)14-25(22)29;/h4-5,7,9-10,13-14,19-20,23,26-27,33H,2-3,6,8,11-12,15-18H2,1H3;1H/b9-4+;/t23-,26-,27+,29-,30-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from DOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118191

(CHEMBL3613173)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)Cc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C31H38N2O2.ClH/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;/h2-6,9,11,16,22,24,27-28,34H,7-8,10,12-15,17-20H2,1H3;1H/t24-,27-,28+,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118177

(CHEMBL3613171)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)\C=C\c1ccoc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C30H36N2O3.ClH/c1-31(28(34)9-4-21-10-13-35-19-21)26-17-29-11-12-32(18-20-2-3-20)27-8-6-23(26)16-30(27,29)15-22-5-7-24(33)14-25(22)29;/h4-5,7,9-10,13-14,19-20,23,26-27,33H,2-3,6,8,11-12,15-18H2,1H3;1H/b9-4+;/t23-,26-,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118178

(CHEMBL3613172)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)CCc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C32H40N2O2.ClH/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;/h2-6,10,12,17,23,25,28-29,35H,7-9,11,13-16,18-21H2,1H3;1H/t25-,28-,29+,31-,32-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118184
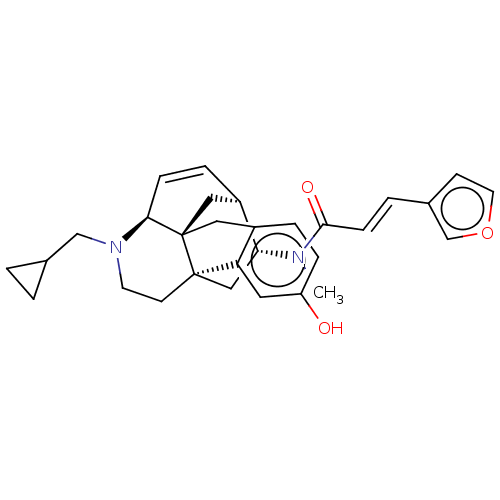
(CHEMBL3613163)Show SMILES Cl.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)\C=C\c1ccoc1 |r,c:26| Show InChI InChI=1S/C30H34N2O3.ClH/c1-31(28(34)9-4-21-10-13-35-19-21)26-17-29-11-12-32(18-20-2-3-20)27-8-6-23(26)16-30(27,29)15-22-5-7-24(33)14-25(22)29;/h4-10,13-14,19-20,23,26-27,33H,2-3,11-12,15-18H2,1H3;1H/b9-4+;/t23-,26-,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118177

(CHEMBL3613171)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)\C=C\c1ccoc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C30H36N2O3.ClH/c1-31(28(34)9-4-21-10-13-35-19-21)26-17-29-11-12-32(18-20-2-3-20)27-8-6-23(26)16-30(27,29)15-22-5-7-24(33)14-25(22)29;/h4-5,7,9-10,13-14,19-20,23,26-27,33H,2-3,6,8,11-12,15-18H2,1H3;1H/b9-4+;/t23-,26-,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.232 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50274347

((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.25.8:18.20.19,THB:6:5:9.25.8:18.20.19| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118182

(CHEMBL3613165)Show SMILES CC1(C)C2CCC1(CS(O)(=O)=O)C(=O)C2.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)Cc1ccccc1 |r,c:42| Show InChI InChI=1S/C31H36N2O2.C10H16O4S/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-6,9-12,16,22,24,27-28,34H,7-8,13-15,17-20H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14)/t24-,27-,28+,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118190
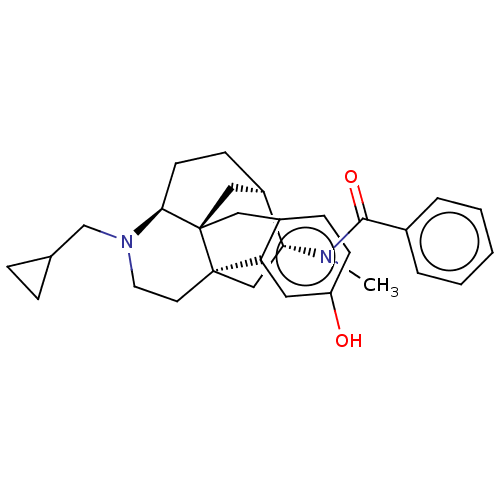
(CHEMBL3613174)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)c1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C30H36N2O2.ClH/c1-31(28(34)21-5-3-2-4-6-21)26-18-29-13-14-32(19-20-7-8-20)27-12-10-23(26)17-30(27,29)16-22-9-11-24(33)15-25(22)29;/h2-6,9,11,15,20,23,26-27,33H,7-8,10,12-14,16-19H2,1H3;1H/t23-,26-,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118182

(CHEMBL3613165)Show SMILES CC1(C)C2CCC1(CS(O)(=O)=O)C(=O)C2.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)Cc1ccccc1 |r,c:42| Show InChI InChI=1S/C31H36N2O2.C10H16O4S/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-6,9-12,16,22,24,27-28,34H,7-8,13-15,17-20H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14)/t24-,27-,28+,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50274347

((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 |r,TLB:4:5:9.25.8:18.20.19,THB:6:5:9.25.8:18.20.19| Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.431 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118183
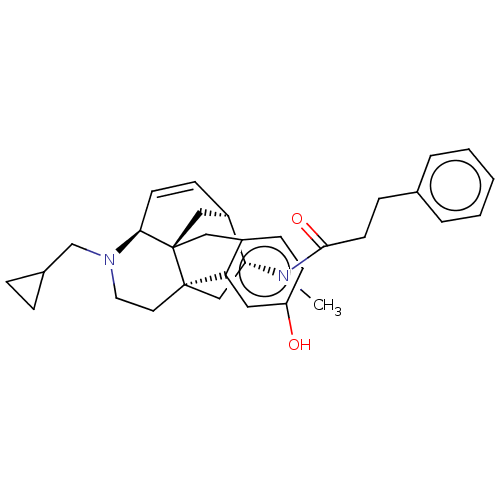
(CHEMBL3613164)Show SMILES Cl.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)CCc1ccccc1 |r,c:26| Show InChI InChI=1S/C32H38N2O2.ClH/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;/h2-6,10-13,17,23,25,28-29,35H,7-9,14-16,18-21H2,1H3;1H/t25-,28-,29+,31-,32-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118183

(CHEMBL3613164)Show SMILES Cl.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)CCc1ccccc1 |r,c:26| Show InChI InChI=1S/C32H38N2O2.ClH/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;/h2-6,10-13,17,23,25,28-29,35H,7-9,14-16,18-21H2,1H3;1H/t25-,28-,29+,31-,32-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118190

(CHEMBL3613174)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)c1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C30H36N2O2.ClH/c1-31(28(34)21-5-3-2-4-6-21)26-18-29-13-14-32(19-20-7-8-20)27-12-10-23(26)17-30(27,29)16-22-9-11-24(33)15-25(22)29;/h2-6,9,11,15,20,23,26-27,33H,7-8,10,12-14,16-19H2,1H3;1H/t23-,26-,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50118184

(CHEMBL3613163)Show SMILES Cl.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)\C=C\c1ccoc1 |r,c:26| Show InChI InChI=1S/C30H34N2O3.ClH/c1-31(28(34)9-4-21-10-13-35-19-21)26-17-29-11-12-32(18-20-2-3-20)27-8-6-23(26)16-30(27,29)15-22-5-7-24(33)14-25(22)29;/h4-10,13-14,19-20,23,26-27,33H,2-3,11-12,15-18H2,1H3;1H/b9-4+;/t23-,26-,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from MOR in guinea pig cerebellum membranes |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50325534

(CHEMBL267495 | nalfurafine)Show SMILES CN([C@@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CC1)c45)C(=O)\C=C\c1ccoc1 Show InChI InChI=1S/C28H32N2O5/c1-29(23(32)7-4-18-9-13-34-16-18)20-8-10-28(33)22-14-19-5-6-21(31)25-24(19)27(28,26(20)35-25)11-12-30(22)15-17-2-3-17/h4-7,9,13,16-17,20,22,26,31,33H,2-3,8,10-12,14-15H2,1H3/b7-4+/t20-,22-,26+,27+,28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128527
BindingDB Entry DOI: 10.7270/Q2HT2TDM |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118180
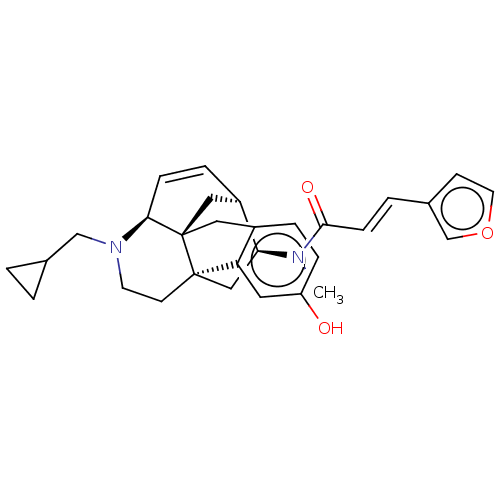
(CHEMBL3613167)Show SMILES Cl.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@@H]2N(C)C(=O)\C=C\c1ccoc1 |r,c:26| Show InChI InChI=1S/C30H34N2O3.ClH/c1-31(28(34)9-4-21-10-13-35-19-21)26-17-29-11-12-32(18-20-2-3-20)27-8-6-23(26)16-30(27,29)15-22-5-7-24(33)14-25(22)29;/h4-10,13-14,19-20,23,26-27,33H,2-3,11-12,15-18H2,1H3;1H/b9-4+;/t23-,26+,27+,29-,30-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50118191

(CHEMBL3613173)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)Cc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C31H38N2O2.ClH/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;/h2-6,9,11,16,22,24,27-28,34H,7-8,10,12-15,17-20H2,1H3;1H/t24-,27-,28+,30-,31-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from DOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50118178

(CHEMBL3613172)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@H]1N(C)C(=O)CCc1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C32H40N2O2.ClH/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;/h2-6,10,12,17,23,25,28-29,35H,7-9,11,13-16,18-21H2,1H3;1H/t25-,28-,29+,31-,32-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from DOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50118186
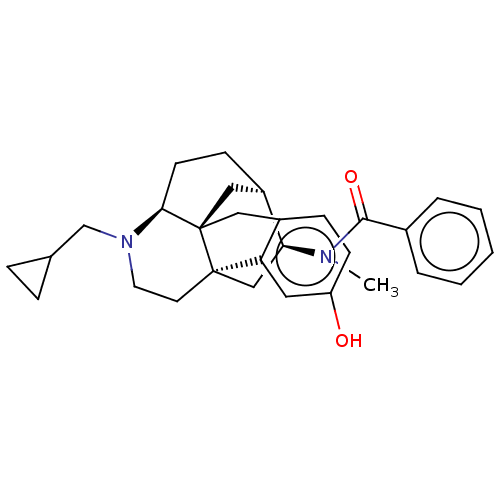
(CHEMBL3613282)Show SMILES Cl.[H][C@]12CC[C@]3([H])N(CC4CC4)CC[C@@]4(C[C@@H]1N(C)C(=O)c1ccccc1)c1cc(O)ccc1C[C@@]34C2 |r| Show InChI InChI=1S/C30H36N2O2.ClH/c1-31(28(34)21-5-3-2-4-6-21)26-18-29-13-14-32(19-20-7-8-20)27-12-10-23(26)17-30(27,29)16-22-9-11-24(33)15-25(22)29;/h2-6,9,11,15,20,23,26-27,33H,7-8,10,12-14,16-19H2,1H3;1H/t23-,26+,27+,29-,30-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from DOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230035
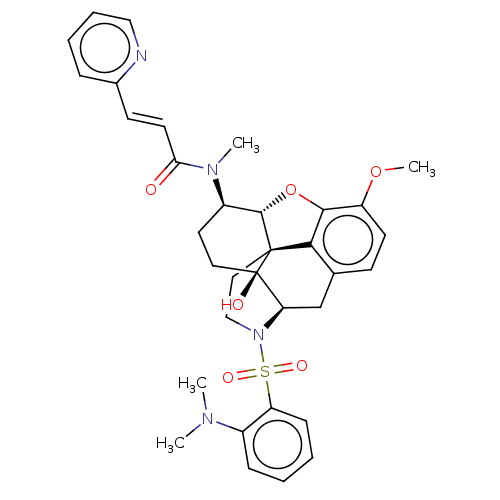
(CHEMBL4091544)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccccn1)S(=O)(=O)c1ccccc1N(C)C)ccc3OC |r,THB:30:9:5.4.6:13| Show InChI InChI=1S/C34H38N4O6S/c1-36(2)24-10-5-6-11-27(24)45(41,42)38-20-18-33-30-22-12-14-26(43-4)31(30)44-32(33)25(16-17-34(33,40)28(38)21-22)37(3)29(39)15-13-23-9-7-8-19-35-23/h5-15,19,25,28,32,40H,16-18,20-21H2,1-4H3/b15-13+/t25-,28-,32+,33+,34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50118182

(CHEMBL3613165)Show SMILES CC1(C)C2CCC1(CS(O)(=O)=O)C(=O)C2.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)Cc1ccccc1 |r,c:42| Show InChI InChI=1S/C31H36N2O2.C10H16O4S/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-6,9-12,16,22,24,27-28,34H,7-8,13-15,17-20H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14)/t24-,27-,28+,30-,31-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from DOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230164
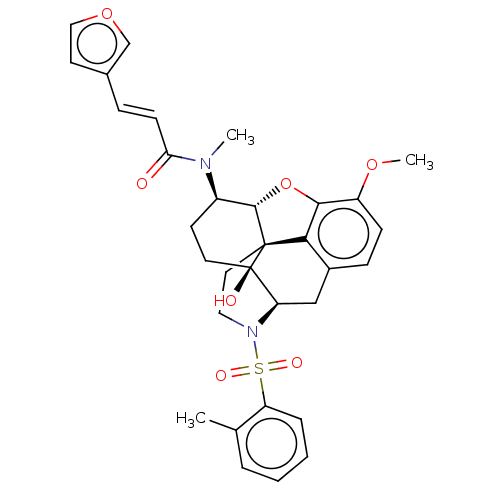
(CHEMBL4091834 | US10377763, Example 2)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1C)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C32H34N2O7S/c1-20-6-4-5-7-25(20)42(37,38)34-16-15-31-28-22-9-10-24(39-3)29(28)41-30(31)23(12-14-32(31,36)26(34)18-22)33(2)27(35)11-8-21-13-17-40-19-21/h4-11,13,17,19,23,26,30,36H,12,14-16,18H2,1-3H3/b11-8+/t23-,26-,30+,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118175

(CHEMBL3613169)Show SMILES CC1(C)C2CCC1(CS(O)(=O)=O)C(=O)C2.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@@H]2N(C)C(=O)Cc1ccccc1 |r,c:42| Show InChI InChI=1S/C31H36N2O2.C10H16O4S/c1-32(29(35)15-21-5-3-2-4-6-21)27-19-30-13-14-33(20-22-7-8-22)28-12-10-24(27)18-31(28,30)17-23-9-11-25(34)16-26(23)30;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-6,9-12,16,22,24,27-28,34H,7-8,13-15,17-20H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14)/t24-,27+,28+,30-,31-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230139
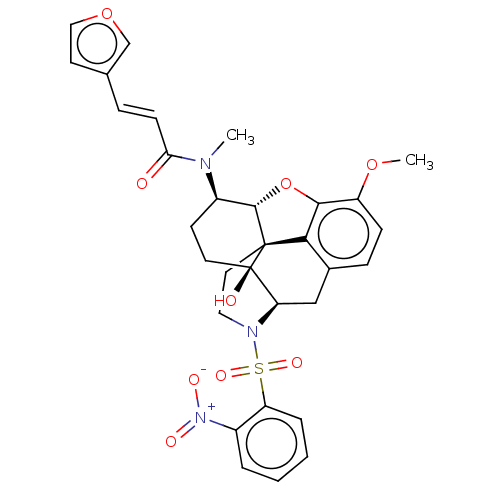
(CHEMBL4105072 | US10377763, Example 8)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1[N+]([O-])=O)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C31H31N3O9S/c1-32(26(35)10-7-19-12-16-42-18-19)22-11-13-31(36)25-17-20-8-9-23(41-2)28-27(20)30(31,29(22)43-28)14-15-33(25)44(39,40)24-6-4-3-5-21(24)34(37)38/h3-10,12,16,18,22,25,29,36H,11,13-15,17H2,1-2H3/b10-7+/t22-,25-,29+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260267
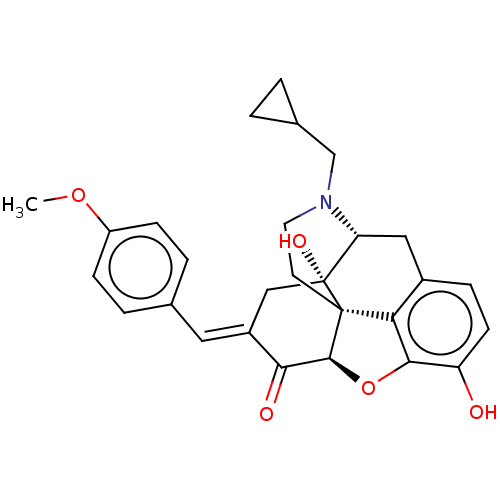
(CHEMBL2059382)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(OC)cc1)C2=O)ccc3O |r,TLB:32:5:9.14.15:17,THB:3:4:9.14.15:17| Show InChI InChI=1S/C28H29NO5/c1-33-20-7-4-16(5-8-20)12-19-14-28(32)22-13-18-6-9-21(30)25-23(18)27(28,26(34-25)24(19)31)10-11-29(22)15-17-2-3-17/h4-9,12,17,22,26,30,32H,2-3,10-11,13-15H2,1H3/b19-12+/t22-,26+,27+,28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230161
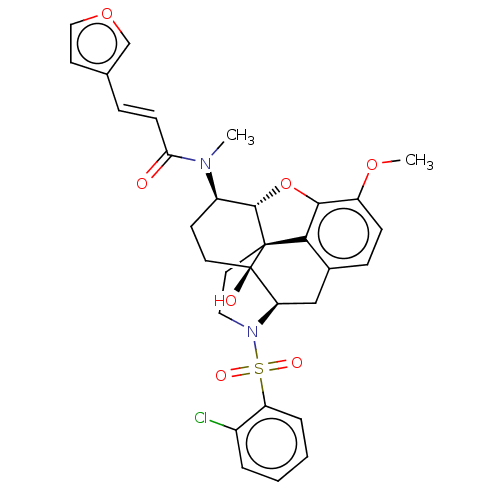
(CHEMBL4089496)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1Cl)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C31H31ClN2O7S/c1-33(26(35)10-7-19-12-16-40-18-19)22-11-13-31(36)25-17-20-8-9-23(39-2)28-27(20)30(31,29(22)41-28)14-15-34(25)42(37,38)24-6-4-3-5-21(24)32/h3-10,12,16,18,22,25,29,36H,11,13-15,17H2,1-2H3/b10-7+/t22-,25-,29+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50592812
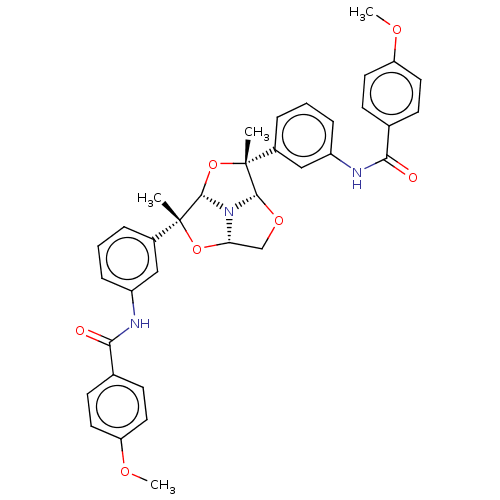
(CHEMBL5194980)Show SMILES [H][C@]12CO[C@]3([H])N1[C@@]([H])(O[C@]3(C)c1cccc(NC(=O)c3ccc(OC)cc3)c1)[C@](C)(O2)c1cccc(NC(=O)c2ccc(OC)cc2)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114505
BindingDB Entry DOI: 10.7270/Q2WM1JFQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230138
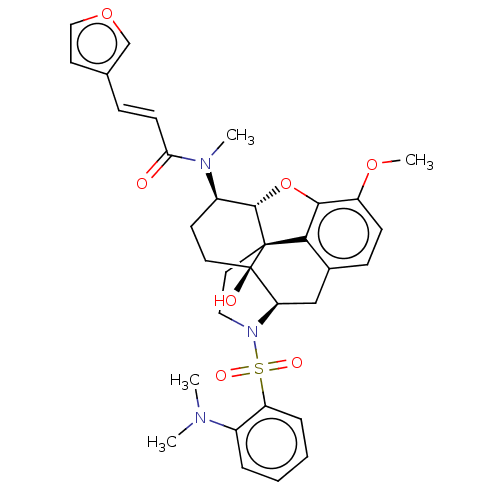
(CHEMBL4094318)Show SMILES Cl.[H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1N(C)C)ccc3OC |r,THB:30:10:6.5.7:14| Show InChI InChI=1S/C33H37N3O7S.ClH/c1-34(2)23-7-5-6-8-26(23)44(39,40)36-17-16-32-29-22-10-11-25(41-4)30(29)43-31(32)24(13-15-33(32,38)27(36)19-22)35(3)28(37)12-9-21-14-18-42-20-21;/h5-12,14,18,20,24,27,31,38H,13,15-17,19H2,1-4H3;1H/b12-9+;/t24-,27-,31+,32+,33-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Mus musculus (Mouse)) | BDBM50118179

(CHEMBL3613168)Show SMILES CC1(C)C2CCC1(CS(O)(=O)=O)C(=O)C2.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@@H]2N(C)C(=O)CCc1ccccc1 |r,c:42| Show InChI InChI=1S/C32H38N2O2.C10H16O4S/c1-33(30(36)14-9-22-5-3-2-4-6-22)28-20-31-15-16-34(21-23-7-8-23)29-13-11-25(28)19-32(29,31)18-24-10-12-26(35)17-27(24)31;1-9(2)7-3-4-10(9,8(11)5-7)6-15(12,13)14/h2-6,10-13,17,23,25,28-29,35H,7-9,14-16,18-21H2,1H3;7H,3-6H2,1-2H3,(H,12,13,14)/t25-,28+,29+,31-,32-;/m1./s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69,593 from KOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50454546
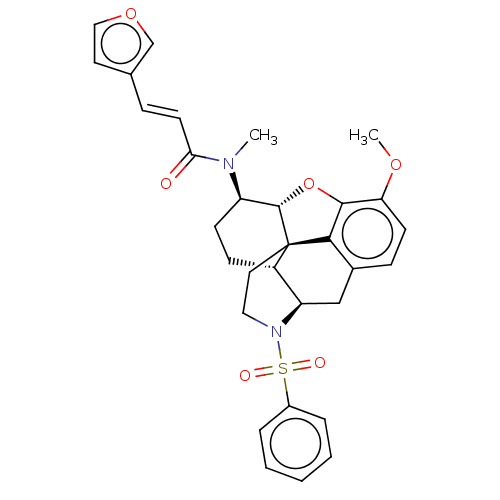
(CHEMBL4215362)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5([H])CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1)ccc3OC |r,THB:29:9:13:6.4.5| Show InChI InChI=1S/C31H32N2O6S/c1-32(27(34)13-8-20-14-17-38-19-20)24-11-10-23-25-18-21-9-12-26(37-2)29-28(21)31(23,30(24)39-29)15-16-33(25)40(35,36)22-6-4-3-5-7-22/h3-9,12-14,17,19,23-25,30H,10-11,15-16,18H2,1-2H3/b13-8+/t23-,24+,25+,30-,31-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincubated for 15 ... |
Bioorg Med Chem Lett 28: 774-777 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.069
BindingDB Entry DOI: 10.7270/Q2BK1G0R |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230158
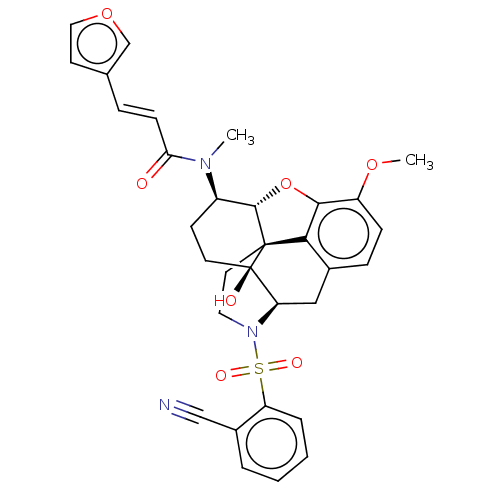
(CHEMBL4073947 | US10377763, Example 11)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1C#N)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C32H31N3O7S/c1-34(27(36)10-7-20-12-16-41-19-20)23-11-13-32(37)26-17-21-8-9-24(40-2)29-28(21)31(32,30(23)42-29)14-15-35(26)43(38,39)25-6-4-3-5-22(25)18-33/h3-10,12,16,19,23,26,30,37H,11,13-15,17H2,1-2H3/b10-7+/t23-,26-,30+,31+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230144
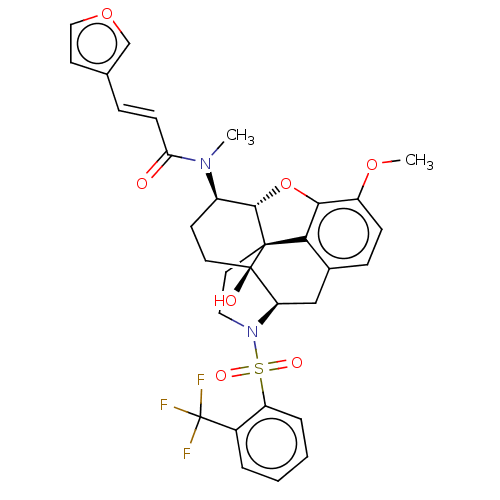
(CHEMBL4081763 | US10377763, Example 5)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1C(F)(F)F)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C32H31F3N2O7S/c1-36(26(38)10-7-19-12-16-43-18-19)22-11-13-31(39)25-17-20-8-9-23(42-2)28-27(20)30(31,29(22)44-28)14-15-37(25)45(40,41)24-6-4-3-5-21(24)32(33,34)35/h3-10,12,16,18,22,25,29,39H,11,13-15,17H2,1-2H3/b10-7+/t22-,25-,29+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50454547
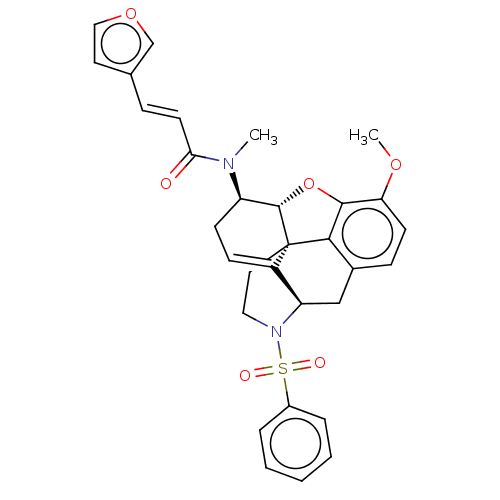
(CHEMBL4213974)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14C5=CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1)ccc3OC |r,t:16,THB:28:9:13:6.4.5| Show InChI InChI=1S/C31H30N2O6S/c1-32(27(34)13-8-20-14-17-38-19-20)24-11-10-23-25-18-21-9-12-26(37-2)29-28(21)31(23,30(24)39-29)15-16-33(25)40(35,36)22-6-4-3-5-7-22/h3-10,12-14,17,19,24-25,30H,11,15-16,18H2,1-2H3/b13-8+/t24-,25-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular Ca2+ release preincubated for 15 ... |
Bioorg Med Chem Lett 28: 774-777 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.069
BindingDB Entry DOI: 10.7270/Q2BK1G0R |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230134
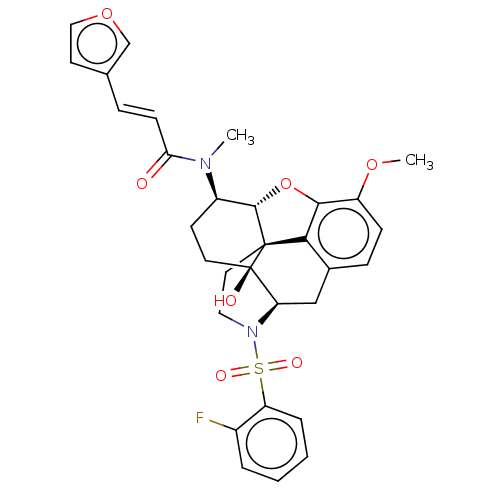
(CHEMBL4087046)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1F)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C31H31FN2O7S/c1-33(26(35)10-7-19-12-16-40-18-19)22-11-13-31(36)25-17-20-8-9-23(39-2)28-27(20)30(31,29(22)41-28)14-15-34(25)42(37,38)24-6-4-3-5-21(24)32/h3-10,12,16,18,22,25,29,36H,11,13-15,17H2,1-2H3/b10-7+/t22-,25-,29+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230141
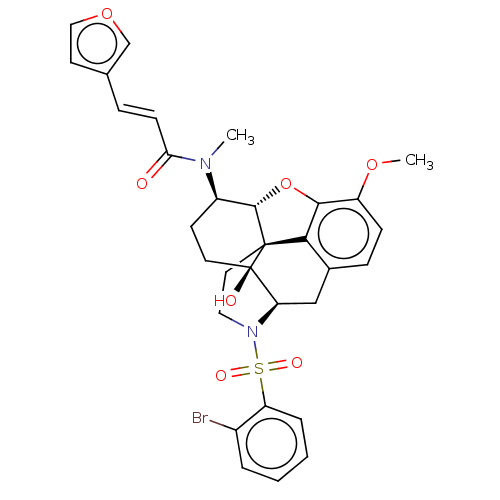
(CHEMBL4083587 | US10377763, Example 20)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1Br)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C31H31BrN2O7S/c1-33(26(35)10-7-19-12-16-40-18-19)22-11-13-31(36)25-17-20-8-9-23(39-2)28-27(20)30(31,29(22)41-28)14-15-34(25)42(37,38)24-6-4-3-5-21(24)32/h3-10,12,16,18,22,25,29,36H,11,13-15,17H2,1-2H3/b10-7+/t22-,25-,29+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50592809
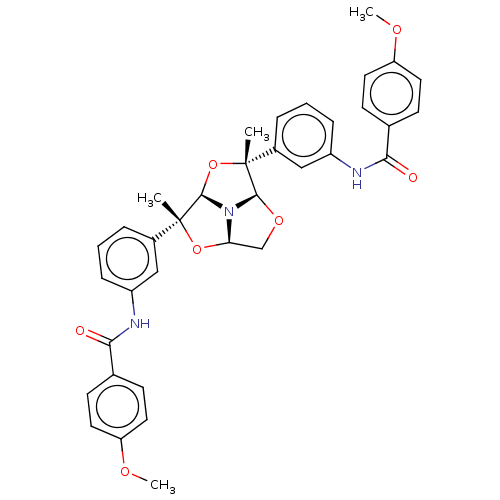
(CHEMBL5172765)Show SMILES [H][C@@]12CO[C@@]3([H])N1[C@]([H])(O[C@]3(C)c1cccc(NC(=O)c3ccc(OC)cc3)c1)[C@](C)(O2)c1cccc(NC(=O)c2ccc(OC)cc2)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50592810
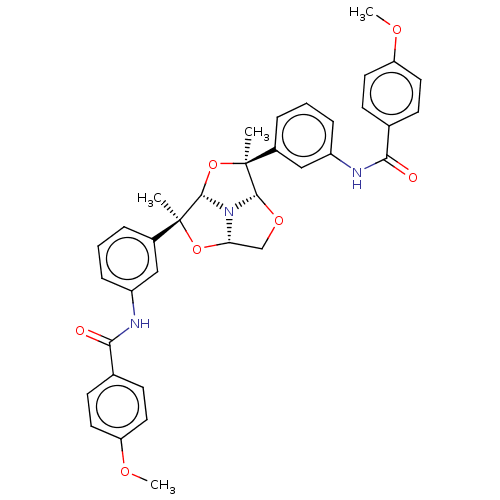
(CHEMBL5174113)Show SMILES [H][C@]12CO[C@]3([H])N1[C@@]([H])(O[C@@]3(C)c1cccc(NC(=O)c3ccc(OC)cc3)c1)[C@@](C)(O2)c1cccc(NC(=O)c2ccc(OC)cc2)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128530
BindingDB Entry DOI: 10.7270/Q28919WZ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50592811
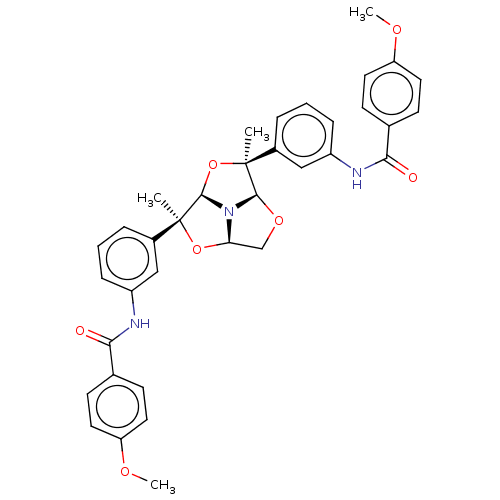
(CHEMBL5170308)Show SMILES [H][C@@]12CO[C@@]3([H])N1[C@]([H])(O[C@@]3(C)c1cccc(NC(=O)c3ccc(OC)cc3)c1)[C@@](C)(O2)c1cccc(NC(=O)c2ccc(OC)cc2)c1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114505
BindingDB Entry DOI: 10.7270/Q2WM1JFQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50118181
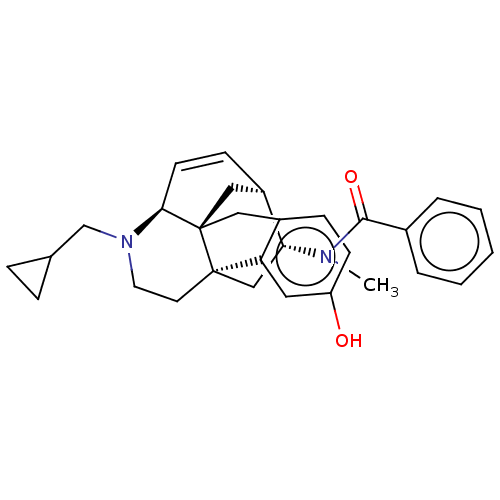
(CHEMBL3613166)Show SMILES Cl.[H][C@]12C[C@@]34Cc5ccc(O)cc5[C@@]3(CCN(CC3CC3)[C@@]4([H])C=C1)C[C@H]2N(C)C(=O)c1ccccc1 |r,c:26| Show InChI InChI=1S/C30H34N2O2.ClH/c1-31(28(34)21-5-3-2-4-6-21)26-18-29-13-14-32(19-20-7-8-20)27-12-10-23(26)17-30(27,29)16-22-9-11-24(33)15-25(22)29;/h2-6,9-12,15,20,23,26-27,33H,7-8,13-14,16-19H2,1H3;1H/t23-,26-,27+,29-,30-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from DOR in mouse whole brain membranes without cerebellum |
Bioorg Med Chem 23: 6271-9 (2015)
Article DOI: 10.1016/j.bmc.2015.08.036
BindingDB Entry DOI: 10.7270/Q2V126M1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50260248
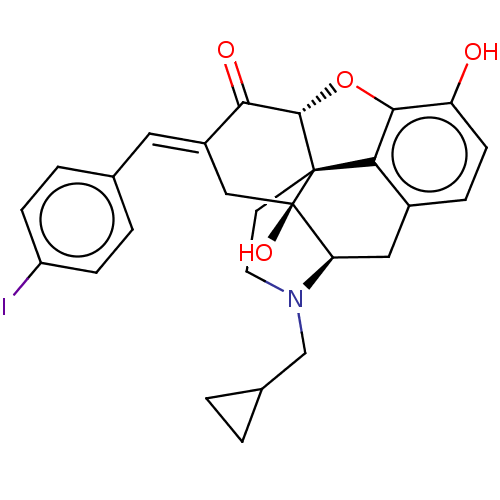
(CHEMBL4092119)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]5(O)C\C(=C/c1ccc(I)cc1)C2=O)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C27H26INO4/c28-19-6-3-15(4-7-19)11-18-13-27(32)21-12-17-5-8-20(30)24-22(17)26(27,25(33-24)23(18)31)9-10-29(21)14-16-1-2-16/h3-8,11,16,21,25,30,32H,1-2,9-10,12-14H2/b18-11+/t21-,25+,26+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by microbeta scintillation counting analysis |
Bioorg Med Chem 25: 4375-4383 (2017)
Article DOI: 10.1016/j.bmc.2017.06.026
BindingDB Entry DOI: 10.7270/Q25T3NZ0 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50230167
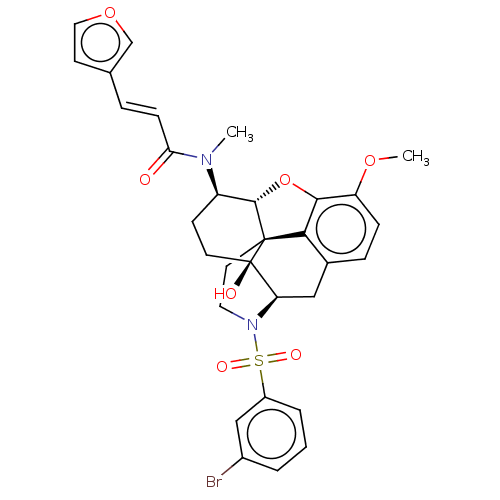
(CHEMBL4071220 | US10377763, Example 21)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14[C@@]5(O)CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1cccc(Br)c1)ccc3OC |r,THB:29:9:5.4.6:13| Show InChI InChI=1S/C31H31BrN2O7S/c1-33(26(35)9-6-19-11-15-40-18-19)23-10-12-31(36)25-16-20-7-8-24(39-2)28-27(20)30(31,29(23)41-28)13-14-34(25)42(37,38)22-5-3-4-21(32)17-22/h3-9,11,15,17-18,23,25,29,36H,10,12-14,16H2,1-2H3/b9-6+/t23-,25-,29+,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... |
J Med Chem 60: 1018-1040 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01418
BindingDB Entry DOI: 10.7270/Q22N54JH |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50454547

(CHEMBL4213974)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC[C@@]14C5=CC[C@H]2N(C)C(=O)\C=C\c1ccoc1)S(=O)(=O)c1ccccc1)ccc3OC |r,t:16,THB:28:9:13:6.4.5| Show InChI InChI=1S/C31H30N2O6S/c1-32(27(34)13-8-20-14-17-38-19-20)24-11-10-23-25-18-21-9-12-26(37-2)29-28(21)31(23,30(24)39-29)15-16-33(25)40(35,36)22-6-4-3-5-7-22/h3-10,12-14,17,19,24-25,30H,11,15-16,18H2,1-2H3/b13-8+/t24-,25-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmcl.2022.128550
BindingDB Entry DOI: 10.7270/Q2GT5S6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data