

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50534550 (CHEMBL4542697) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Displacement of [3H]NMS from human muscarinic M5 receptor | Bioorg Med Chem Lett 26: 4487-4491 (2016) Article DOI: 10.1016/j.bmcl.2016.07.071 BindingDB Entry DOI: 10.7270/Q2MG7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse COX-2 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024253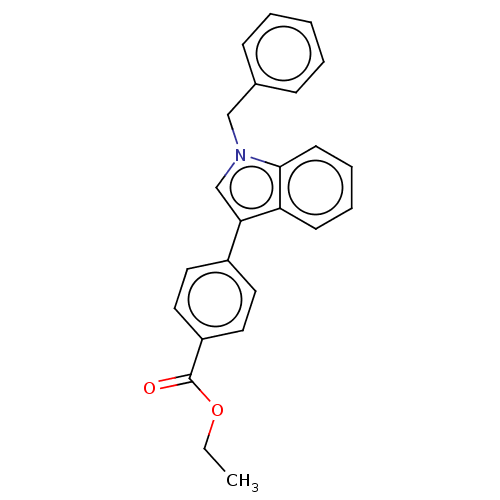 (CHEMBL3334887) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033168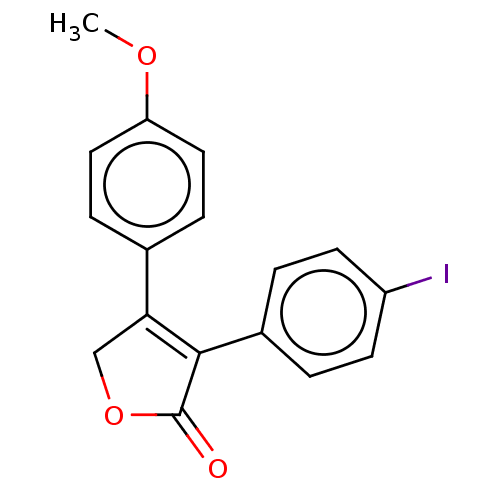 (CHEMBL3357106) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033167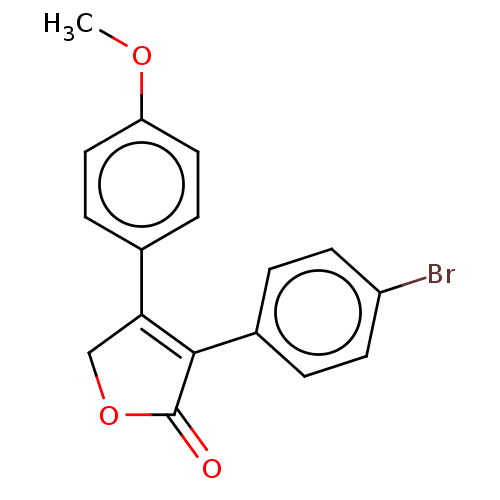 (CHEMBL3357105) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024254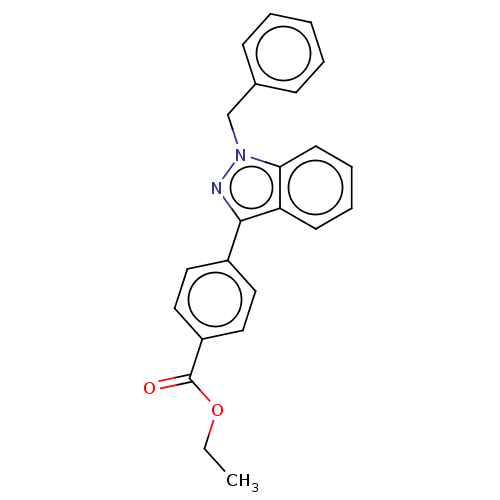 (CHEMBL125021) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024252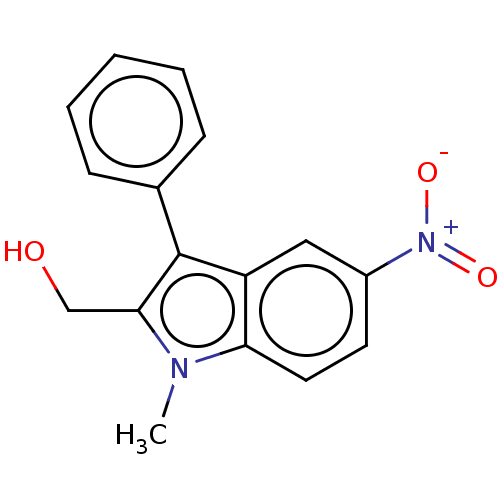 (CHEMBL1609104) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50033277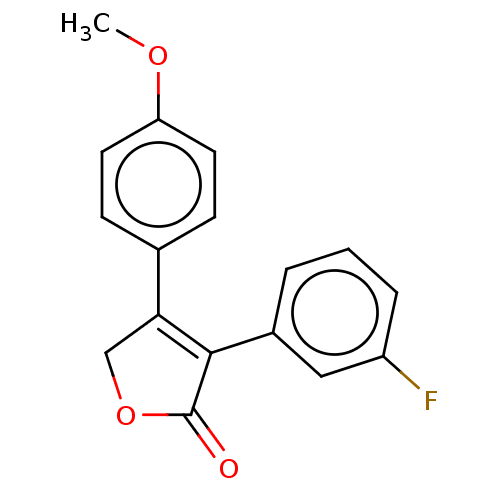 (CHEMBL3357103) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells by cell-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50042441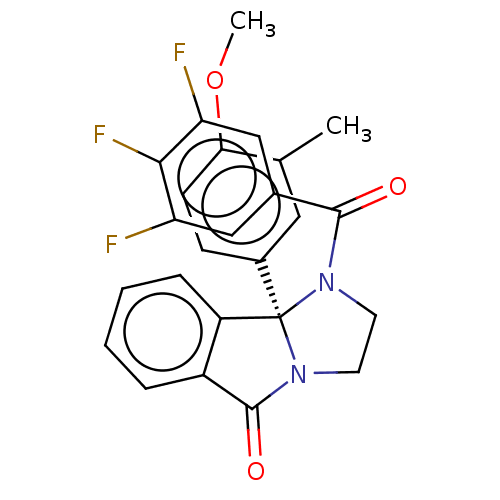 (CHEMBL3353290) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M5 receptor assessed as inhibition of acetylcholine-induced calcium response | Bioorg Med Chem Lett 26: 4487-4491 (2016) Article DOI: 10.1016/j.bmcl.2016.07.071 BindingDB Entry DOI: 10.7270/Q2MG7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024255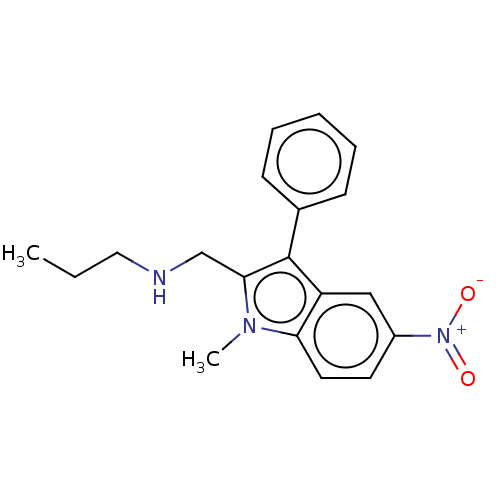 (CHEMBL3334944) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50445222 (CHEMBL3105228) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M5 receptor assessed as inhibition of acetylcholine-induced calcium response | Bioorg Med Chem Lett 26: 4487-4491 (2016) Article DOI: 10.1016/j.bmcl.2016.07.071 BindingDB Entry DOI: 10.7270/Q2MG7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033170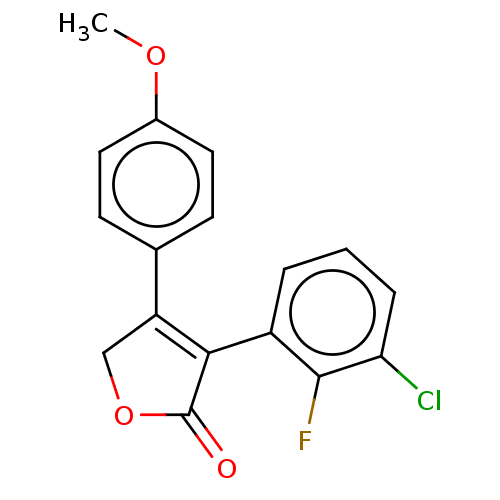 (CHEMBL3357108) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024258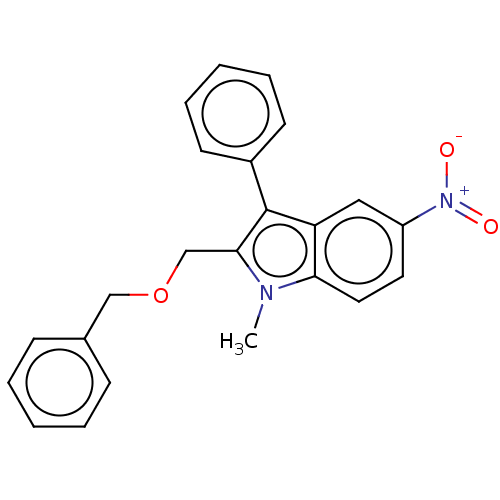 (CHEMBL3334941) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50249435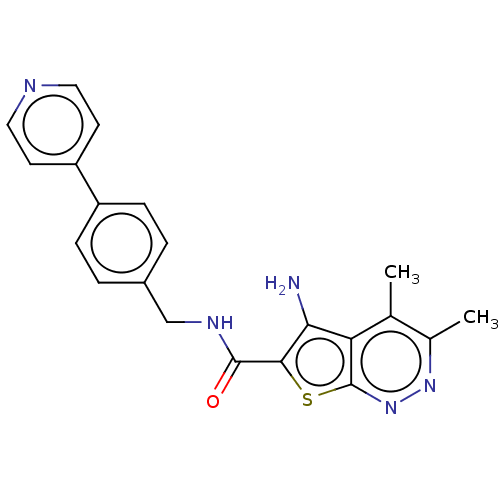 (CHEMBL4070692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Induction of CYP3A4 in cryopreserved human hepatocytes measured after 48 hrs | Bioorg Med Chem Lett 27: 2296-2301 (2017) Article DOI: 10.1016/j.bmcl.2017.04.043 BindingDB Entry DOI: 10.7270/Q23B62J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033277 (CHEMBL3357103) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50033166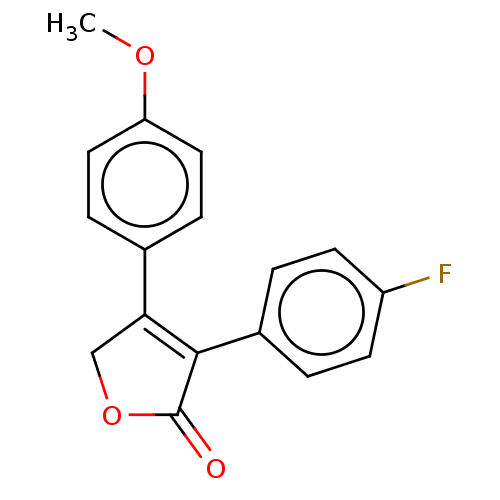 (CHEMBL3357104) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells by cell-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50230576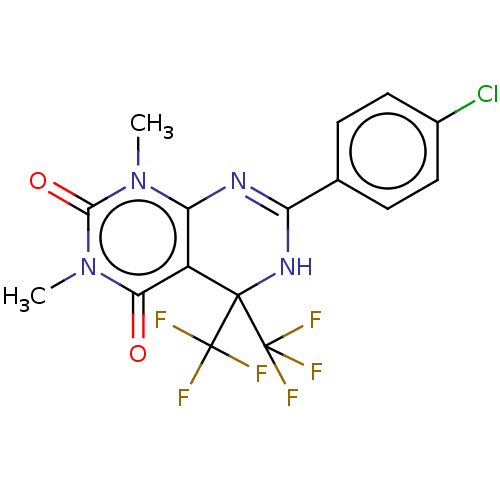 (CHEMBL1341270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50033167 (CHEMBL3357105) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse COX-2 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50230575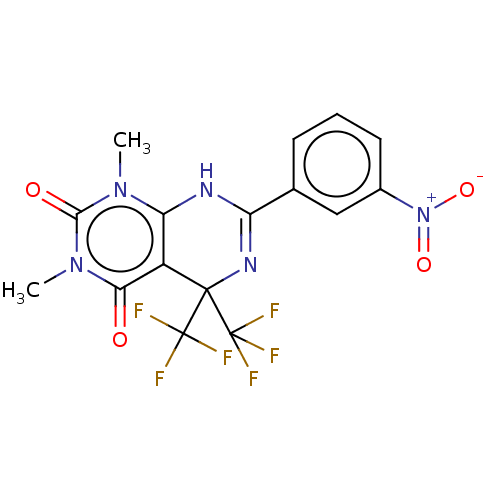 (CHEMBL4079193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate preincubated for 15 mins followed by NADPH addition measured after 8 min... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033173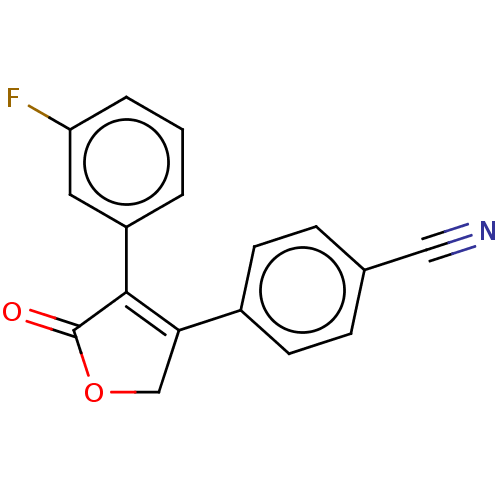 (CHEMBL3357111) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033166 (CHEMBL3357104) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 603 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024259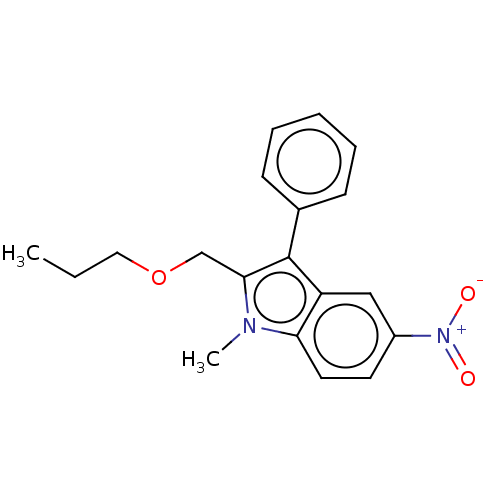 (CHEMBL3334940) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 646 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50534550 (CHEMBL4542697) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M5 receptor assessed as inhibition of acetylcholine-induced calcium response | Bioorg Med Chem Lett 26: 4487-4491 (2016) Article DOI: 10.1016/j.bmcl.2016.07.071 BindingDB Entry DOI: 10.7270/Q2MG7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50534550 (CHEMBL4542697) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 661 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M5 receptor assessed as inhibition of acetylcholine-induced calcium response | Bioorg Med Chem Lett 26: 4487-4491 (2016) Article DOI: 10.1016/j.bmcl.2016.07.071 BindingDB Entry DOI: 10.7270/Q2MG7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230575 (CHEMBL4079193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230576 (CHEMBL1341270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 692 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of exendin-4-induced cA... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50033281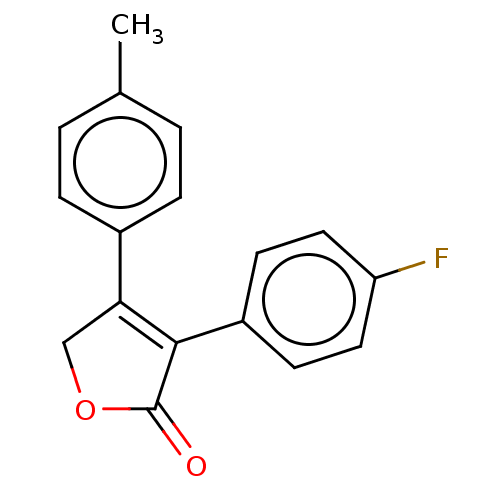 (CHEMBL3357099) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of COX-1 in human OVCAR3 cells by cell-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024260 (CHEMBL3334939) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 785 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50249405 (CHEMBL4102040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 27: 2296-2301 (2017) Article DOI: 10.1016/j.bmcl.2017.04.043 BindingDB Entry DOI: 10.7270/Q23B62J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 4 (Homo sapiens (Human)) | BDBM50024257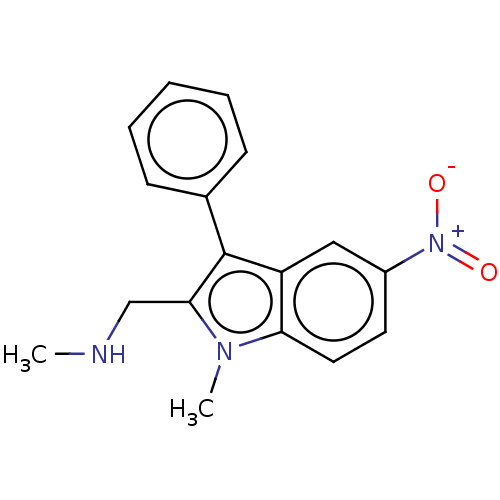 (CHEMBL3334942) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 793 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwest Agriculture& Forestry University Curated by ChEMBL | Assay Description Antagonist activity at PAR4 in PAR-4-AP-stimulated human platelets compound pretreated for 5 mins by fluorescent PAC1 integrin alpha2bb3 activation a... | Bioorg Med Chem Lett 24: 4708-13 (2014) Article DOI: 10.1016/j.bmcl.2014.08.021 BindingDB Entry DOI: 10.7270/Q29P336C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50534551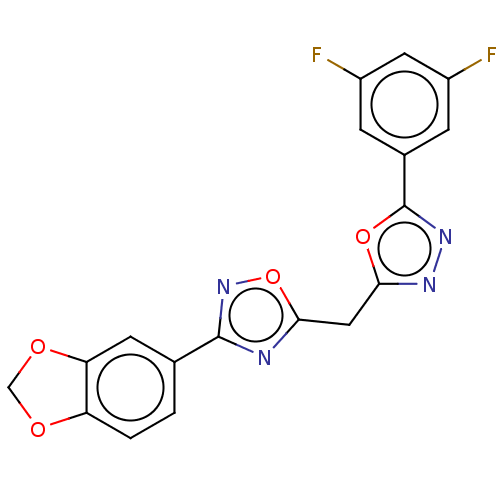 (CHEMBL4448840) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic M2 receptor assessed as inhibition of acetylcholine-induced calcium response | Bioorg Med Chem Lett 26: 4487-4491 (2016) Article DOI: 10.1016/j.bmcl.2016.07.071 BindingDB Entry DOI: 10.7270/Q2MG7T0M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033281 (CHEMBL3357099) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033283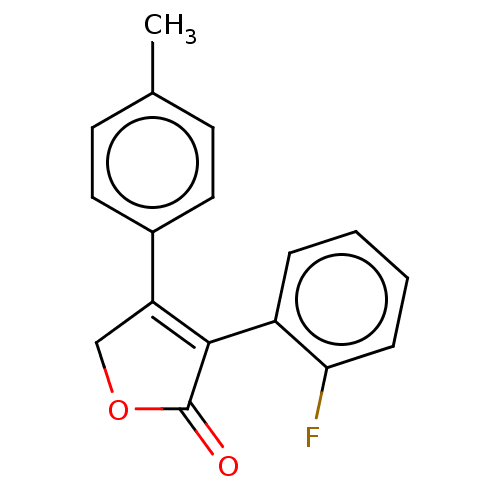 (CHEMBL3357097) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50033178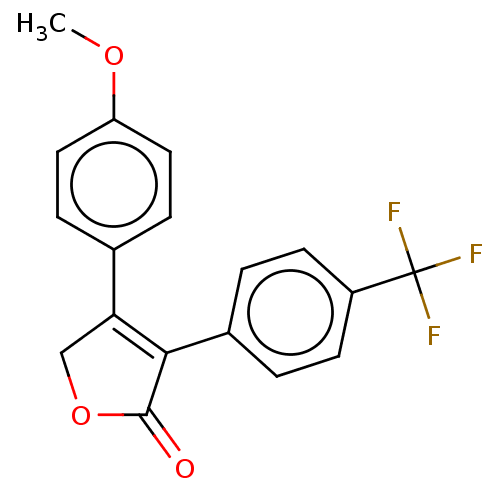 (CHEMBL3357116) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Inhibition of ovine COX-1 assessed as inhibition of [14C]arachidonic acid to radiolabeled prostaglandins preincubated for 15 mins by TLC-based assay | ACS Med Chem Lett 5: 1254-8 (2014) Article DOI: 10.1021/ml500344j BindingDB Entry DOI: 10.7270/Q2BK1DZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50445222 (CHEMBL3105228) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50445222 (CHEMBL3105228) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50233360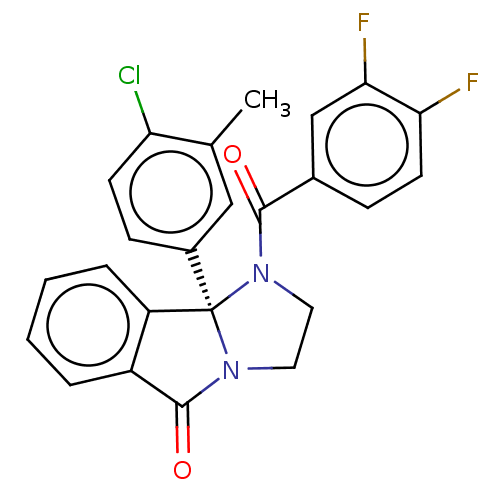 (CHEMBL4062497) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50233360 (CHEMBL4062497) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50233372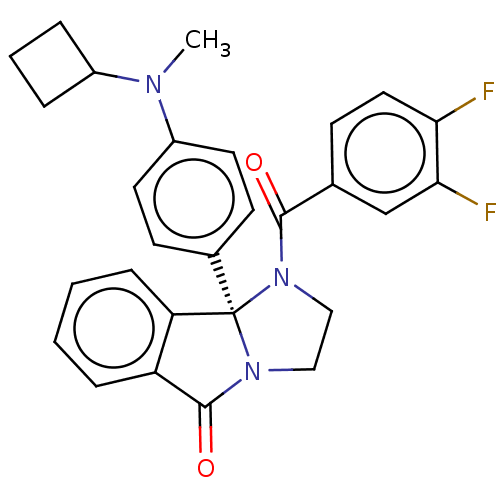 (CHEMBL4100518) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230573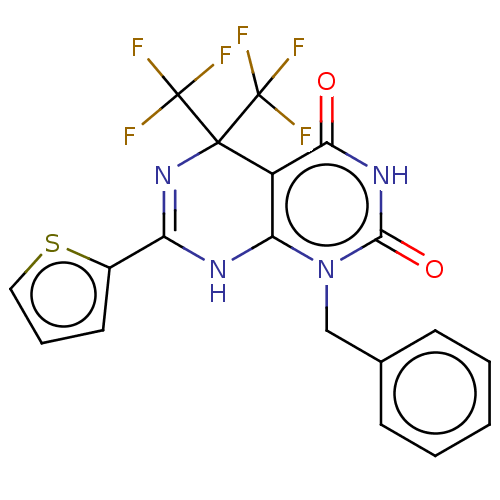 (CHEMBL4068337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50230573 (CHEMBL4068337) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Antagonist activity at human GLP-1R expressed in TREx293 cells co-expressing cAMP sensitive luciferase assessed as inhibition of GLP1 (7 to 36 residu... | J Med Chem 60: 1611-1616 (2017) Article DOI: 10.1021/acs.jmedchem.6b01706 BindingDB Entry DOI: 10.7270/Q2NG4SVK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50249435 (CHEMBL4070692) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | Bioorg Med Chem Lett 27: 2296-2301 (2017) Article DOI: 10.1016/j.bmcl.2017.04.043 BindingDB Entry DOI: 10.7270/Q23B62J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50233372 (CHEMBL4100518) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50233363 (CHEMBL4065335) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University School of Medicine Curated by ChEMBL | Assay Description Negative allosteric modulation of human M5 receptor expressed in CHO cells assessed as inhibition of acetyl choline-induced calcium mobilization prei... | Bioorg Med Chem Lett 27: 1356-1359 (2017) Article DOI: 10.1016/j.bmcl.2017.02.020 BindingDB Entry DOI: 10.7270/Q241709W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 452 total ) | Next | Last >> |