

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116936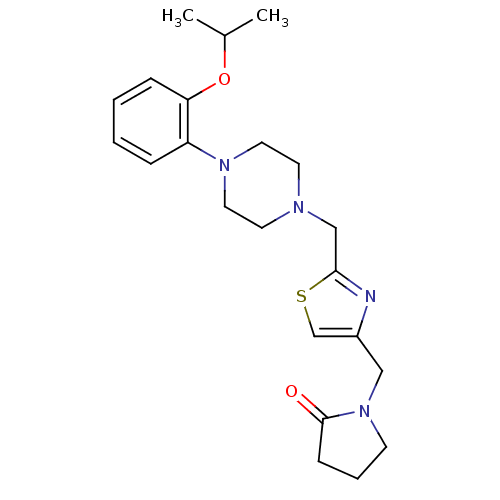 (1-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116940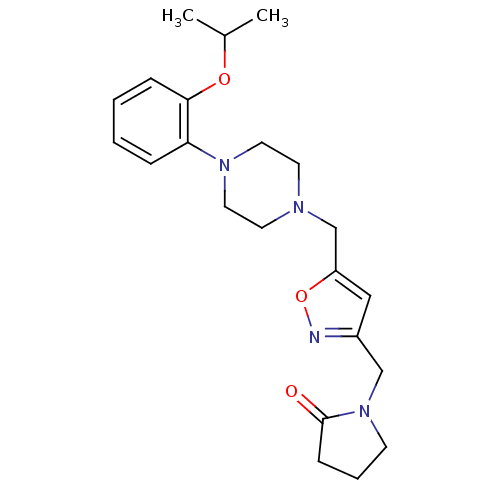 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116928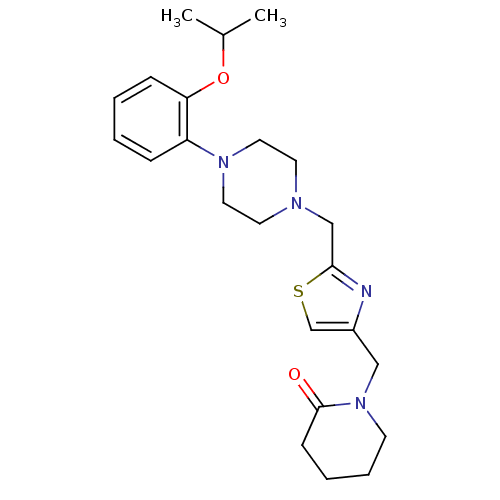 (1-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM404301 (US10017501, Compound 1020-289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249309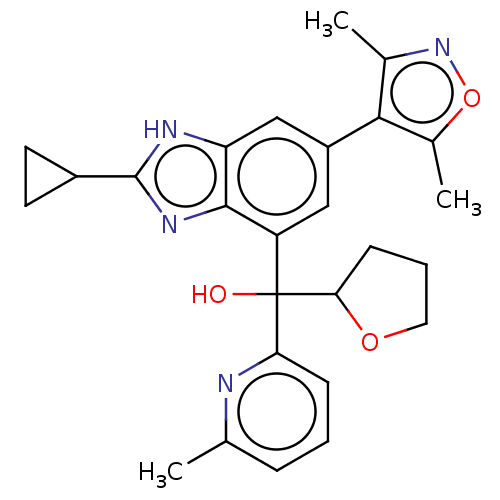 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249314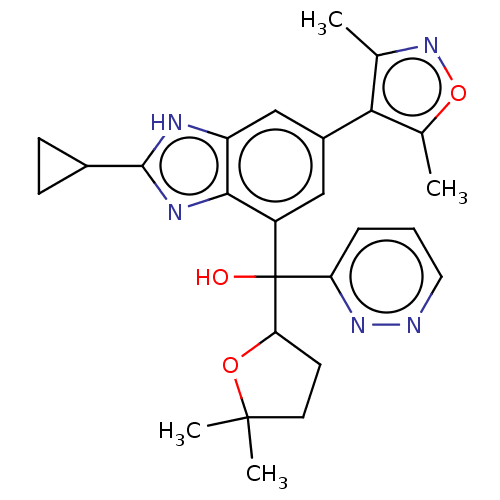 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249309 (US10017501, Compound 1020-114 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249314 (US10017501, Compound 1020-298 | US9458145, 1020-29...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249312 (US10017501, Compound 1020-257 | US9458145, 1020-25...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249311 (US10017501, Compound 1020-239 | US9458145, 1020-23...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50029266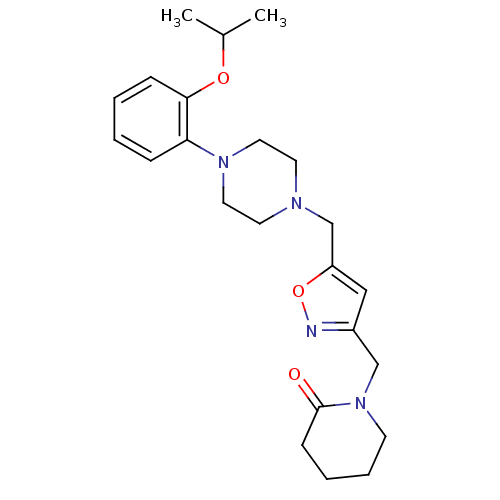 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [75-147] (Homo sapiens (Human)) | BDBM249310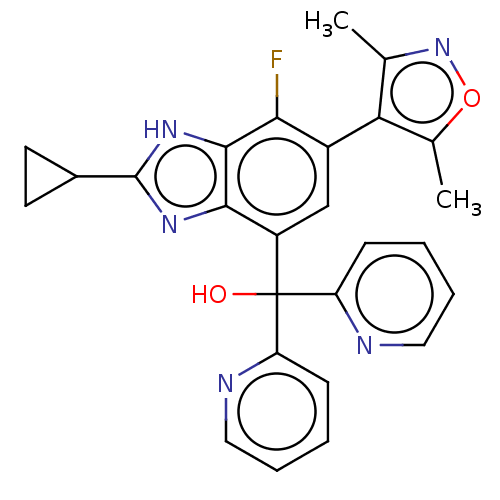 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249306 (US10017501, Compound 1020-104 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249305 (US10017501, Compound 1020-103 | US9458145, 1020-10...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249308 (US10017501, Compound 1020-113 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016697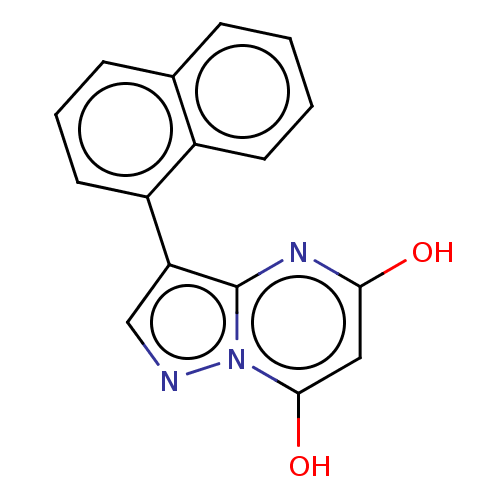 (CHEMBL3277223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249307 (US10017501, Compound 1020-112 | US9458145, 1020-11...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116938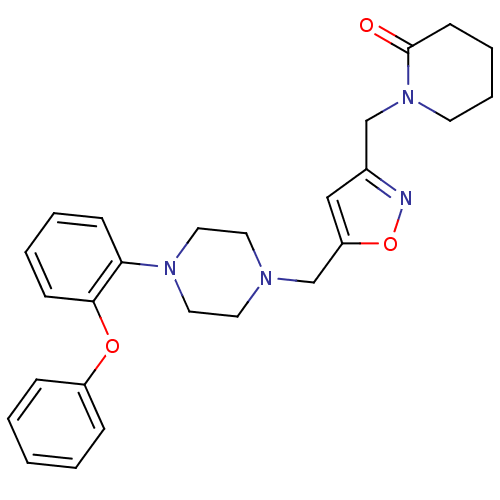 (1-{5-[4-(2-Phenoxy-phenyl)-piperazin-1-ylmethyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016697 (CHEMBL3277223) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016702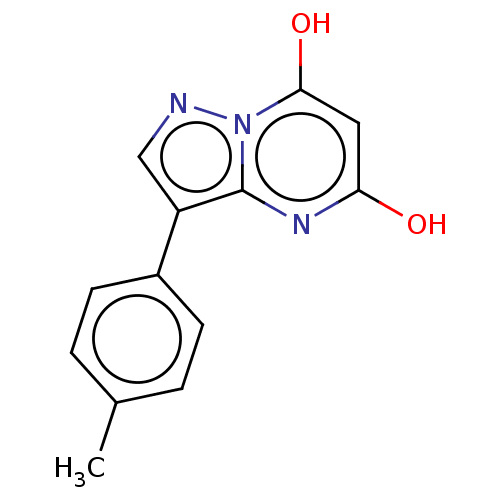 (CHEMBL3276844) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016707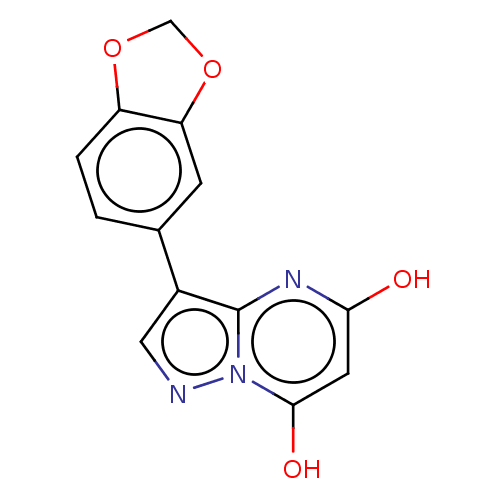 (CHEMBL3276846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016705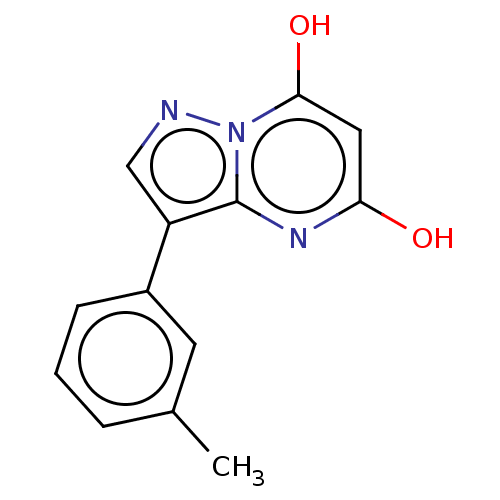 (CHEMBL3276845) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016700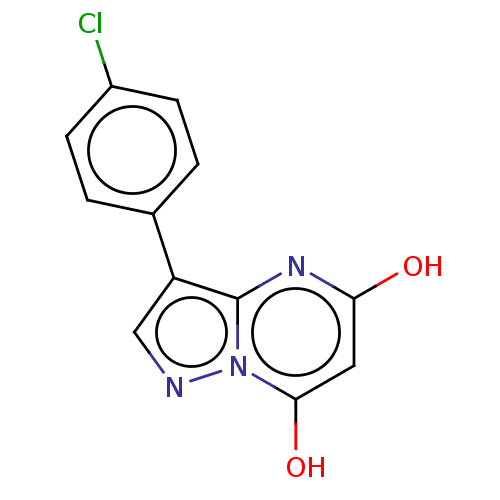 (CHEMBL3276842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016705 (CHEMBL3276845) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 [368-440] (Homo sapiens (Human)) | BDBM249310 (US10017501, Compound 1020-224 | US9458145, 1020-22...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences Inc US Patent | Assay Description Binding of the two tandem bromodomains, BRD4-1 and BRD4-2, to an acetylated histone H4 peptide was measured using a homogeneous time resolved fluores... | US Patent US10017501 (2018) BindingDB Entry DOI: 10.7270/Q2CV4M3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016707 (CHEMBL3276846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016699 (CHEMBL3276841) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016700 (CHEMBL3276842) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016702 (CHEMBL3276844) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016696 (CHEMBL3276840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016699 (CHEMBL3276841) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116937 (1-{5-[4-(2-Phenoxy-phenyl)-piperazin-1-ylmethyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016696 (CHEMBL3276840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50116937 (1-{5-[4-(2-Phenoxy-phenyl)-piperazin-1-ylmethyl]-i...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1D adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50116928 (1-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1D adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016669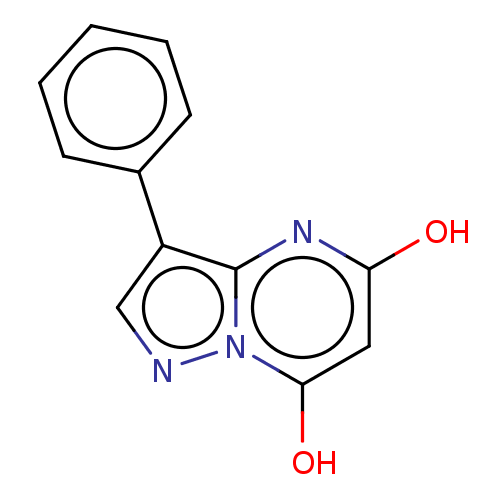 (CHEMBL3276839) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016669 (CHEMBL3276839) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Non-competitive inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50116940 (1-{5-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1D adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016701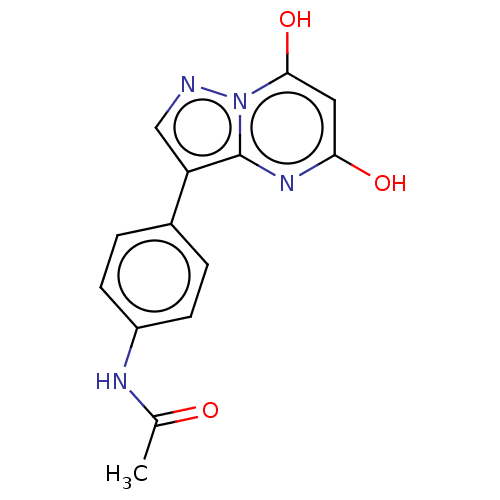 (CHEMBL3276843) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using hypoxanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116935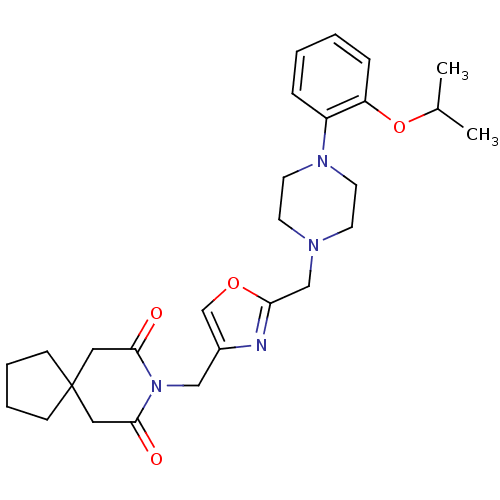 (8-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50016701 (CHEMBL3276843) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Mixed-type inhibition of bovine milk xanthine oxidase using xanthine as substrate by Lineweaver-Burk plot analysis | J Med Chem 19: 291-6 (1976) BindingDB Entry DOI: 10.7270/Q2XP76G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50116931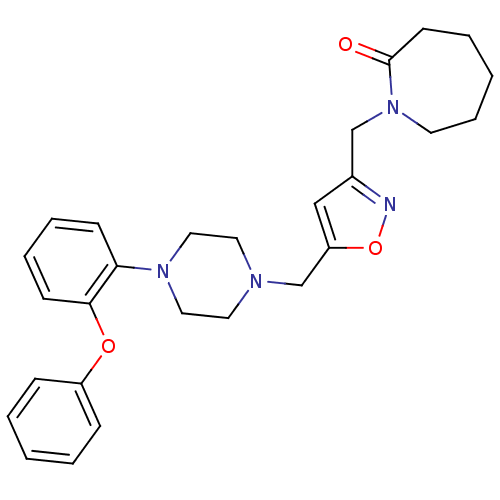 (1-{5-[4-(2-Phenoxy-phenyl)-piperazin-1-ylmethyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1A adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50116936 (1-{2-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Displacement of [125I]-HEAT from human Alpha-1D adrenergic receptor | Bioorg Med Chem Lett 12: 2443-6 (2002) BindingDB Entry DOI: 10.7270/Q2FB528D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1053 total ) | Next | Last >> |