 Found 17043 hits with Last Name = 'ni' and Initial = 'y'
Found 17043 hits with Last Name = 'ni' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50079482
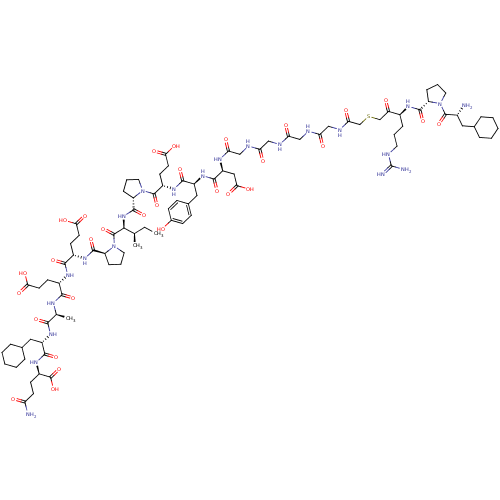
(Arginyl Ketomethylene analogue | CHEMBL410589)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CSCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C92H140N22O30S/c1-4-49(2)78(90(142)114-38-14-21-65(114)86(138)106-58(29-33-75(125)126)81(133)105-57(28-32-74(123)124)80(132)102-50(3)79(131)109-61(40-52-17-9-6-10-18-52)83(135)108-60(91(143)144)27-31-68(94)117)111-87(139)66-22-13-37-113(66)89(141)59(30-34-76(127)128)107-82(134)62(41-53-23-25-54(115)26-24-53)110-84(136)63(42-77(129)130)103-72(121)46-100-70(119)44-98-69(118)43-99-71(120)45-101-73(122)48-145-47-67(116)56(19-11-35-97-92(95)96)104-85(137)64-20-12-36-112(64)88(140)55(93)39-51-15-7-5-8-16-51/h23-26,49-52,55-66,78,115H,4-22,27-48,93H2,1-3H3,(H2,94,117)(H,98,118)(H,99,120)(H,100,119)(H,101,122)(H,102,132)(H,103,121)(H,104,137)(H,105,133)(H,106,138)(H,107,134)(H,108,135)(H,109,131)(H,110,136)(H,111,139)(H,123,124)(H,125,126)(H,127,128)(H,129,130)(H,143,144)(H4,95,96,97)/t49-,50+,55-,56+,57+,58+,59+,60-,61+,62+,63+,64+,65+,66+,78+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079489
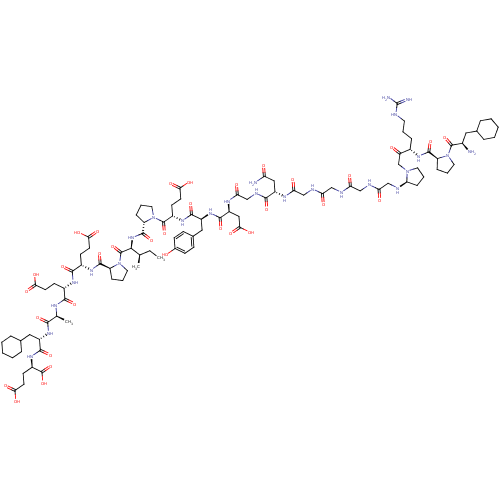
(Arginyl Ketomethylene analogue | CHEMBL428116)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CN[C@@H]1CCCN1CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C100H153N25O33/c1-4-53(2)85(98(156)125-41-14-22-70(125)94(152)116-62(30-34-81(136)137)89(147)115-61(29-33-80(134)135)88(146)111-54(3)86(144)119-65(43-56-18-9-6-10-19-56)91(149)118-64(99(157)158)32-36-83(140)141)121-95(153)71-23-13-40-124(71)97(155)63(31-35-82(138)139)117-90(148)66(44-57-25-27-58(126)28-26-57)120-92(150)68(46-84(142)143)113-79(133)51-110-87(145)67(45-73(102)128)112-78(132)50-109-77(131)49-108-76(130)48-107-75(129)47-106-74-24-15-38-122(74)52-72(127)60(20-11-37-105-100(103)104)114-93(151)69-21-12-39-123(69)96(154)59(101)42-55-16-7-5-8-17-55/h25-28,53-56,59-71,74,85,106,126H,4-24,29-52,101H2,1-3H3,(H2,102,128)(H,107,129)(H,108,130)(H,109,131)(H,110,145)(H,111,146)(H,112,132)(H,113,133)(H,114,151)(H,115,147)(H,116,152)(H,117,148)(H,118,149)(H,119,144)(H,120,150)(H,121,153)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,157,158)(H4,103,104,105)/t53-,54+,59-,60+,61+,62+,63+,64-,65+,66+,67+,68+,69+,70+,71+,74+,85+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079476
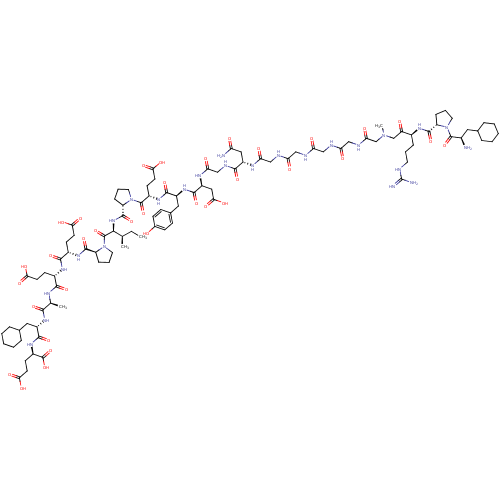
(Arginyl Ketomethylene analogue | CHEMBL437873)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C99H151N25O34/c1-5-52(2)84(97(156)124-39-15-22-69(124)93(152)115-61(29-33-80(136)137)88(147)114-60(28-32-79(134)135)87(146)110-53(3)85(144)118-64(41-55-18-10-7-11-19-55)90(149)117-63(98(157)158)31-35-82(140)141)120-94(153)70-23-14-38-123(70)96(155)62(30-34-81(138)139)116-89(148)65(42-56-24-26-57(125)27-25-56)119-91(150)67(44-83(142)143)112-77(132)49-109-86(145)66(43-72(101)127)111-76(131)48-107-74(129)46-105-73(128)45-106-75(130)47-108-78(133)51-121(4)50-71(126)59(20-12-36-104-99(102)103)113-92(151)68-21-13-37-122(68)95(154)58(100)40-54-16-8-6-9-17-54/h24-27,52-55,58-70,84,125H,5-23,28-51,100H2,1-4H3,(H2,101,127)(H,105,128)(H,106,130)(H,107,129)(H,108,133)(H,109,145)(H,110,146)(H,111,131)(H,112,132)(H,113,151)(H,114,147)(H,115,152)(H,116,148)(H,117,149)(H,118,144)(H,119,150)(H,120,153)(H,134,135)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,157,158)(H4,102,103,104)/t52-,53+,58-,59+,60+,61+,62+,63-,64+,65+,66+,67+,68+,69+,70+,84+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50300196
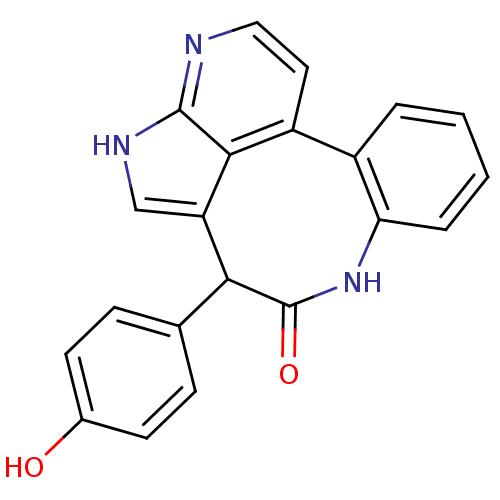
(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50079479
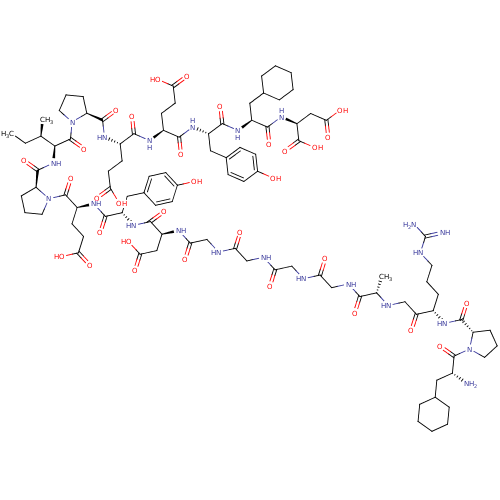
(Arginyl Ketomethylene analogue | CHEMBL407043)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)[C@H](C)NCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C98H144N22O32/c1-4-52(2)83(96(150)120-40-14-21-71(120)92(146)111-63(32-35-79(130)131)85(139)110-62(31-34-78(128)129)86(140)113-67(44-57-25-29-59(122)30-26-57)88(142)114-65(42-55-17-9-6-10-18-55)89(143)116-69(97(151)152)46-82(136)137)117-93(147)72-22-13-39-119(72)95(149)64(33-36-80(132)133)112-87(141)66(43-56-23-27-58(121)28-24-56)115-90(144)68(45-81(134)135)108-77(127)51-106-75(125)49-104-74(124)48-105-76(126)50-107-84(138)53(3)103-47-73(123)61(19-11-37-102-98(100)101)109-91(145)70-20-12-38-118(70)94(148)60(99)41-54-15-7-5-8-16-54/h23-30,52-55,60-72,83,103,121-122H,4-22,31-51,99H2,1-3H3,(H,104,124)(H,105,126)(H,106,125)(H,107,138)(H,108,127)(H,109,145)(H,110,139)(H,111,146)(H,112,141)(H,113,140)(H,114,142)(H,115,144)(H,116,143)(H,117,147)(H,128,129)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,151,152)(H4,100,101,102)/t52-,53+,60-,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,83+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079485
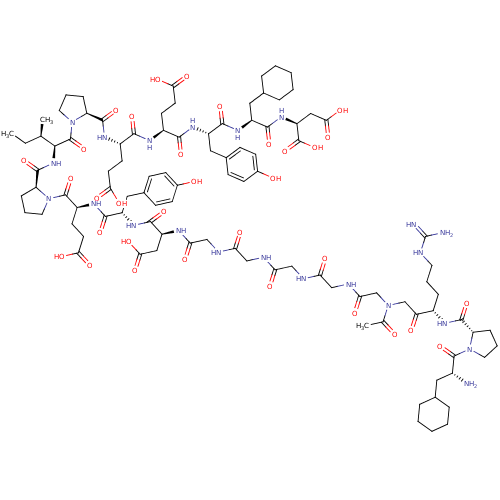
(Arginyl Ketomethylene analogue | CHEMBL414489)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C99H144N22O33/c1-4-53(2)85(97(152)121-40-14-21-72(121)93(148)111-64(32-35-81(133)134)86(141)110-63(31-34-80(131)132)87(142)113-68(44-58-25-29-60(124)30-26-58)89(144)114-66(42-56-17-9-6-10-18-56)90(145)116-70(98(153)154)46-84(139)140)117-94(149)73-22-13-39-120(73)96(151)65(33-36-82(135)136)112-88(143)67(43-57-23-27-59(123)28-24-57)115-91(146)69(45-83(137)138)108-78(129)50-106-76(127)48-104-75(126)47-105-77(128)49-107-79(130)52-118(54(3)122)51-74(125)62(19-11-37-103-99(101)102)109-92(147)71-20-12-38-119(71)95(150)61(100)41-55-15-7-5-8-16-55/h23-30,53,55-56,61-73,85,123-124H,4-22,31-52,100H2,1-3H3,(H,104,126)(H,105,128)(H,106,127)(H,107,130)(H,108,129)(H,109,147)(H,110,141)(H,111,148)(H,112,143)(H,113,142)(H,114,144)(H,115,146)(H,116,145)(H,117,149)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,139,140)(H,153,154)(H4,101,102,103)/t53-,61-,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,73+,85+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079478
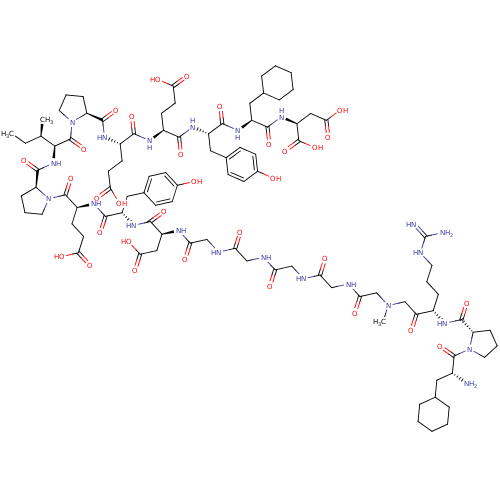
(Arginyl Ketomethylene analogue | CHEMBL414760)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C98H144N22O32/c1-4-53(2)84(96(150)120-40-14-21-71(120)92(146)110-63(32-35-80(131)132)85(139)109-62(31-34-79(129)130)86(140)112-67(44-57-25-29-59(122)30-26-57)88(142)113-65(42-55-17-9-6-10-18-55)89(143)115-69(97(151)152)46-83(137)138)116-93(147)72-22-13-39-119(72)95(149)64(33-36-81(133)134)111-87(141)66(43-56-23-27-58(121)28-24-56)114-90(144)68(45-82(135)136)107-77(127)50-105-75(125)48-103-74(124)47-104-76(126)49-106-78(128)52-117(3)51-73(123)61(19-11-37-102-98(100)101)108-91(145)70-20-12-38-118(70)94(148)60(99)41-54-15-7-5-8-16-54/h23-30,53-55,60-72,84,121-122H,4-22,31-52,99H2,1-3H3,(H,103,124)(H,104,126)(H,105,125)(H,106,128)(H,107,127)(H,108,145)(H,109,139)(H,110,146)(H,111,141)(H,112,140)(H,113,142)(H,114,144)(H,115,143)(H,116,147)(H,129,130)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,151,152)(H4,100,101,102)/t53-,60-,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,84+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50300196

(10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....)Show SMILES Oc1ccc(cc1)C1c2c[nH]c3nccc(-c4ccccc4NC1=O)c23 Show InChI InChI=1S/C21H15N3O2/c25-13-7-5-12(6-8-13)18-16-11-23-20-19(16)15(9-10-22-20)14-3-1-2-4-17(14)24-21(18)26/h1-11,18,25H,(H,22,23)(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK3 |
J Med Chem 52: 7938-41 (2009)
Checked by Author
Article DOI: 10.1021/jm901383u
BindingDB Entry DOI: 10.7270/Q2GF0TK0 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50000558
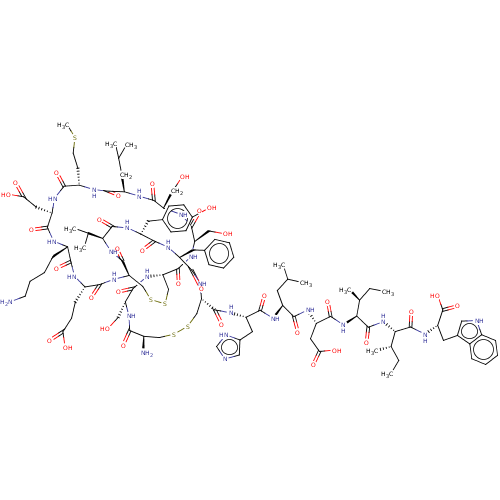
(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079491
(Arginyl Ketomethylene analogue | CHEMBL412457)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CSCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C95H134N22O32S/c1-5-51(4)81(93(147)117-38-14-21-68(117)91(145)110-60(29-34-78(130)131)83(137)107-59(28-33-77(128)129)84(138)113-65(42-54-22-24-55(118)25-23-54)88(142)112-63(39-50(2)3)86(140)111-62(94(148)149)26-31-70(97)120)115-85(139)61(30-35-79(132)133)108-82(136)58(27-32-76(126)127)109-87(141)64(41-53-17-10-7-11-18-53)114-89(143)66(43-80(134)135)105-74(124)47-103-72(122)45-101-71(121)44-102-73(123)46-104-75(125)49-150-48-69(119)57(19-12-36-100-95(98)99)106-90(144)67-20-13-37-116(67)92(146)56(96)40-52-15-8-6-9-16-52/h6-11,15-18,22-25,50-51,56-68,81,118H,5,12-14,19-21,26-49,96H2,1-4H3,(H2,97,120)(H,101,121)(H,102,123)(H,103,122)(H,104,125)(H,105,124)(H,106,144)(H,107,137)(H,108,136)(H,109,141)(H,110,145)(H,111,140)(H,112,142)(H,113,138)(H,114,143)(H,115,139)(H,126,127)(H,128,129)(H,130,131)(H,132,133)(H,134,135)(H,148,149)(H4,98,99,100)/t51-,56-,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,81+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50351401

(CHEMBL1819091)Show SMILES CC[C@@]1(N)CCCN(C1)c1cc2n(C)c(=O)n(C)c(=O)c2n1Cc1cc(F)ccc1C#N |r| Show InChI InChI=1S/C23H27FN6O2/c1-4-23(26)8-5-9-29(14-23)19-11-18-20(21(31)28(3)22(32)27(18)2)30(19)13-16-10-17(24)7-6-15(16)12-25/h6-7,10-11H,4-5,8-9,13-14,26H2,1-3H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis |
Bioorg Med Chem 19: 5490-9 (2011)
Article DOI: 10.1016/j.bmc.2011.07.042
BindingDB Entry DOI: 10.7270/Q2T72HT6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50351399
(CHEMBL1819089)Show SMILES Cn1c2cc(N3CCC[C@@H](N)C3)n(Cc3cc(F)ccc3C#N)c2c(=O)n(C)c1=O |r| Show InChI InChI=1S/C21H23FN6O2/c1-25-17-9-18(27-7-3-4-16(24)12-27)28(19(17)20(29)26(2)21(25)30)11-14-8-15(22)6-5-13(14)10-23/h5-6,8-9,16H,3-4,7,11-12,24H2,1-2H3/t16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis |
Bioorg Med Chem 19: 5490-9 (2011)
Article DOI: 10.1016/j.bmc.2011.07.042
BindingDB Entry DOI: 10.7270/Q2T72HT6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079480
(Arginyl Ketomethylene analogue | CHEMBL415375)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C98H138N22O32/c1-4-53(2)84(96(150)120-40-14-21-71(120)92(146)110-63(32-35-80(131)132)85(139)109-62(31-34-79(129)130)86(140)112-67(44-57-25-29-59(122)30-26-57)88(142)113-65(42-55-17-9-6-10-18-55)89(143)115-69(97(151)152)46-83(137)138)116-93(147)72-22-13-39-119(72)95(149)64(33-36-81(133)134)111-87(141)66(43-56-23-27-58(121)28-24-56)114-90(144)68(45-82(135)136)107-77(127)50-105-75(125)48-103-74(124)47-104-76(126)49-106-78(128)52-117(3)51-73(123)61(19-11-37-102-98(100)101)108-91(145)70-20-12-38-118(70)94(148)60(99)41-54-15-7-5-8-16-54/h5,7-8,15-16,23-30,53,55,60-72,84,121-122H,4,6,9-14,17-22,31-52,99H2,1-3H3,(H,103,124)(H,104,126)(H,105,125)(H,106,128)(H,107,127)(H,108,145)(H,109,139)(H,110,146)(H,111,141)(H,112,140)(H,113,142)(H,114,144)(H,115,143)(H,116,147)(H,129,130)(H,131,132)(H,133,134)(H,135,136)(H,137,138)(H,151,152)(H4,100,101,102)/t53-,60-,61+,62+,63+,64+,65+,66+,67+,68+,69+,70+,71+,72+,84+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET1 binding to the Endothelin A receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Sus scrofa) | BDBM50287890
(CHEMBL427778 | Suc-Glu-Ala-Val-Tyr-Phe-Ala-His-Leu...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](CCC(O)=O)NC(=O)CCC(O)=O)C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C77H105N15O21/c1-11-41(7)64(75(110)89-58(77(112)113)33-47-36-79-51-21-17-16-20-50(47)51)92-76(111)65(42(8)12-2)91-73(108)57(35-62(99)100)87-70(105)53(30-39(3)4)85-72(107)56(34-48-37-78-38-80-48)84-66(101)43(9)82-69(104)54(31-45-18-14-13-15-19-45)86-71(106)55(32-46-22-24-49(93)25-23-46)88-74(109)63(40(5)6)90-67(102)44(10)81-68(103)52(26-28-60(95)96)83-59(94)27-29-61(97)98/h13-25,36-44,52-58,63-65,79,93H,11-12,26-35H2,1-10H3,(H,78,80)(H,81,103)(H,82,104)(H,83,94)(H,84,101)(H,85,107)(H,86,106)(H,87,105)(H,88,109)(H,89,110)(H,90,102)(H,91,108)(H,92,111)(H,95,96)(H,97,98)(H,99,100)(H,112,113)/t41-,42-,43-,44-,52-,53-,54-,55-,56-,57-,58-,63-,64-,65-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit [125I]ET-3 binding to the Endothelin B receptor from porcine lung membranes was evaluated |
Bioorg Med Chem Lett 6: 2323-2328 (1996)
Article DOI: 10.1016/0960-894X(96)00421-0
BindingDB Entry DOI: 10.7270/Q21J9B8K |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50074627
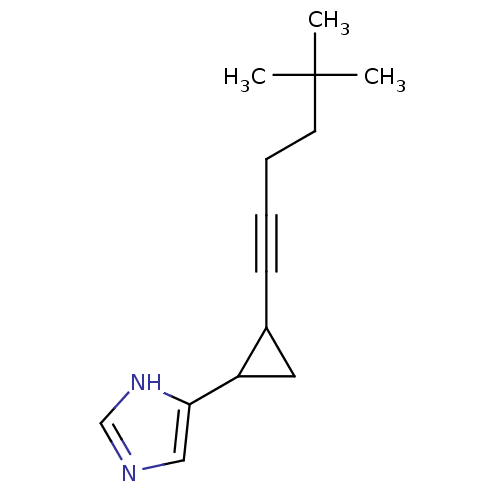
(4-[2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl]-1H-imi...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158590
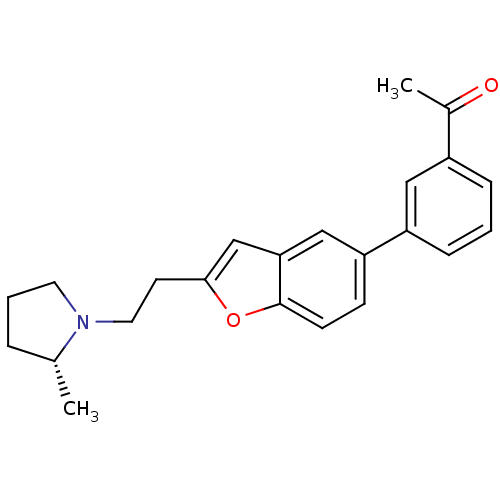
(1-(3-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-b...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cccc(c1)C(C)=O Show InChI InChI=1S/C23H25NO2/c1-16-5-4-11-24(16)12-10-22-15-21-14-20(8-9-23(21)26-22)19-7-3-6-18(13-19)17(2)25/h3,6-9,13-16H,4-5,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine receptor H3
(Dog) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079490
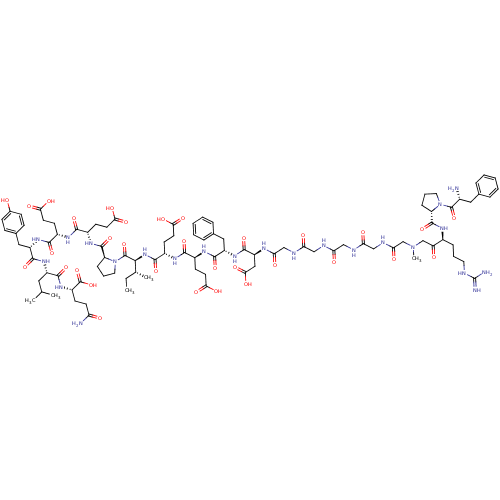
(Arginyl Ketomethylene analogue | CHEMBL437999)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CN(C)CC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C96H137N23O32/c1-6-52(4)82(94(149)119-39-15-22-69(119)92(147)111-61(30-35-79(132)133)84(139)108-60(29-34-78(130)131)85(140)114-66(43-55-23-25-56(120)26-24-55)89(144)113-64(40-51(2)3)87(142)112-63(95(150)151)27-32-71(98)122)116-86(141)62(31-36-80(134)135)109-83(138)59(28-33-77(128)129)110-88(143)65(42-54-18-11-8-12-19-54)115-90(145)67(44-81(136)137)106-75(126)48-104-73(124)46-102-72(123)45-103-74(125)47-105-76(127)50-117(5)49-70(121)58(20-13-37-101-96(99)100)107-91(146)68-21-14-38-118(68)93(148)57(97)41-53-16-9-7-10-17-53/h7-12,16-19,23-26,51-52,57-69,82,120H,6,13-15,20-22,27-50,97H2,1-5H3,(H2,98,122)(H,102,123)(H,103,125)(H,104,124)(H,105,127)(H,106,126)(H,107,146)(H,108,139)(H,109,138)(H,110,143)(H,111,147)(H,112,142)(H,113,144)(H,114,140)(H,115,145)(H,116,141)(H,128,129)(H,130,131)(H,132,133)(H,134,135)(H,136,137)(H,150,151)(H4,99,100,101)/t52-,57-,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,69+,82+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50079488
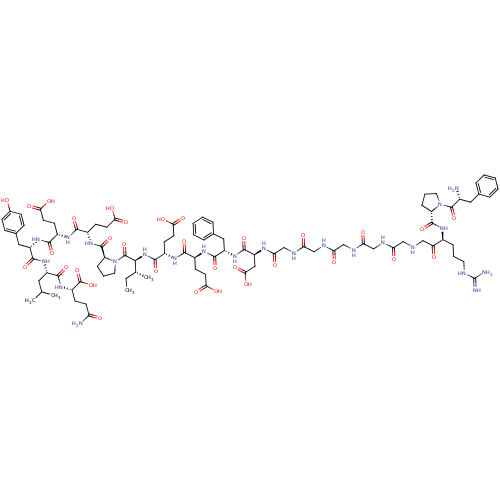
(Arginyl Ketomethylene analogue | CHEMBL414974)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)CNC(=O)CNC(=O)CNC(=O)CNCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C95H135N23O32/c1-5-51(4)81(93(148)118-38-14-21-68(118)91(146)111-60(29-34-78(131)132)83(138)108-59(28-33-77(129)130)84(139)114-65(42-54-22-24-55(119)25-23-54)88(143)113-63(39-50(2)3)86(141)112-62(94(149)150)26-31-70(97)121)116-85(140)61(30-35-79(133)134)109-82(137)58(27-32-76(127)128)110-87(142)64(41-53-17-10-7-11-18-53)115-89(144)66(43-80(135)136)106-75(126)49-105-74(125)48-104-73(124)47-103-72(123)46-102-71(122)45-100-44-69(120)57(19-12-36-101-95(98)99)107-90(145)67-20-13-37-117(67)92(147)56(96)40-52-15-8-6-9-16-52/h6-11,15-18,22-25,50-51,56-68,81,100,119H,5,12-14,19-21,26-49,96H2,1-4H3,(H2,97,121)(H,102,122)(H,103,123)(H,104,124)(H,105,125)(H,106,126)(H,107,145)(H,108,138)(H,109,137)(H,110,142)(H,111,146)(H,112,141)(H,113,143)(H,114,139)(H,115,144)(H,116,140)(H,127,128)(H,129,130)(H,131,132)(H,133,134)(H,135,136)(H,149,150)(H4,98,99,101)/t51-,56-,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,68+,81+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council Canada
Curated by ChEMBL
| Assay Description
Inhibitory constant against thrombin |
J Med Chem 42: 3109-15 (1999)
Article DOI: 10.1021/jm9807297
BindingDB Entry DOI: 10.7270/Q2VQ31WT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158588
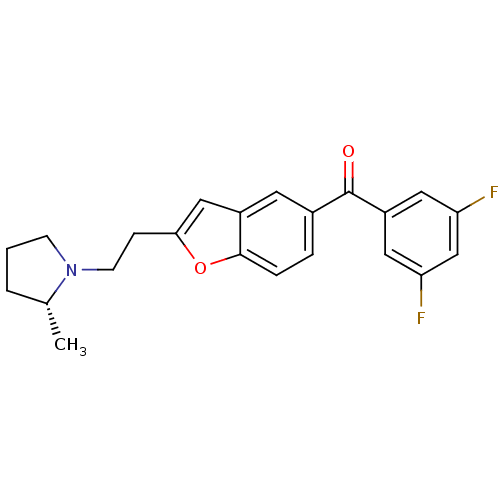
((3,5-Difluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolid...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1cc(F)cc(F)c1 Show InChI InChI=1S/C22H21F2NO2/c1-14-3-2-7-25(14)8-6-20-12-16-9-15(4-5-21(16)27-20)22(26)17-10-18(23)13-19(24)11-17/h4-5,9-14H,2-3,6-8H2,1H3/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50096629

(3-(4-Amino-cyclohexyl)-2-oxo-3-[((6S,8aS)-4-oxo-2-...)Show SMILES [H][C@@]12CC[C@H](N1C(=O)CN(C2)S(=O)(=O)Cc1ccccc1)C(=O)NC([C@H]1CC[C@H](N)CC1)C(=O)C(O)=O |wU:4.23,28.31,1.0,wD:25.27,(5.13,2.97,;5.13,1.42,;6.6,1.89,;7.51,.65,;6.6,-.59,;5.13,-.12,;3.8,-.9,;3.8,-2.44,;2.46,-.12,;2.46,1.42,;3.8,2.19,;1.11,2.19,;.34,.84,;1.88,3.55,;-.23,2.96,;-.25,4.5,;-1.58,5.28,;-1.58,6.83,;-.25,7.6,;1.08,6.83,;1.08,5.28,;7.07,-2.07,;6.04,-3.21,;8.57,-2.39,;9.05,-3.87,;8,-5.01,;6.51,-4.68,;5.47,-5.81,;5.95,-7.27,;4.92,-8.43,;7.44,-7.61,;8.47,-6.48,;10.55,-4.19,;11.58,-3.06,;11.02,-5.67,;12.42,-6.3,;9.87,-6.69,)| Show InChI InChI=1S/C24H32N4O7S/c25-17-8-6-16(7-9-17)21(22(30)24(32)33)26-23(31)19-11-10-18-12-27(13-20(29)28(18)19)36(34,35)14-15-4-2-1-3-5-15/h1-5,16-19,21H,6-14,25H2,(H,26,31)(H,32,33)/t16-,17-,18-,19-,21?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc.
Curated by ChEMBL
| Assay Description
In vitro activity of the compound against human alpha thrombin was determined |
Bioorg Med Chem Lett 11: 287-90 (2001)
BindingDB Entry DOI: 10.7270/Q2P55MS6 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158596
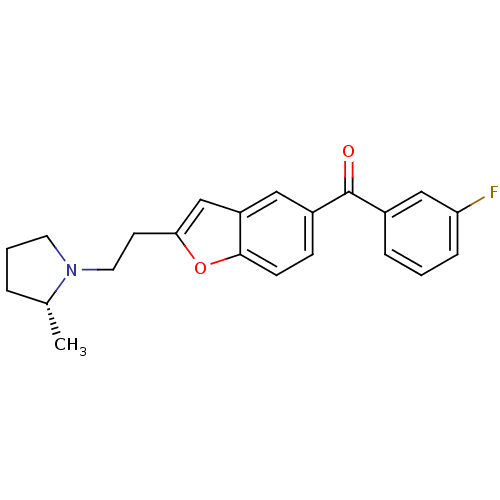
((3-Fluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1cccc(F)c1 Show InChI InChI=1S/C22H22FNO2/c1-15-4-3-10-24(15)11-9-20-14-18-12-17(7-8-21(18)26-20)22(25)16-5-2-6-19(23)13-16/h2,5-8,12-15H,3-4,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072528
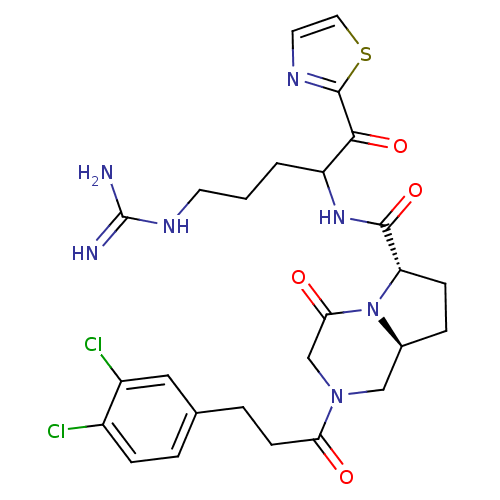
((6S,8aS)-2-[3-(3,4-Dichloro-phenyl)-propionyl]-4-o...)Show SMILES NC(=N)NCCCC(NC(=O)[C@@H]1CC[C@H]2CN(CC(=O)N12)C(=O)CCc1ccc(Cl)c(Cl)c1)C(=O)c1nccs1 Show InChI InChI=1S/C26H31Cl2N7O4S/c27-17-6-3-15(12-18(17)28)4-8-21(36)34-13-16-5-7-20(35(16)22(37)14-34)24(39)33-19(2-1-9-32-26(29)30)23(38)25-31-10-11-40-25/h3,6,10-12,16,19-20H,1-2,4-5,7-9,13-14H2,(H,33,39)(H4,29,30,32)/t16-,19?,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Therapeutic Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound against thrombin |
Bioorg Med Chem Lett 8: 3193-8 (1999)
BindingDB Entry DOI: 10.7270/Q2HT2NGF |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092959
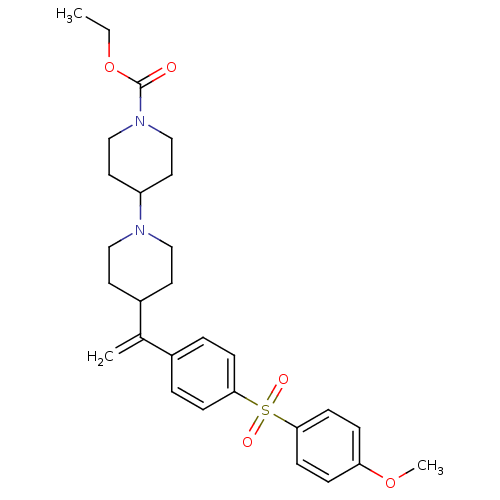
(4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C(=C)c1ccc(cc1)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C28H36N2O5S/c1-4-35-28(31)30-19-15-24(16-20-30)29-17-13-23(14-18-29)21(2)22-5-9-26(10-6-22)36(32,33)27-11-7-25(34-3)8-12-27/h5-12,23-24H,2,4,13-20H2,1,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 17: 2260-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.058
BindingDB Entry DOI: 10.7270/Q2668H0S |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50522827
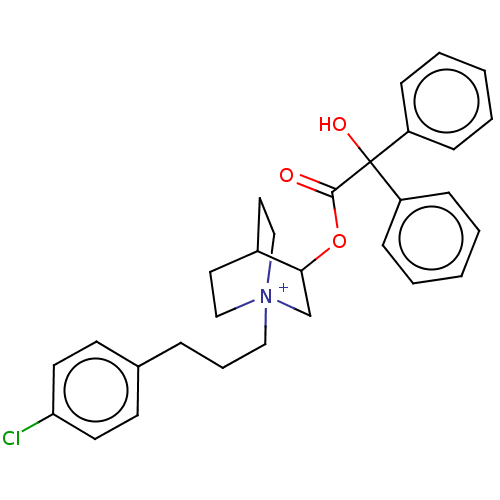
(CHEMBL4519930)Show SMILES OC(C(=O)OC1C[N+]2(CCCc3ccc(Cl)cc3)CCC1CC2)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C30H33ClNO3.BrH/c31-27-15-13-23(14-16-27)8-7-19-32-20-17-24(18-21-32)28(22-32)35-29(33)30(34,25-9-3-1-4-10-25)26-11-5-2-6-12-26;/h1-6,9-16,24,28,34H,7-8,17-22H2;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method |
Bioorg Med Chem 27: 3339-3346 (2019)
Article DOI: 10.1016/j.bmc.2019.06.016
BindingDB Entry DOI: 10.7270/Q2DR2ZXC |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50074629

(4-[(1R,2R)-2-(5,5-Dimethyl-hex-1-ynyl)-cyclopropyl...)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 313: 165-75 (2005)
Article DOI: 10.1124/jpet.104.078303
BindingDB Entry DOI: 10.7270/Q2SN07JC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200642

((S)-4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphtha...)Show SMILES C[C@H]1CCCN1CCc1ccc2cc(ccc2c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C24H24N2/c1-18-3-2-13-26(18)14-12-19-4-9-24-16-23(11-10-22(24)15-19)21-7-5-20(17-25)6-8-21/h4-11,15-16,18H,2-3,12-14H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells |
Bioorg Med Chem Lett 17: 1443-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.073
BindingDB Entry DOI: 10.7270/Q2416WR3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50096623

(3-(4-Amino-cyclohexyl)-2-oxo-3-[(4-oxo-2-phenylmet...)Show SMILES [H][C@@]12CC[C@H](N1C(=O)CN(C2)S(=O)(=O)Cc1ccccc1)C(=O)NC([C@H]1CC[C@H](N)CC1)C(=O)C(=O)OC |wU:4.23,28.31,1.0,wD:25.27,(3.92,9.02,;3.92,7.48,;5.38,7.95,;6.28,6.69,;5.38,5.45,;3.92,5.93,;2.59,5.15,;2.59,3.6,;1.25,5.93,;1.25,7.48,;2.59,8.23,;-.09,8.23,;-.87,6.9,;.66,9.6,;-1.43,9.02,;-1.45,10.56,;-2.79,11.34,;-2.79,12.91,;-1.45,13.66,;-.11,12.91,;-.11,11.34,;5.84,3.98,;4.82,2.84,;7.33,3.65,;7.81,2.16,;6.77,1.02,;5.28,1.36,;4.24,.22,;4.72,-1.24,;3.7,-2.39,;6.21,-1.58,;7.25,-.42,;9.3,1.85,;10.34,2.98,;9.81,.32,;8.66,-.81,;11.36,-.02,;11.85,-1.53,)| Show InChI InChI=1S/C25H34N4O7S/c1-36-25(33)23(31)22(17-7-9-18(26)10-8-17)27-24(32)20-12-11-19-13-28(14-21(30)29(19)20)37(34,35)15-16-5-3-2-4-6-16/h2-6,17-20,22H,7-15,26H2,1H3,(H,27,32)/t17-,18-,19-,20-,22?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BioChem Pharma Inc.
Curated by ChEMBL
| Assay Description
In vitro activity of the compound against human alpha thrombin was determined |
Bioorg Med Chem Lett 11: 287-90 (2001)
BindingDB Entry DOI: 10.7270/Q2P55MS6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50004744

(CHEMBL2370453 | Hirudin analogue)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)CCCCC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O |wU:132.137,4.4,65.73,49.54,26.34,96.105,17.22,37.38,166.171,81.88,145.149,174.179,wD:107.108,75.79,57.62,8.13,154.158,114.125,136.140,2.2,(23.18,-7.9,;24.72,-8.01,;25.58,-6.74,;24.91,-5.35,;27.12,-6.85,;27.79,-8.23,;26.93,-9.51,;25.39,-9.4,;27.6,-10.89,;29.14,-11.01,;30,-9.73,;31.54,-9.84,;32.21,-11.23,;32.4,-8.57,;26.74,-12.17,;27.41,-13.55,;28.94,-13.67,;26.54,-14.83,;25.01,-14.72,;24.14,-15.99,;22.61,-15.88,;21.94,-14.5,;21.74,-17.16,;27.22,-16.22,;26.35,-17.49,;24.82,-17.38,;27.02,-18.88,;26.16,-20.15,;26.83,-21.54,;25.97,-22.81,;26.64,-24.2,;28.18,-24.31,;29.04,-23.03,;28.37,-21.65,;28.56,-18.99,;29.42,-17.71,;28.75,-16.33,;30.96,-17.82,;31.63,-19.21,;30.77,-20.48,;31.44,-21.87,;29.23,-20.37,;31.82,-16.55,;33.36,-16.66,;34.03,-18.04,;34.22,-15.38,;35.76,-15.49,;36.62,-14.22,;35.95,-12.83,;38.16,-14.33,;38.83,-15.72,;40.37,-15.83,;41.04,-17.21,;41.82,-15.3,;39.02,-13.06,;40.56,-13.17,;41.23,-14.55,;41.42,-11.89,;40.75,-10.51,;41.62,-9.23,;42.92,-8.42,;40.94,-7.85,;42.96,-12,;43.82,-10.73,;43.15,-9.34,;45.36,-10.84,;46.03,-12.22,;47.57,-12.34,;48.38,-13.64,;49.88,-13.27,;49.99,-11.74,;48.56,-11.16,;46.22,-9.56,;47.76,-9.67,;49.12,-10.4,;48.62,-8.4,;47.95,-7.01,;46.42,-6.9,;50.16,-8.51,;51.02,-7.24,;50.35,-5.85,;52.56,-7.35,;53.23,-8.73,;54.77,-8.84,;55.44,-10.23,;54.58,-11.5,;56.98,-10.34,;53.42,-6.07,;54.96,-6.18,;55.63,-7.57,;55.82,-4.91,;57.36,-5.02,;58.22,-3.74,;59.76,-3.86,;60.62,-2.58,;59.95,-1.19,;62.16,-2.69,;62.83,-4.08,;61.97,-5.35,;62.64,-6.74,;64.18,-6.85,;64.85,-8.23,;63.98,-9.51,;66.38,-8.34,;63.02,-1.42,;64.56,-1.53,;65.23,-2.91,;65.42,-.25,;64.9,1.2,;66.12,2.14,;67.39,1.28,;66.96,-.2,;67.91,-1.42,;67.33,-2.84,;69.43,-1.21,;70.01,.22,;71.54,.43,;72.48,-.79,;74.01,-.57,;74.59,.85,;73.64,2.07,;72.12,1.86,;70.38,-2.42,;71.9,-2.21,;72.85,-3.43,;73.21,-1.4,;27.98,-5.57,;29.52,-5.68,;27.31,-4.19,;25.8,-3.92,;25.58,-2.39,;26.97,-1.72,;28.04,-2.83,;29.56,-2.62,;30.51,-3.83,;30.14,-1.19,;31.67,-.98,;32.61,-2.2,;32.03,-3.62,;32.98,-4.84,;32.4,-6.27,;34.5,-4.63,;32.25,.45,;31.3,1.66,;33.77,.66,;34.35,2.08,;33.41,3.3,;31.88,3.09,;30.94,4.3,;31.52,5.73,;29.41,4.09,;35.88,2.29,;36.83,1.08,;36.46,3.72,;37.99,3.93,;38.93,2.72,;40.46,2.93,;41.4,1.71,;42.93,1.92,;43.51,3.35,;45.03,3.56,;42.56,4.56,;41.04,4.35,;38.57,5.36,;37.62,6.57,;40.09,5.57,;40.67,7,;39.73,8.21,;40.31,9.64,;39.36,10.85,;41.83,9.85,;42.2,7.21,;43.14,5.99,;42.78,8.63,;44.3,8.84,;44.88,10.27,;46.41,10.48,;46.99,11.91,;46.04,13.12,;48.51,12.12,;45.25,7.63,;44.67,6.2,;46.77,7.84,)| Show InChI InChI=1S/C115H162N28O40/c1-6-59(4)96(113(181)143-45-17-24-83(143)111(179)132-70(35-41-92(157)158)100(168)129-69(34-40-91(155)156)101(169)135-75(48-63-27-29-65(146)30-28-63)105(173)134-73(46-58(2)3)103(171)133-72(114(182)183)32-38-86(117)149)141-102(170)71(36-42-93(159)160)130-99(167)68(33-39-90(153)154)131-104(172)74(47-61-18-9-7-10-19-61)136-108(176)79(53-95(163)164)127-89(152)55-123-97(165)78(52-94(161)162)139-107(175)77(51-87(118)150)138-106(174)76(50-64-54-121-57-124-64)137-109(177)81(56-144)140-98(166)67(31-37-85(116)148)126-88(151)26-14-13-25-84(147)66(22-15-43-122-115(119)120)128-110(178)82-23-16-44-142(82)112(180)80(125-60(5)145)49-62-20-11-8-12-21-62/h7-12,18-21,27-30,54,57-59,66-83,96,144,146H,6,13-17,22-26,31-53,55-56H2,1-5H3,(H2,116,148)(H2,117,149)(H2,118,150)(H,121,124)(H,123,165)(H,125,145)(H,126,151)(H,127,152)(H,128,178)(H,129,168)(H,130,167)(H,131,172)(H,132,179)(H,133,171)(H,134,173)(H,135,169)(H,136,176)(H,137,177)(H,138,174)(H,139,175)(H,140,166)(H,141,170)(H,153,154)(H,155,156)(H,157,158)(H,159,160)(H,161,162)(H,163,164)(H,182,183)(H4,119,120,122)/t59-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,96-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada
Curated by ChEMBL
| Assay Description
Compound was evaluated for their ability to inhibit the alpha-thrombin-mediated hydrolysis of the fluorescent substrate Tos-Gly-Pro-Arg-Amc |
J Med Chem 35: 3331-41 (1992)
BindingDB Entry DOI: 10.7270/Q2FX78D9 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092961
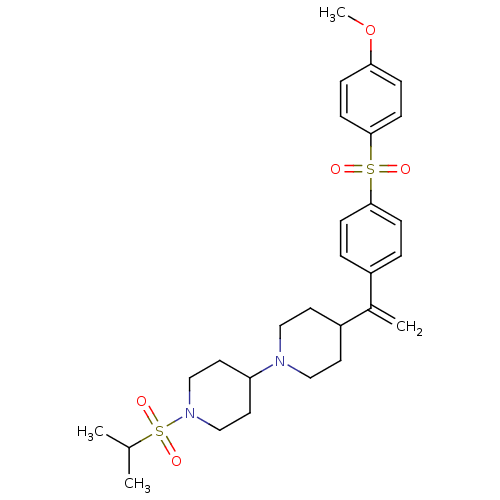
(1-(isopropylsulfonyl)-4-(4-(1-(4-(4-methoxyphenyls...)Show SMILES COc1ccc(cc1)S(=O)(=O)c1ccc(cc1)C(=C)C1CCN(CC1)C1CCN(CC1)S(=O)(=O)C(C)C Show InChI InChI=1S/C28H38N2O5S2/c1-21(2)37(33,34)30-19-15-25(16-20-30)29-17-13-24(14-18-29)22(3)23-5-9-27(10-6-23)36(31,32)28-11-7-26(35-4)8-12-28/h5-12,21,24-25H,3,13-20H2,1-2,4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Jiao Tong University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 17: 2260-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.058
BindingDB Entry DOI: 10.7270/Q2668H0S |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50522833
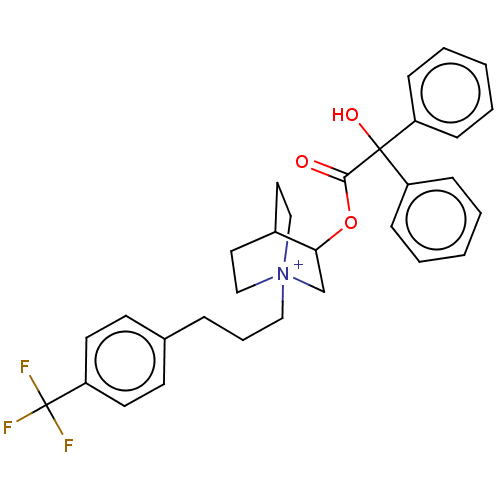
(CHEMBL4516342)Show SMILES OC(C(=O)OC1C[N+]2(CCCc3ccc(cc3)C(F)(F)F)CCC1CC2)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C31H33F3NO3.BrH/c32-31(33,34)27-15-13-23(14-16-27)8-7-19-35-20-17-24(18-21-35)28(22-35)38-29(36)30(37,25-9-3-1-4-10-25)26-11-5-2-6-12-26;/h1-6,9-16,24,28,37H,7-8,17-22H2;1H/q+1;/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M2R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method |
Bioorg Med Chem 27: 3339-3346 (2019)
Article DOI: 10.1016/j.bmc.2019.06.016
BindingDB Entry DOI: 10.7270/Q2DR2ZXC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200641
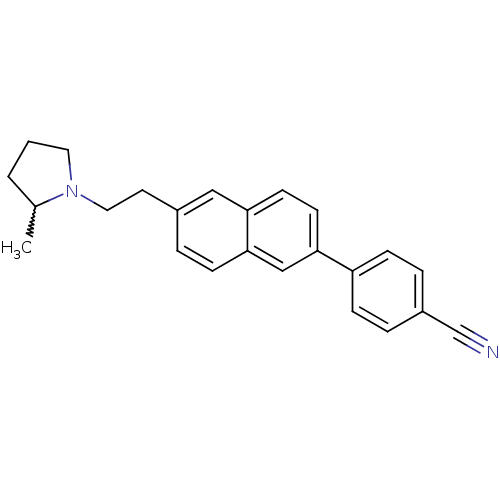
(4-(6-(2-(2-methylpyrrolidin-1-yl)ethyl)naphthalen-...)Show SMILES CC1CCCN1CCc1ccc2cc(ccc2c1)-c1ccc(cc1)C#N |w:1.0| Show InChI InChI=1S/C24H24N2/c1-18-3-2-13-26(18)14-12-19-4-9-24-16-23(11-10-22(24)15-19)21-7-5-20(17-25)6-8-21/h4-11,15-16,18H,2-3,12-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells |
Bioorg Med Chem Lett 17: 1443-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.073
BindingDB Entry DOI: 10.7270/Q2416WR3 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158609
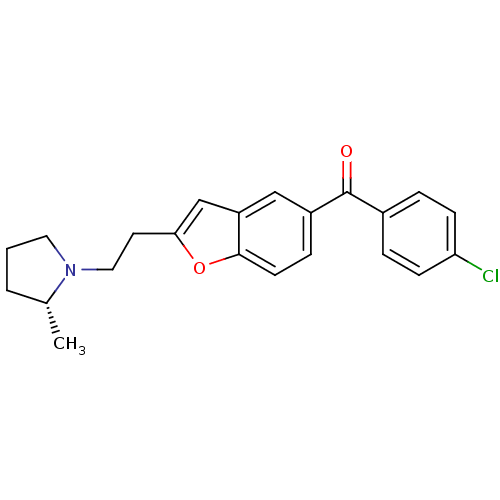
((4-Chloro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C22H22ClNO2/c1-15-3-2-11-24(15)12-10-20-14-18-13-17(6-9-21(18)26-20)22(25)16-4-7-19(23)8-5-16/h4-9,13-15H,2-3,10-12H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50287476
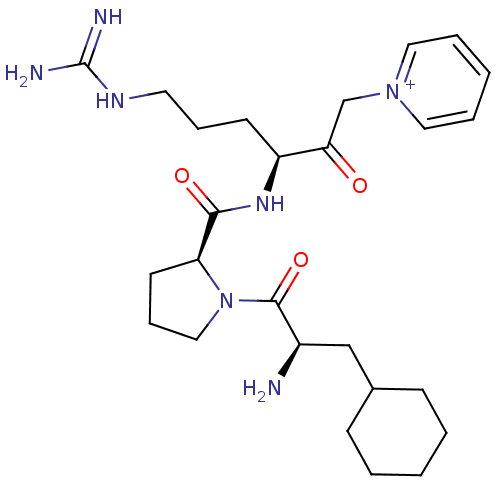
(1-((S)-3-{[(S)-1-((R)-2-Amino-3-cyclohexyl-propion...)Show SMILES N[C@H](CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)C[n+]1ccccc1 Show InChI InChI=1S/C26H41N7O3/c27-20(17-19-9-3-1-4-10-19)25(36)33-16-8-12-22(33)24(35)31-21(11-7-13-30-26(28)29)23(34)18-32-14-5-2-6-15-32/h2,5-6,14-15,19-22H,1,3-4,7-13,16-18,27H2,(H4-,28,29,30,31,35)/p+1/t20-,21+,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was measured for the inhibition of alpha-human thrombin by amidolytic assay |
Bioorg Med Chem Lett 6: 1677-1682 (1996)
Article DOI: 10.1016/0960-894X(96)00291-0
BindingDB Entry DOI: 10.7270/Q2CF9Q2H |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158607

((3-Chloro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C22H22ClNO2/c1-15-4-3-10-24(15)11-9-20-14-18-12-17(7-8-21(18)26-20)22(25)16-5-2-6-19(23)13-16/h2,5-8,12-15H,3-4,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50222968

(Cipralisant | GT-2331)Show InChI InChI=1S/C14H20N2/c1-14(2,3)7-5-4-6-11-8-12(11)13-9-15-10-16-13/h9-12H,5,7-8H2,1-3H3,(H,15,16)/t11-,12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards rat cortical H3 receptor |
Bioorg Med Chem Lett 13: 1325-8 (2003)
BindingDB Entry DOI: 10.7270/Q2HM59NM |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50239981
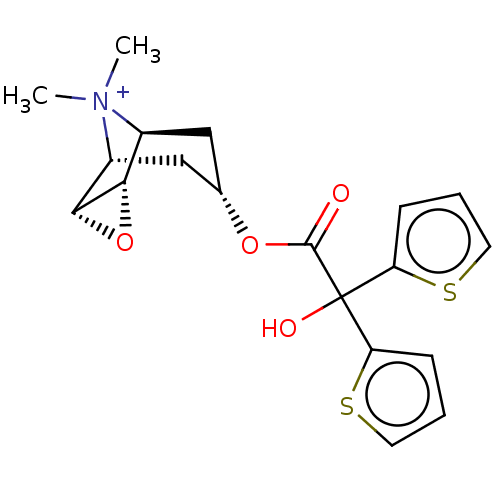
(Braltus | Spiriva | Spiriva Respimat | Tiotropium)Show SMILES [H][C@]12O[C@@]1([H])[C@]1([H])C[C@@]([H])(C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r,TLB:2:1:13:8.10.7,2:3:13:8.10.7,16:8:13:1.3| Show InChI InChI=1S/C19H22NO4S2/c1-20(2)12-9-11(10-13(20)17-16(12)24-17)23-18(21)19(22,14-5-3-7-25-14)15-6-4-8-26-15/h3-8,11-13,16-17,22H,9-10H2,1-2H3/q+1/t11-,12-,13+,16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Advanced Industrial Science and Technology (AIST)
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from human M3R expressed in CHOK1 cell membranes incubated for 2 hrs by microbeta scintillation counting method |
Bioorg Med Chem 27: 3339-3346 (2019)
Article DOI: 10.1016/j.bmc.2019.06.016
BindingDB Entry DOI: 10.7270/Q2DR2ZXC |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158599
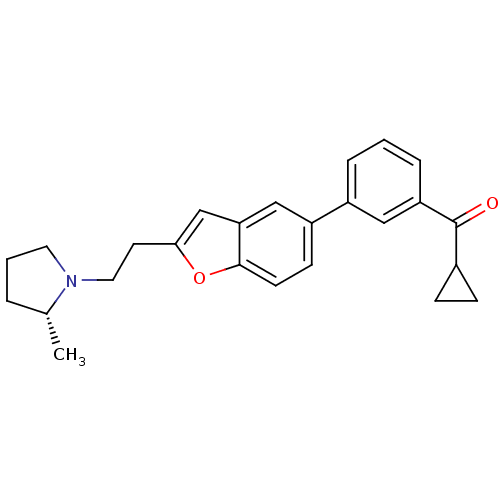
(CHEMBL369502 | Cyclopropyl-(3-{2-[2-((R)-2-methyl-...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)-c1cccc(c1)C(=O)C1CC1 Show InChI InChI=1S/C25H27NO2/c1-17-4-3-12-26(17)13-11-23-16-22-15-20(9-10-24(22)28-23)19-5-2-6-21(14-19)25(27)18-7-8-18/h2,5-6,9-10,14-18H,3-4,7-8,11-13H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158592

(CHEMBL368699 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(C)cc1 Show InChI InChI=1S/C23H25NO2/c1-16-5-7-18(8-6-16)23(25)19-9-10-22-20(14-19)15-21(26-22)11-13-24-12-3-4-17(24)2/h5-10,14-15,17H,3-4,11-13H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27210

((2R)-N,3-dimethyl-2-(methylamino)-N-[(1R,2S,5S,6S,...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@H](NC)C(C)C)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-18(2)26(30-5)27(33)32(7)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(6)19(3)23(29)10-11-25(22)29/h8,18-19,21-26,30H,9-17H2,1-7H3/t19-,21-,22+,23+,24-,25-,26+,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-alpha methyl histamine from human cloned histamine H3 receptor expressed in C6 cells |
J Med Chem 52: 4640-9 (2009)
Article DOI: 10.1021/jm900480x
BindingDB Entry DOI: 10.7270/Q2Z31ZNT |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158603

((4-Fluoro-3-methyl-phenyl)-{2-[2-((R)-2-methyl-pyr...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(F)c(C)c1 Show InChI InChI=1S/C23H24FNO2/c1-15-12-17(5-7-21(15)24)23(26)18-6-8-22-19(13-18)14-20(27-22)9-11-25-10-3-4-16(25)2/h5-8,12-14,16H,3-4,9-11H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27208
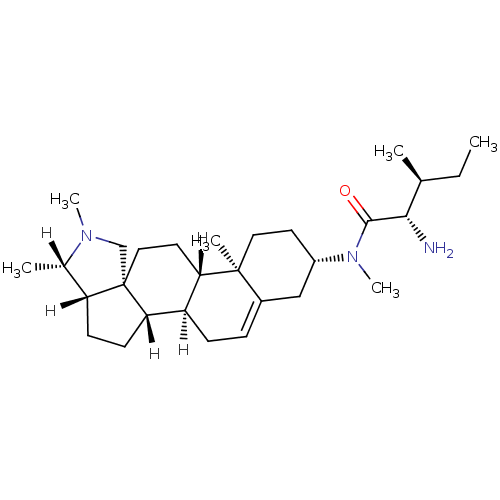
((2S,3S)-2-amino-N,3-dimethyl-N-[(1R,2S,5S,6S,9R,12...)Show SMILES [H][C@@]1(C)N(C)C[C@]23CC[C@@]4([H])[C@@]([H])(CC=C5C[C@H](CC[C@]45C)N(C)C(=O)[C@@H](N)[C@@H](C)CC)[C@]2([H])CC[C@]13[H] |r,t:14| Show InChI InChI=1S/C29H49N3O/c1-7-18(2)26(30)27(33)32(6)21-12-14-28(4)20(16-21)8-9-22-24(28)13-15-29-17-31(5)19(3)23(29)10-11-25(22)29/h8,18-19,21-26H,7,9-17,30H2,1-6H3/t18-,19-,21-,22+,23+,24-,25-,26-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Abbott Laboratories
| Assay Description
Competition radioligand binding assays were performed with increasing concentrations of test compound in the presence of [3H]ligand. All binding reac... |
J Med Chem 51: 5423-30 (2008)
Article DOI: 10.1021/jm8003625
BindingDB Entry DOI: 10.7270/Q21G0JK0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50158605
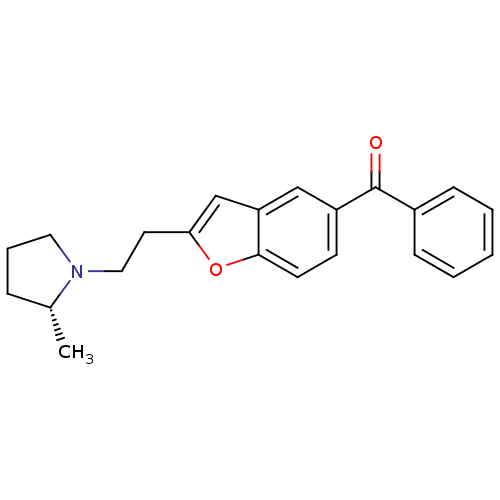
(CHEMBL362662 | {2-[2-((R)-2-Methyl-pyrrolidin-1-yl...)Show SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccccc1 Show InChI InChI=1S/C22H23NO2/c1-16-6-5-12-23(16)13-11-20-15-19-14-18(9-10-21(19)25-20)22(24)17-7-3-2-4-8-17/h2-4,7-10,14-16H,5-6,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor |
J Med Chem 48: 38-55 (2005)
Article DOI: 10.1021/jm040118g
BindingDB Entry DOI: 10.7270/Q2571CST |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50397757
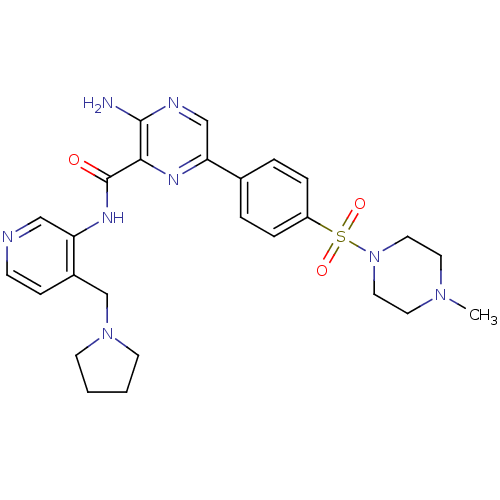
(CHEMBL2177173)Show SMILES CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1CN1CCCC1 Show InChI InChI=1S/C26H32N8O3S/c1-32-12-14-34(15-13-32)38(36,37)21-6-4-19(5-7-21)23-17-29-25(27)24(30-23)26(35)31-22-16-28-9-8-20(22)18-33-10-2-3-11-33/h4-9,16-17H,2-3,10-15,18H2,1H3,(H2,27,29)(H,31,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GSK3beta using biotin- AAEELDSRAGS(PO3H2)PQL as substrate and [gamma32P]ATP after 20 mins by scintillation proximity ... |
J Med Chem 55: 9107-19 (2012)
Article DOI: 10.1021/jm201724m
BindingDB Entry DOI: 10.7270/Q2X0685T |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(GUINEA PIG) | BDBM22541

(Clobenpropit | N''-[(4-chlorophenyl)methyl]{[3-(1H...)Show SMILES NC(SCCCc1cnc[nH]1)=NCc1ccc(Cl)cc1 |w:11.12| Show InChI InChI=1S/C14H17ClN4S/c15-12-5-3-11(4-6-12)8-18-14(16)20-7-1-2-13-9-17-10-19-13/h3-6,9-10H,1-2,7-8H2,(H2,16,18)(H,17,19) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 305: 887-96 (2003)
Article DOI: 10.1124/jpet.102.047183
BindingDB Entry DOI: 10.7270/Q22J69FK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50200646
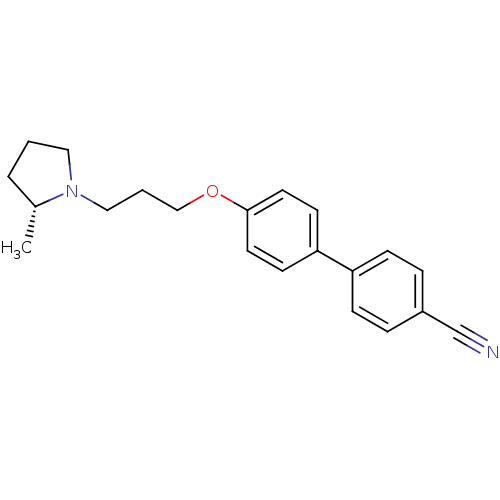
(4'-[3-((R)-2-methyl-pyrrolidin-1-yl)-propoxy]-biph...)Show InChI InChI=1S/C21H24N2O/c1-17-4-2-13-23(17)14-3-15-24-21-11-9-20(10-12-21)19-7-5-18(16-22)6-8-19/h5-12,17H,2-4,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human cloned histamine H3 receptor expressed in C6 cells |
Bioorg Med Chem Lett 17: 1443-6 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.073
BindingDB Entry DOI: 10.7270/Q2416WR3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































