 Found 246 hits with Last Name = 'norton' and Initial = 'mb'
Found 246 hits with Last Name = 'norton' and Initial = 'mb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128131
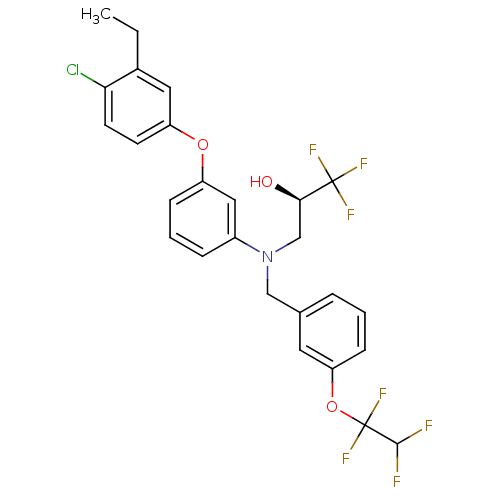
((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...)Show SMILES CCc1cc(Oc2cccc(c2)N(C[C@@H](O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)ccc1Cl Show InChI InChI=1S/C26H23ClF7NO3/c1-2-17-12-20(9-10-22(17)27)37-19-7-4-6-18(13-19)35(15-23(36)25(30,31)32)14-16-5-3-8-21(11-16)38-26(33,34)24(28)29/h3-13,23-24,36H,2,14-15H2,1H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer with <1 nM [CETP] for 18 ... |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049644
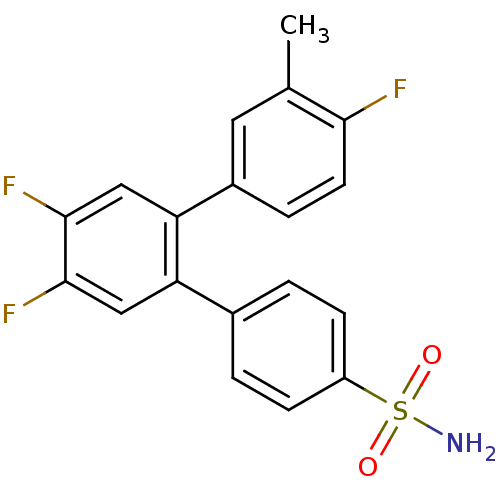
(4,4',5'-Trifluoro-3-methyl-[1,1';2',1'']terphenyl-...)Show SMILES Cc1cc(ccc1F)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H14F3NO2S/c1-11-8-13(4-7-17(11)20)16-10-19(22)18(21)9-15(16)12-2-5-14(6-3-12)26(23,24)25/h2-10H,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049619
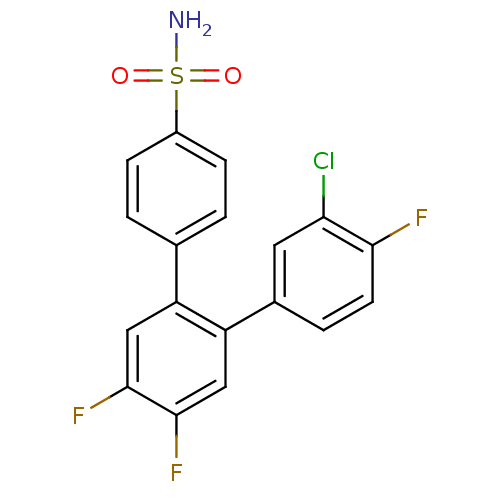
(3-Chloro-4,4',5'-trifluoro-[1,1';2',1'']terphenyl-...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C18H11ClF3NO2S/c19-15-7-11(3-6-16(15)20)14-9-18(22)17(21)8-13(14)10-1-4-12(5-2-10)26(23,24)25/h1-9H,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029625

(1-methyl-4-[2-(4-methylsulfonylphenyl)-1-cyclopent...)Show SMILES Cc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:8| Show InChI InChI=1S/C19H20O2S/c1-14-6-8-15(9-7-14)18-4-3-5-19(18)16-10-12-17(13-11-16)22(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029623
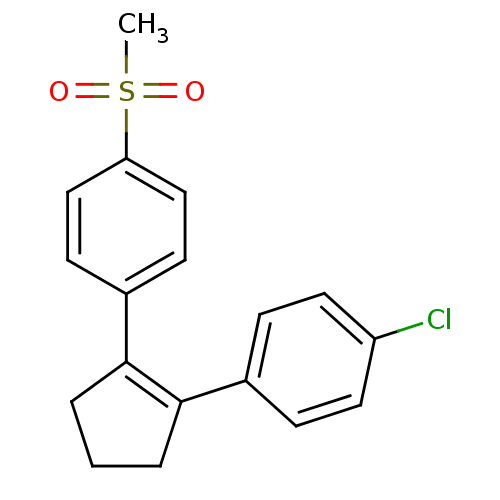
(1-chloro-4-[2-(4-methylsulfonylphenyl)-1-cyclopent...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)cc1 |t:11| Show InChI InChI=1S/C18H17ClO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049625
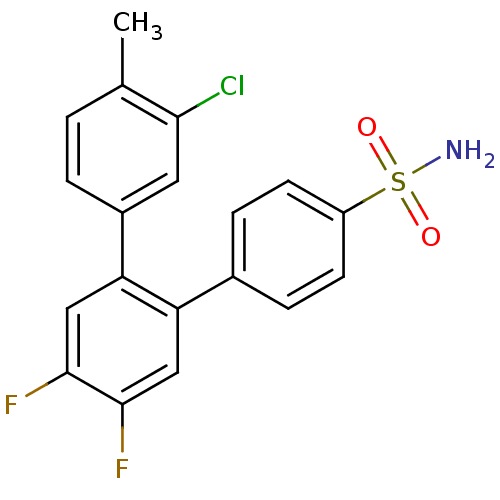
(3-Chloro-4',5'-difluoro-4-methyl-[1,1';2',1'']terp...)Show SMILES Cc1ccc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H14ClF2NO2S/c1-11-2-3-13(8-17(11)20)16-10-19(22)18(21)9-15(16)12-4-6-14(7-5-12)26(23,24)25/h2-10H,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049653
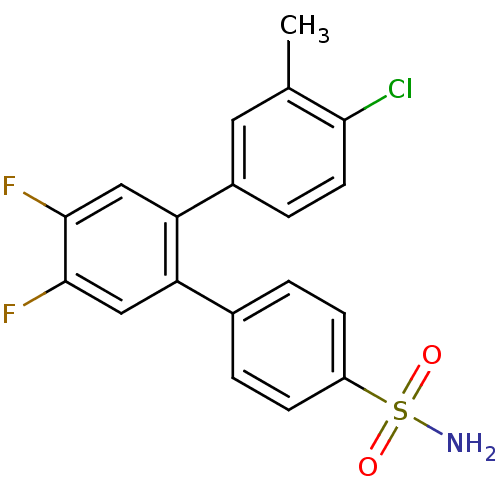
(4-Chloro-4',5'-difluoro-3-methyl-[1,1';2',1'']terp...)Show SMILES Cc1cc(ccc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H14ClF2NO2S/c1-11-8-13(4-7-17(11)20)16-10-19(22)18(21)9-15(16)12-2-5-14(6-3-12)26(23,24)25/h2-10H,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049622
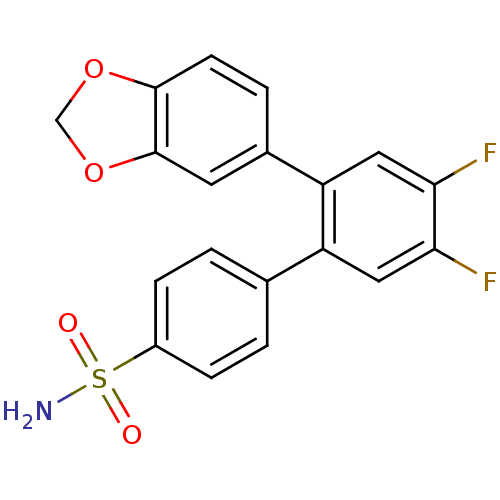
(2'-Benzo[1,3]dioxol-5-yl-4',5'-difluoro-biphenyl-4...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc2OCOc2c1 Show InChI InChI=1S/C19H13F2NO4S/c20-16-8-14(11-1-4-13(5-2-11)27(22,23)24)15(9-17(16)21)12-3-6-18-19(7-12)26-10-25-18/h1-9H,10H2,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049645
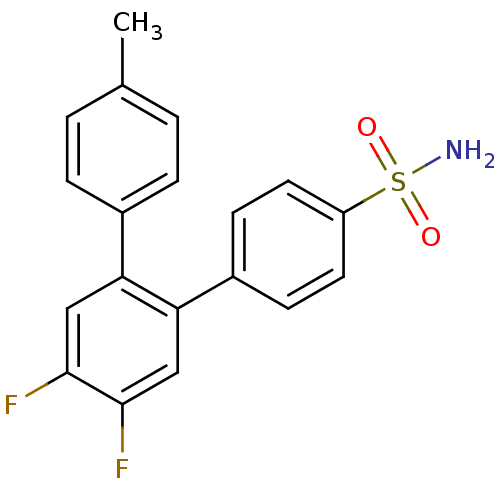
(4',5'-Difluoro-4''-methyl-[1,1';2',1'']terphenyl-4...)Show SMILES Cc1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H15F2NO2S/c1-12-2-4-13(5-3-12)16-10-18(20)19(21)11-17(16)14-6-8-15(9-7-14)25(22,23)24/h2-11H,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049659
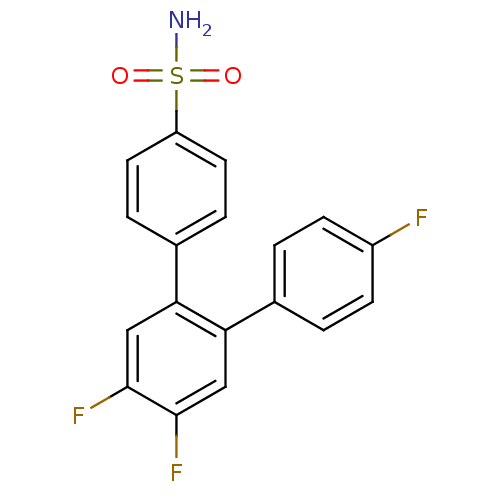
(4',5',4''-Trifluoro-[1,1';2',1'']terphenyl-4-sulfo...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(F)cc1 Show InChI InChI=1S/C18H12F3NO2S/c19-13-5-1-11(2-6-13)15-9-17(20)18(21)10-16(15)12-3-7-14(8-4-12)25(22,23)24/h1-10H,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049623

(4,4',5'-Trifluoro-4''-methanesulfonyl-3-methyl-[1,...)Show SMILES Cc1cc(ccc1F)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H15F3O2S/c1-12-9-14(5-8-18(12)21)17-11-20(23)19(22)10-16(17)13-3-6-15(7-4-13)26(2,24)25/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049656
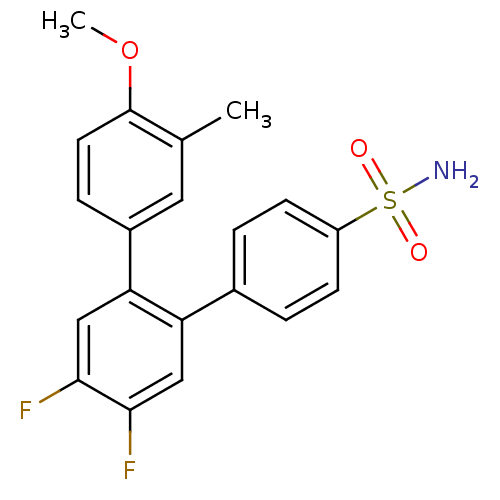
(4',5'-Difluoro-4-methoxy-3-methyl-[1,1';2',1'']ter...)Show SMILES COc1ccc(cc1C)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H17F2NO3S/c1-12-9-14(5-8-20(12)26-2)17-11-19(22)18(21)10-16(17)13-3-6-15(7-4-13)27(23,24)25/h3-11H,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029613
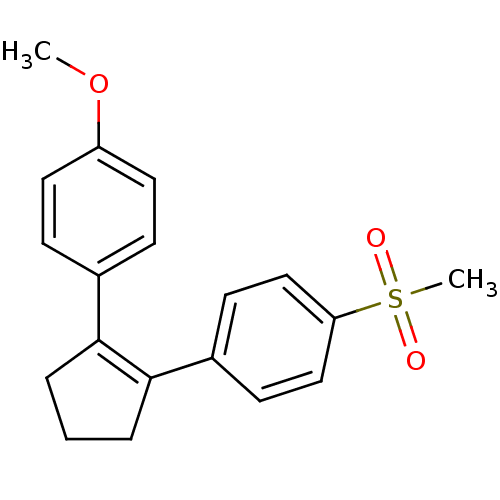
(1-Methoxy-4-(2-(4-(methanesulfonyl)phenyl)cyclopen...)Show SMILES COc1ccc(cc1)C1=C(CCC1)c1ccc(cc1)S(C)(=O)=O |t:9| Show InChI InChI=1S/C19H20O3S/c1-22-16-10-6-14(7-11-16)18-4-3-5-19(18)15-8-12-17(13-9-15)23(2,20)21/h6-13H,3-5H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049662
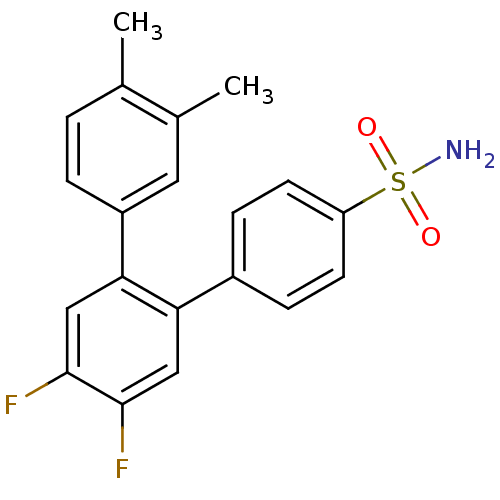
(4',5'-Difluoro-3,4-dimethyl-[1,1';2',1'']terphenyl...)Show SMILES Cc1ccc(cc1C)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H17F2NO2S/c1-12-3-4-15(9-13(12)2)18-11-20(22)19(21)10-17(18)14-5-7-16(8-6-14)26(23,24)25/h3-11H,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049651

(4-Chloro-[1,1'';2'',1'''']terphenyl-4''''-sulfonic...)Show InChI InChI=1S/C18H14ClNO2S/c19-15-9-5-13(6-10-15)17-3-1-2-4-18(17)14-7-11-16(12-8-14)23(20,21)22/h1-12H,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049617
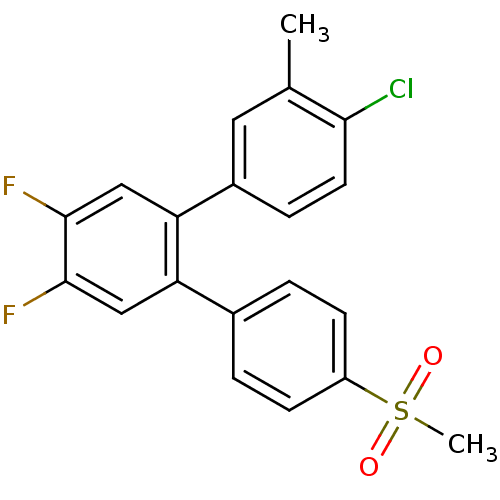
(4-Chloro-4',5'-difluoro-4''-methanesulfonyl-3-meth...)Show SMILES Cc1cc(ccc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H15ClF2O2S/c1-12-9-14(5-8-18(12)21)17-11-20(23)19(22)10-16(17)13-3-6-15(7-4-13)26(2,24)25/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049646
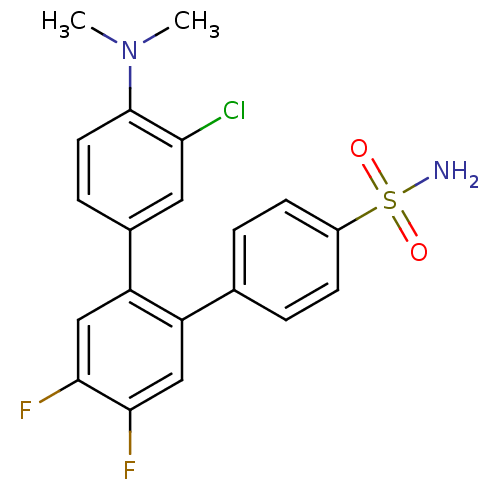
(3-Chloro-4-dimethylamino-4',5'-difluoro-[1,1';2',1...)Show SMILES CN(C)c1ccc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H17ClF2N2O2S/c1-25(2)20-8-5-13(9-17(20)21)16-11-19(23)18(22)10-15(16)12-3-6-14(7-4-12)28(24,26)27/h3-11H,1-2H3,(H2,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50037941
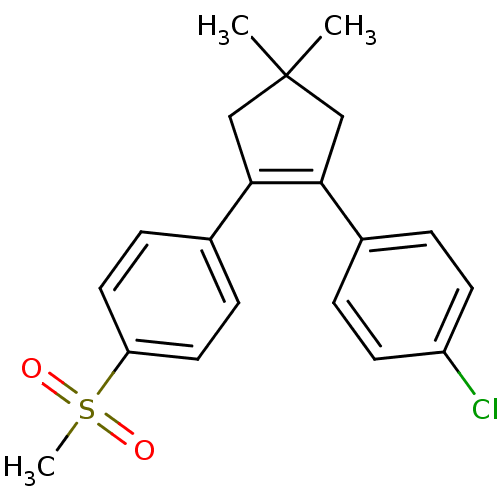
(1-[4,4-dimethyl-2-(4-methylsulfonylphenyl)-1-cyclo...)Show SMILES CC1(C)CC(=C(C1)c1ccc(cc1)S(C)(=O)=O)c1ccc(Cl)cc1 |c:4| Show InChI InChI=1S/C20H21ClO2S/c1-20(2)12-18(14-4-8-16(21)9-5-14)19(13-20)15-6-10-17(11-7-15)24(3,22)23/h4-11H,12-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049660

(4',5'-Difluoro-4''-methanesulfonyl-4-methyl-[1,1';...)Show SMILES Cc1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H16F2O2S/c1-13-3-5-14(6-4-13)17-11-19(21)20(22)12-18(17)15-7-9-16(10-8-15)25(2,23)24/h3-12H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049649
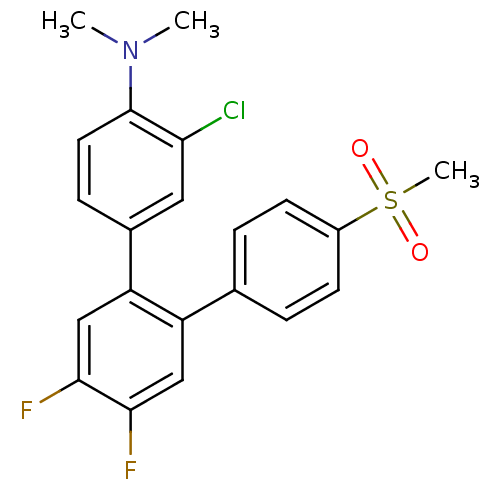
((3-Chloro-4',5'-difluoro-4''-methanesulfonyl-[1,1'...)Show SMILES CN(C)c1ccc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H18ClF2NO2S/c1-25(2)21-9-6-14(10-18(21)22)17-12-20(24)19(23)11-16(17)13-4-7-15(8-5-13)28(3,26)27/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049642
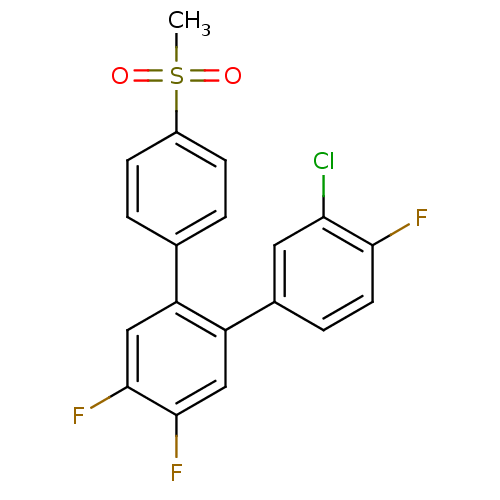
(3-Chloro-4,4',5'-trifluoro-4''-methanesulfonyl-[1,...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C19H12ClF3O2S/c1-26(24,25)13-5-2-11(3-6-13)14-9-18(22)19(23)10-15(14)12-4-7-17(21)16(20)8-12/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029593

(CHEMBL7162 | N-(2-(cyclohexyloxy)-4-nitrophenyl)me...)Show InChI InChI=1S/C13H18N2O5S/c1-21(18,19)14-12-8-7-10(15(16)17)9-13(12)20-11-5-3-2-4-6-11/h7-9,11,14H,2-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049635

(5-(4,5-Difluoro-4'-methanesulfonyl-biphenyl-2-yl)-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc2OCOc2c1 Show InChI InChI=1S/C20H14F2O4S/c1-27(23,24)14-5-2-12(3-6-14)15-9-17(21)18(22)10-16(15)13-4-7-19-20(8-13)26-11-25-19/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049636
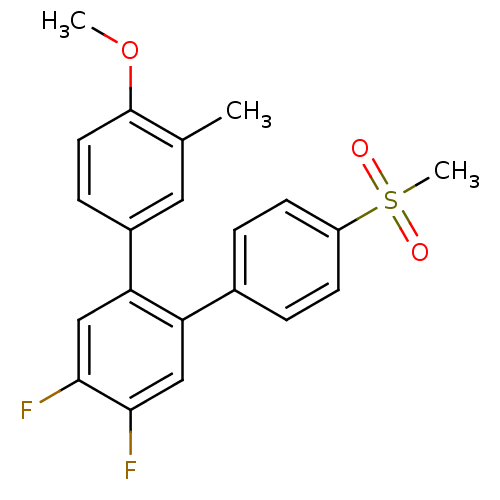
(4',5'-Difluoro-4''-methanesulfonyl-4-methoxy-3-met...)Show SMILES COc1ccc(cc1C)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H18F2O3S/c1-13-10-15(6-9-21(13)26-2)18-12-20(23)19(22)11-17(18)14-4-7-16(8-5-14)27(3,24)25/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049652
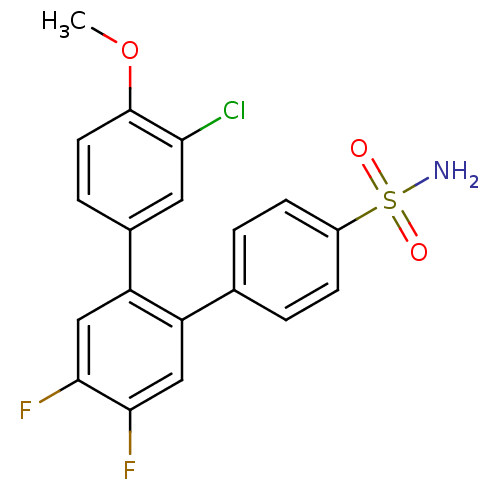
(3-Chloro-4',5'-difluoro-4-methoxy-[1,1';2',1'']ter...)Show SMILES COc1ccc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H14ClF2NO3S/c1-26-19-7-4-12(8-16(19)20)15-10-18(22)17(21)9-14(15)11-2-5-13(6-3-11)27(23,24)25/h2-10H,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049639
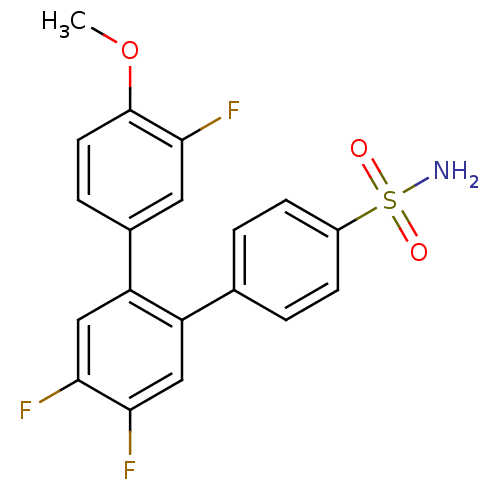
(3,4',5'-Trifluoro-4-methoxy-[1,1';2',1'']terphenyl...)Show SMILES COc1ccc(cc1F)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H14F3NO3S/c1-26-19-7-4-12(8-18(19)22)15-10-17(21)16(20)9-14(15)11-2-5-13(6-3-11)27(23,24)25/h2-10H,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049637
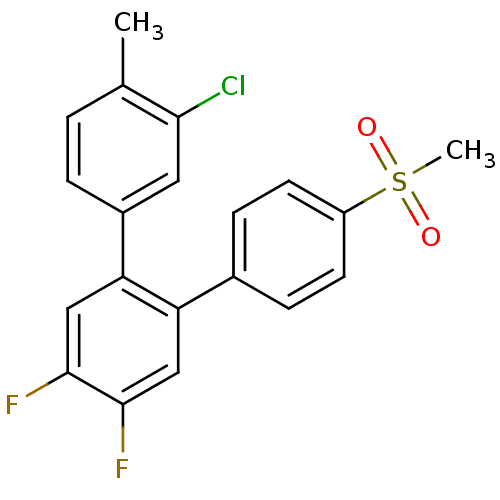
(3-Chloro-4',5'-difluoro-4''-methanesulfonyl-4-meth...)Show SMILES Cc1ccc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H15ClF2O2S/c1-12-3-4-14(9-18(12)21)17-11-20(23)19(22)10-16(17)13-5-7-15(8-6-13)26(2,24)25/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049632

(4',5',4''-Trifluoro-4-methanesulfonyl-[1,1';2',1''...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc(F)cc1 Show InChI InChI=1S/C19H13F3O2S/c1-25(23,24)15-8-4-13(5-9-15)17-11-19(22)18(21)10-16(17)12-2-6-14(20)7-3-12/h2-11H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50037945

(1-[2-(4-fluorophenyl)-4,4-dimethyl-1-cyclopentenyl...)Show SMILES CC1(C)CC(=C(C1)c1ccc(cc1)S(C)(=O)=O)c1ccc(F)cc1 |c:4| Show InChI InChI=1S/C20H21FO2S/c1-20(2)12-18(14-4-8-16(21)9-5-14)19(13-20)15-6-10-17(11-7-15)24(3,22)23/h4-11H,12-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049633
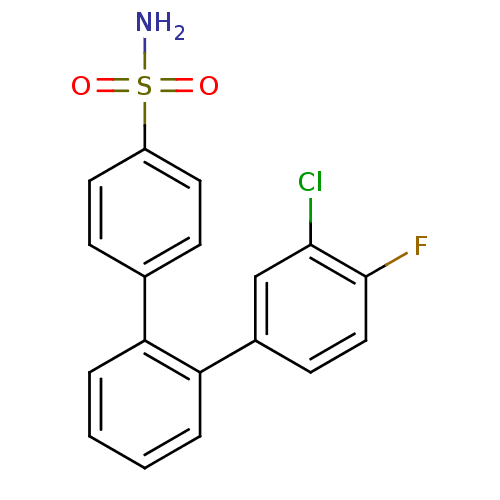
(3-Chloro-4-fluoro-[1,1'';2'',1'''']terphenyl-4''''...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1ccccc1-c1ccc(F)c(Cl)c1 Show InChI InChI=1S/C18H13ClFNO2S/c19-17-11-13(7-10-18(17)20)16-4-2-1-3-15(16)12-5-8-14(9-6-12)24(21,22)23/h1-11H,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049618

(3-Chloro-4-methoxy-[1,1'';2'',1'''']terphenyl-4'''...)Show SMILES COc1ccc(cc1Cl)-c1ccccc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16ClNO3S/c1-24-19-11-8-14(12-18(19)20)17-5-3-2-4-16(17)13-6-9-15(10-7-13)25(21,22)23/h2-12H,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049641

(3-Chloro-4',5'-difluoro-4''-methanesulfonyl-4-meth...)Show SMILES COc1ccc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H15ClF2O3S/c1-26-20-8-5-13(9-17(20)21)16-11-19(23)18(22)10-15(16)12-3-6-14(7-4-12)27(2,24)25/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128175

(1,1,1-Trifluoro-3-{[3-(3-isopropyl-phenoxy)-phenyl...)Show SMILES CC(C)c1cccc(Oc2cccc(c2)N(CC(O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)c1 Show InChI InChI=1S/C27H26F7NO3/c1-17(2)19-7-4-9-21(13-19)37-22-10-5-8-20(14-22)35(16-24(36)26(30,31)32)15-18-6-3-11-23(12-18)38-27(33,34)25(28)29/h3-14,17,24-25,36H,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049634
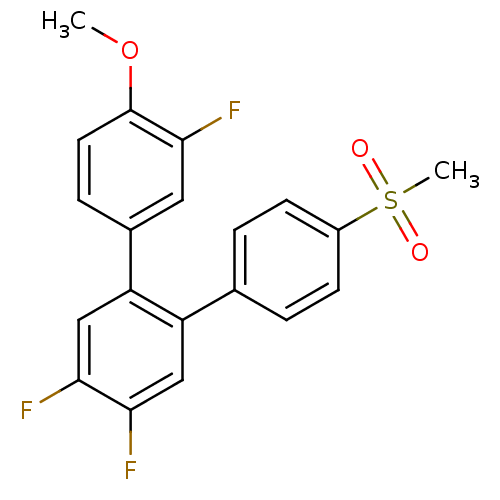
(3,4',5'-Trifluoro-4''-methanesulfonyl-4-methoxy-[1...)Show SMILES COc1ccc(cc1F)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C20H15F3O3S/c1-26-20-8-5-13(9-19(20)23)16-11-18(22)17(21)10-15(16)12-3-6-14(7-4-12)27(2,24)25/h3-11H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049621
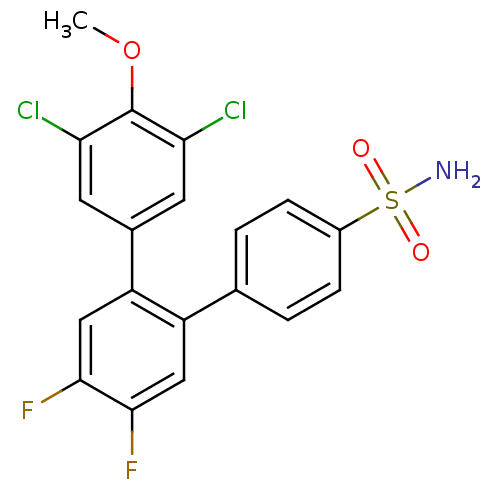
(3,5-Dichloro-4',5'-difluoro-4-methoxy-[1,1';2',1''...)Show SMILES COc1c(Cl)cc(cc1Cl)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H13Cl2F2NO3S/c1-27-19-15(20)6-11(7-16(19)21)14-9-18(23)17(22)8-13(14)10-2-4-12(5-3-10)28(24,25)26/h2-9H,1H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049640

(4',5'-Difluoro-4''-methanesulfonyl-3,4-dimethyl-[1...)Show SMILES Cc1ccc(cc1C)-c1cc(F)c(F)cc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C21H18F2O2S/c1-13-4-5-16(10-14(13)2)19-12-21(23)20(22)11-18(19)15-6-8-17(9-7-15)26(3,24)25/h4-12H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50029614
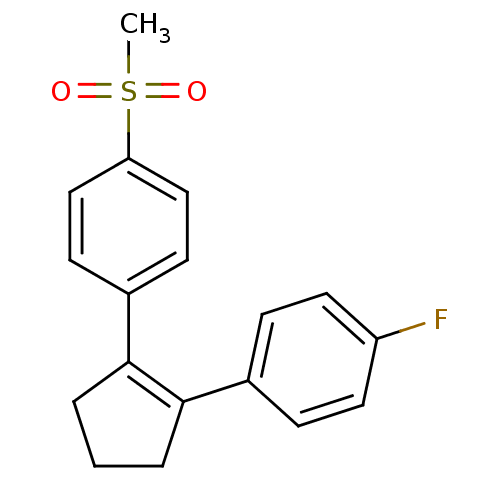
((SC-57666)1-[2-(4-fluorophenyl)-1-cyclopentenyl]-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C18H17FO2S/c1-22(20,21)16-11-7-14(8-12-16)18-4-2-3-17(18)13-5-9-15(19)10-6-13/h5-12H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128164
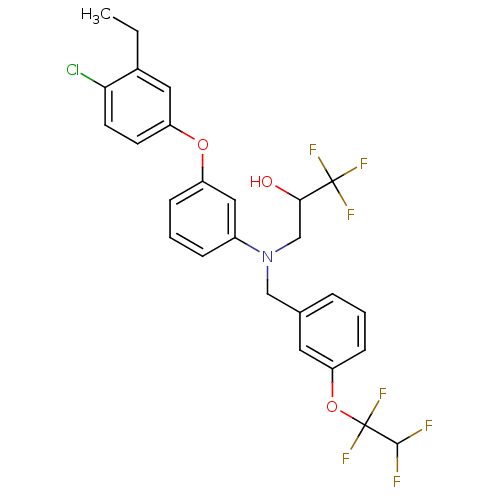
(3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-chlo...)Show SMILES CCc1cc(Oc2cccc(c2)N(CC(O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)ccc1Cl Show InChI InChI=1S/C26H23ClF7NO3/c1-2-17-12-20(9-10-22(17)27)37-19-7-4-6-18(13-19)35(15-23(36)25(30,31)32)14-16-5-3-8-21(11-16)38-26(33,34)24(28)29/h3-13,23-24,36H,2,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128141

(1,1,1-Trifluoro-3-{[3-(4-fluoro-phenoxy)-phenyl]-[...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2ccc(F)cc2)c1)C(F)(F)F Show InChI InChI=1S/C24H19F8NO3/c25-16-7-9-18(10-8-16)35-19-5-2-4-17(12-19)33(14-21(34)23(28,29)30)13-15-3-1-6-20(11-15)36-24(31,32)22(26)27/h1-12,21-22,34H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128135

(3-((3-(2,3-dichlorophenoxy)phenyl)(3-(1,1,2,2-tetr...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2cccc(Cl)c2Cl)c1)C(F)(F)F Show InChI InChI=1S/C24H18Cl2F7NO3/c25-18-8-3-9-19(21(18)26)36-16-6-2-5-15(11-16)34(13-20(35)23(29,30)31)12-14-4-1-7-17(10-14)37-24(32,33)22(27)28/h1-11,20,22,35H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128154
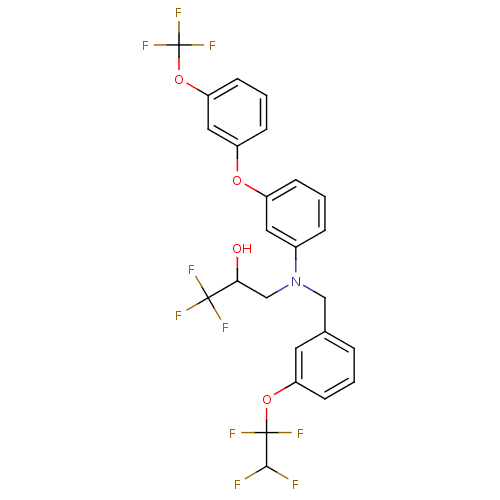
(1,1,1-Trifluoro-3-{[3-(1,1,2,2-tetrafluoro-ethoxy)...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2cccc(OC(F)(F)F)c2)c1)C(F)(F)F Show InChI InChI=1S/C25H19F10NO4/c26-22(27)24(31,32)39-19-8-1-4-15(10-19)13-36(14-21(37)23(28,29)30)16-5-2-6-17(11-16)38-18-7-3-9-20(12-18)40-25(33,34)35/h1-12,21-22,37H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049647
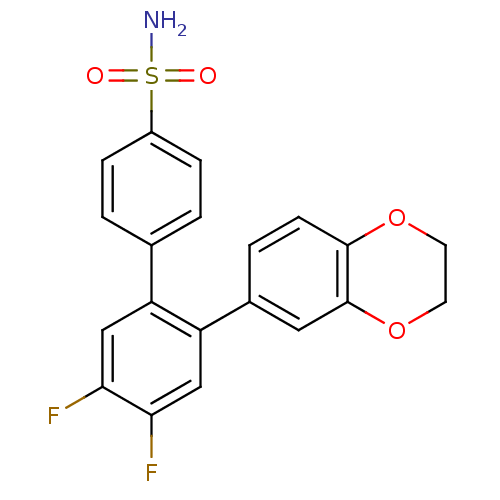
(2'-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-4',5'-diflu...)Show SMILES NS(=O)(=O)c1ccc(cc1)-c1cc(F)c(F)cc1-c1ccc2OCCOc2c1 Show InChI InChI=1S/C20H15F2NO4S/c21-17-10-15(12-1-4-14(5-2-12)28(23,24)25)16(11-18(17)22)13-3-6-19-20(9-13)27-8-7-26-19/h1-6,9-11H,7-8H2,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049661
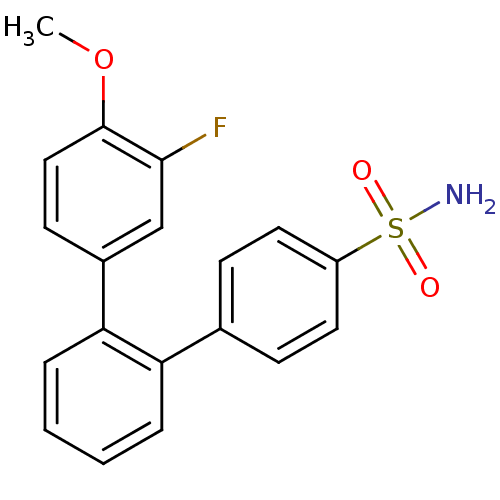
(3''''-Fluoro-4''''-methoxy-[1,1'';2'',1'''']terphe...)Show SMILES COc1ccc(cc1F)-c1ccccc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H16FNO3S/c1-24-19-11-8-14(12-18(19)20)17-5-3-2-4-16(17)13-6-9-15(10-7-13)25(21,22)23/h2-12H,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50037942
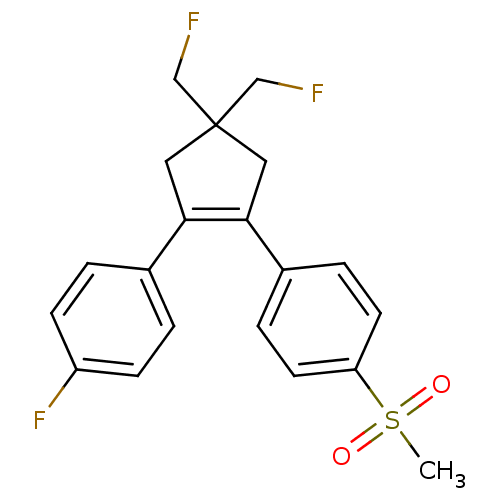
(1-[4,4-bis(fluoromethyl)-2-(4-fluorophenyl)cyclope...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CC(CF)(CF)C1)c1ccc(F)cc1 |t:11| Show InChI InChI=1S/C20H19F3O2S/c1-26(24,25)17-8-4-15(5-9-17)19-11-20(12-21,13-22)10-18(19)14-2-6-16(23)7-3-14/h2-9H,10-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049663
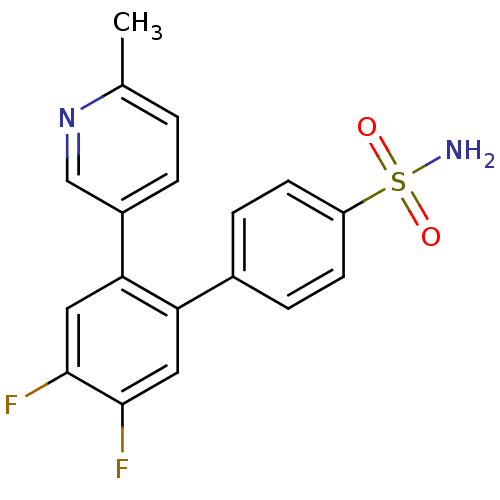
(4',5'-Difluoro-2'-(6-methyl-pyridin-3-yl)-biphenyl...)Show SMILES Cc1ccc(cn1)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C18H14F2N2O2S/c1-11-2-3-13(10-22-11)16-9-18(20)17(19)8-15(16)12-4-6-14(7-5-12)25(21,23)24/h2-10H,1H3,(H2,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50037951
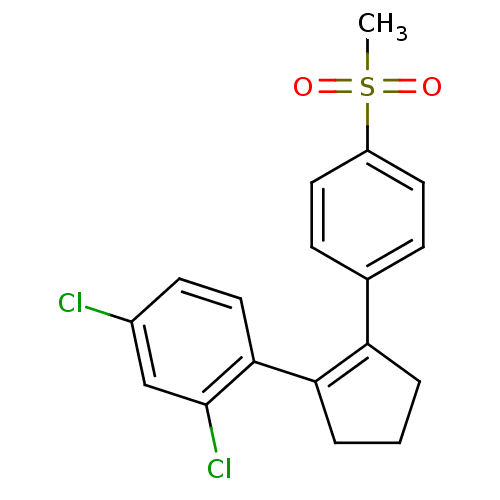
(2,4-Dichloro-1-[2-(4-methanesulfonyl-phenyl)-cyclo...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(CCC1)c1ccc(Cl)cc1Cl |t:11| Show InChI InChI=1S/C18H16Cl2O2S/c1-23(21,22)14-8-5-12(6-9-14)15-3-2-4-16(15)17-10-7-13(19)11-18(17)20/h5-11H,2-4H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research & Development
Curated by ChEMBL
| Assay Description
Tested for inhibition against Prostaglandin G/H synthase 2 |
J Med Chem 37: 3878-81 (1994)
BindingDB Entry DOI: 10.7270/Q26D5S1G |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128176
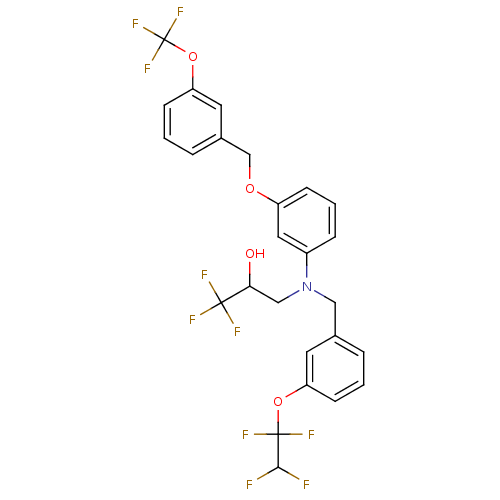
(1,1,1-Trifluoro-3-{[3-(1,1,2,2-tetrafluoro-ethoxy)...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(OCc2cccc(OC(F)(F)F)c2)c1)C(F)(F)F Show InChI InChI=1S/C26H21F10NO4/c27-23(28)25(32,33)40-20-8-1-4-16(10-20)13-37(14-22(38)24(29,30)31)18-6-3-7-19(12-18)39-15-17-5-2-9-21(11-17)41-26(34,35)36/h1-12,22-23,38H,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128146

(3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(3-ethy...)Show SMILES CCc1cccc(Oc2cccc(c2)N(CC(O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)c1 Show InChI InChI=1S/C26H24F7NO3/c1-2-17-6-3-9-20(12-17)36-21-10-5-8-19(14-21)34(16-23(35)25(29,30)31)15-18-7-4-11-22(13-18)37-26(32,33)24(27)28/h3-14,23-24,35H,2,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049657

(4''-Fluoro-[1,1';2',1'']terphenyl-4-sulfonic acid ...)Show InChI InChI=1S/C18H14FNO2S/c19-15-9-5-13(6-10-15)17-3-1-2-4-18(17)14-7-11-16(12-8-14)23(20,21)22/h1-12H,(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50049616

(4',5'-Difluoro-3,4-dimethoxy-[1,1';2',1'']terpheny...)Show SMILES COc1ccc(cc1OC)-c1cc(F)c(F)cc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C20H17F2NO4S/c1-26-19-8-5-13(9-20(19)27-2)16-11-18(22)17(21)10-15(16)12-3-6-14(7-4-12)28(23,24)25/h3-11H,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human recombinant prostaglandin G/H synthase 2 |
J Med Chem 39: 1846-56 (1996)
Article DOI: 10.1021/jm950878e
BindingDB Entry DOI: 10.7270/Q2Z89BGW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data