 Found 1303 hits with Last Name = 'nuss' and Initial = 'm'
Found 1303 hits with Last Name = 'nuss' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.427 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.654 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50240709
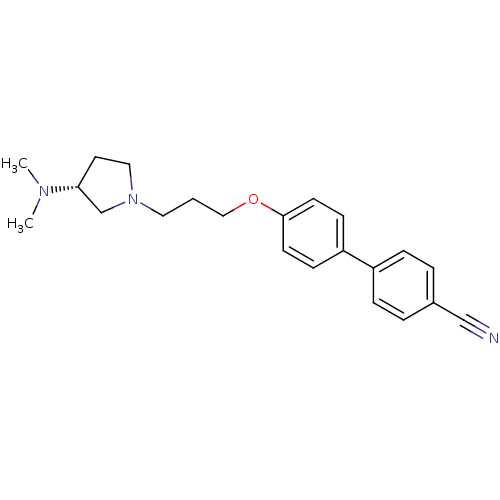
((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Hrh3 protein
(RAT) | BDBM86490
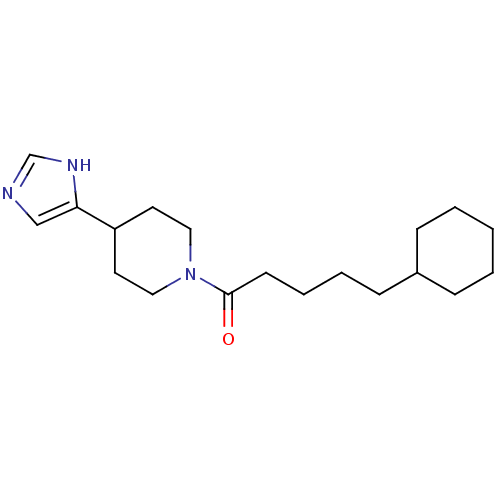
(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 47.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 61.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 72.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 89.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 499 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 734 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM86490

(CAS_0 | CHEMBL14812 | GT 2016 | NSC_0)Show InChI InChI=1S/C19H31N3O/c23-19(9-5-4-8-16-6-2-1-3-7-16)22-12-10-17(11-13-22)18-14-20-15-21-18/h14-17H,1-13H2,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50240709

((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...)Show SMILES CN(C)[C@@H]1CCN(CCCOc2ccc(cc2)-c2ccc(cc2)C#N)C1 |r| Show InChI InChI=1S/C22H27N3O/c1-24(2)21-12-14-25(17-21)13-3-15-26-22-10-8-20(9-11-22)19-6-4-18(16-23)5-7-19/h4-11,21H,3,12-15,17H2,1-2H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM27213

(4-[3-(4-cyclopropanecarbonylphenoxy)propyl]-1H-imi...)Show InChI InChI=1S/C16H18N2O2/c19-16(12-3-4-12)13-5-7-15(8-6-13)20-9-1-2-14-10-17-11-18-14/h5-8,10-12H,1-4,9H2,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM7966

(2-(1H-imidazol-4-yl)ethan-1-amine | CHEMBL544208 |...)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM22914

(CHEMBL260374 | N-cyclohexyl-4-(1H-imidazol-5-yl)pi...)Show InChI InChI=1S/C15H24N4S/c20-15(18-13-4-2-1-3-5-13)19-8-6-12(7-9-19)14-10-16-11-17-14/h10-13H,1-9H2,(H,16,17)(H,18,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by PDSP Ki Database
| |
Eur J Pharmacol 487: 183-97 (2004)
Article DOI: 10.1016/j.ejphar.2004.01.015
BindingDB Entry DOI: 10.7270/Q2NZ866C |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM104963
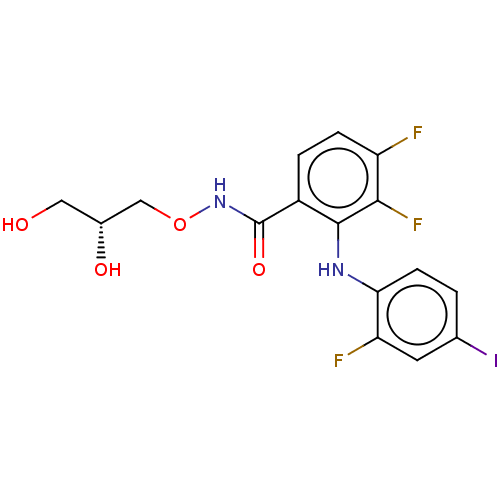
(CHEMBL507361 | US11147816, PD0325901 | US11701360,...)Show SMILES OC[C@@H](O)CONC(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F |r| Show InChI InChI=1S/C16H14F3IN2O4/c17-11-3-2-10(16(25)22-26-7-9(24)6-23)15(14(11)19)21-13-4-1-8(20)5-12(13)18/h1-5,9,21,23-24H,6-7H2,(H,22,25)/t9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
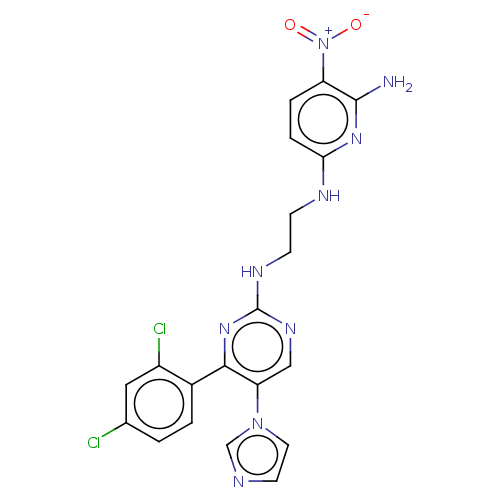
(Homo sapiens (Human)) | BDBM50243389
(CHEMBL3185148)Show SMILES Nc1nc(NCCNc2ncc(c(n2)-c2ccc(Cl)cc2Cl)-n2ccnc2)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-12-1-2-13(14(22)9-12)18-16(30-8-7-24-11-30)10-27-20(29-18)26-6-5-25-17-4-3-15(31(32)33)19(23)28-17/h1-4,7-11H,5-6H2,(H3,23,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50313013
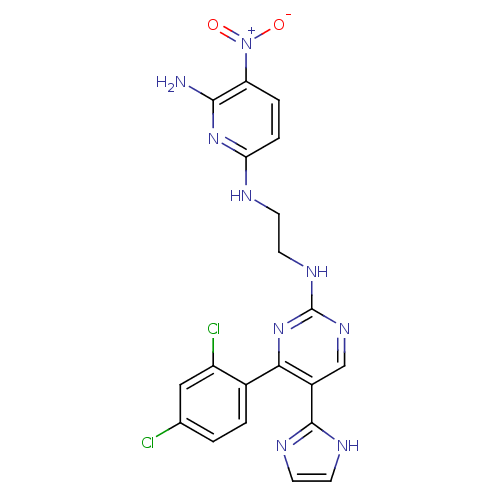
(CHEMBL1080901 | CT-98024 | N2-(2-(4-(2,4-dichlorop...)Show SMILES Nc1nc(NCCNc2ncc(-c3ncc[nH]3)c(n2)-c2ccc(Cl)cc2Cl)ccc1[N+]([O-])=O Show InChI InChI=1S/C20H17Cl2N9O2/c21-11-1-2-12(14(22)9-11)17-13(19-25-6-7-26-19)10-28-20(30-17)27-8-5-24-16-4-3-15(31(32)33)18(23)29-16/h1-4,6-7,9-10H,5,8H2,(H,25,26)(H3,23,24,29)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3beta using biotin-CREB peptide substrate after 1 hr by scintillation counting method |
J Med Chem 60: 8482-8514 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00922
BindingDB Entry DOI: 10.7270/Q25T3NW3 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50420996
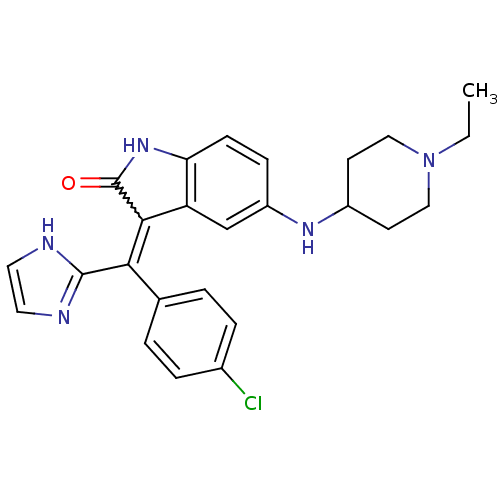
(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of Flt3 |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391802
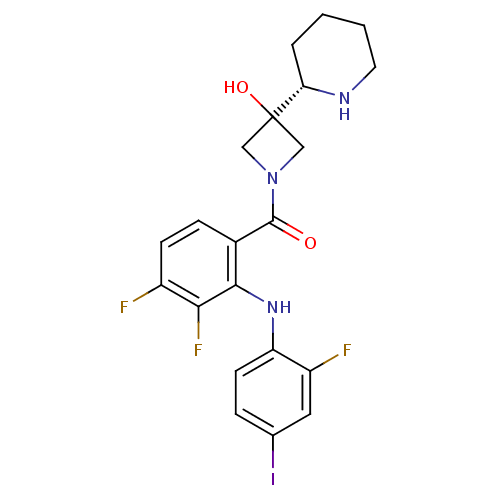
((3,4-difluoro-2-(2-fluoro-4-iodophenylamino)phenyl...)Show SMILES OC1(CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F)[C@@H]1CCCCN1 |r| Show InChI InChI=1S/C21H21F3IN3O2/c22-14-6-5-13(19(18(14)24)27-16-7-4-12(25)9-15(16)23)20(29)28-10-21(30,11-28)17-3-1-2-8-26-17/h4-7,9,17,26-27,30H,1-3,8,10-11H2/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402421
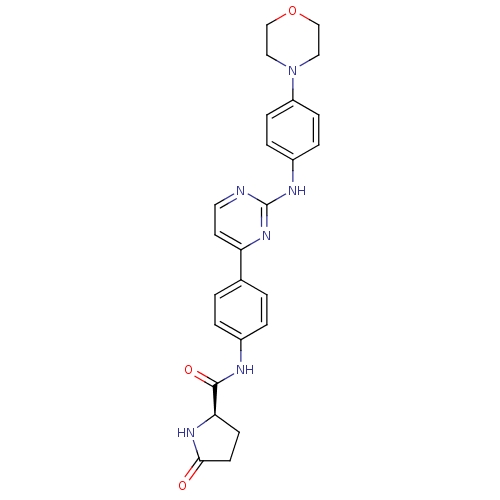
(CHEMBL2208035)Show SMILES O=C(Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1)[C@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C25H26N6O3/c32-23-10-9-22(29-23)24(33)27-18-3-1-17(2-4-18)21-11-12-26-25(30-21)28-19-5-7-20(8-6-19)31-13-15-34-16-14-31/h1-8,11-12,22H,9-10,13-16H2,(H,27,33)(H,29,32)(H,26,28,30)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380931
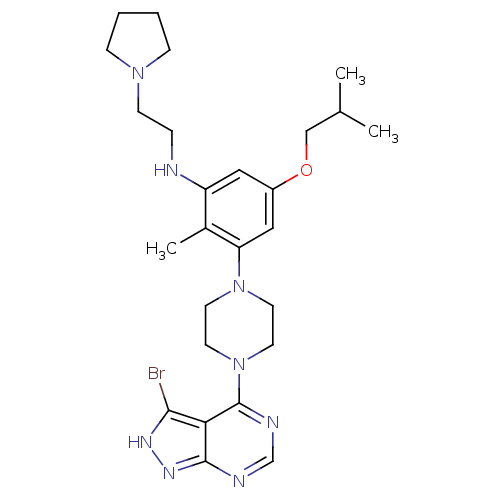
(CHEMBL2016886)Show SMILES CC(C)COc1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C26H37BrN8O/c1-18(2)16-36-20-14-21(28-6-9-33-7-4-5-8-33)19(3)22(15-20)34-10-12-35(13-11-34)26-23-24(27)31-32-25(23)29-17-30-26/h14-15,17-18,28H,4-13,16H2,1-3H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380935
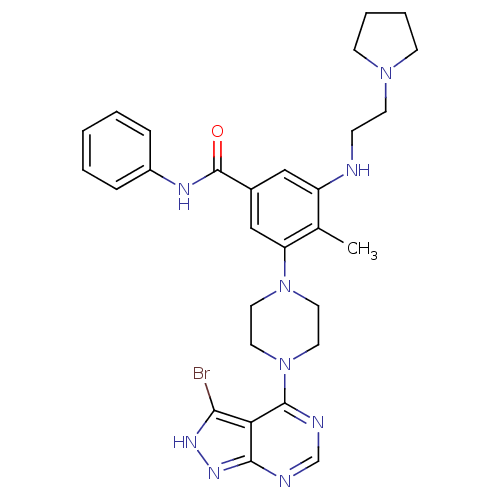
(CHEMBL2016887)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C29H34BrN9O/c1-20-23(31-9-12-37-10-5-6-11-37)17-21(29(40)34-22-7-3-2-4-8-22)18-24(20)38-13-15-39(16-14-38)28-25-26(30)35-36-27(25)32-19-33-28/h2-4,7-8,17-19,31H,5-6,9-16H2,1H3,(H,34,40)(H,32,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380929
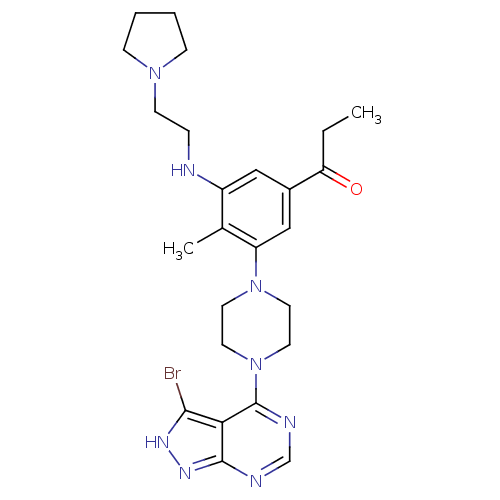
(CHEMBL2016892)Show SMILES CCC(=O)c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H33BrN8O/c1-3-21(35)18-14-19(27-6-9-32-7-4-5-8-32)17(2)20(15-18)33-10-12-34(13-11-33)25-22-23(26)30-31-24(22)28-16-29-25/h14-16,27H,3-13H2,1-2H3,(H,28,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50380939
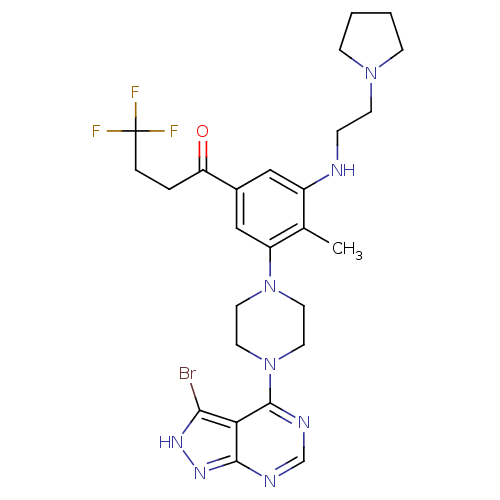
(CHEMBL2016893)Show SMILES Cc1c(NCCN2CCCC2)cc(cc1N1CCN(CC1)c1ncnc2n[nH]c(Br)c12)C(=O)CCC(F)(F)F Show InChI InChI=1S/C26H32BrF3N8O/c1-17-19(31-6-9-36-7-2-3-8-36)14-18(21(39)4-5-26(28,29)30)15-20(17)37-10-12-38(13-11-37)25-22-23(27)34-35-24(22)32-16-33-25/h14-16,31H,2-13H2,1H3,(H,32,33,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50380930
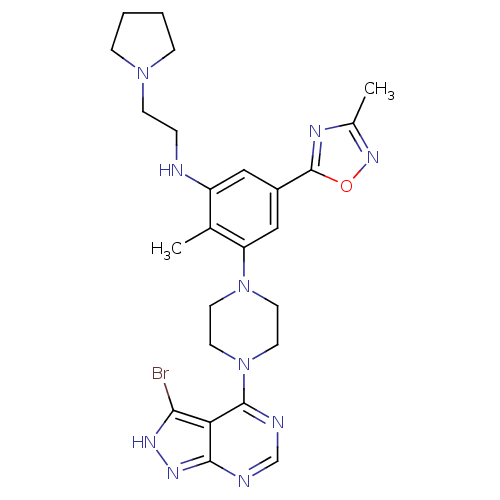
(CHEMBL2016890)Show SMILES Cc1noc(n1)-c1cc(NCCN2CCCC2)c(C)c(c1)N1CCN(CC1)c1ncnc2n[nH]c(Br)c12 Show InChI InChI=1S/C25H31BrN10O/c1-16-19(27-5-8-34-6-3-4-7-34)13-18(25-30-17(2)33-37-25)14-20(16)35-9-11-36(12-10-35)24-21-22(26)31-32-23(21)28-15-29-24/h13-15,27H,3-12H2,1-2H3,(H,28,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K after 3 hrs by luciferase based chemiluminescence assay |
Bioorg Med Chem Lett 22: 2693-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.011
BindingDB Entry DOI: 10.7270/Q2WD41MT |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged Flt1 using poly(Glu,Tyr) as substrate after 60 mins by alphascreen assay |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50420996

(CHEMBL2086760)Show SMILES CCN1CCC(CC1)Nc1ccc2NC(=O)C(=C(c3ncc[nH]3)c3ccc(Cl)cc3)c2c1 Show InChI InChI=1S/C25H26ClN5O/c1-2-31-13-9-18(10-14-31)29-19-7-8-21-20(15-19)23(25(32)30-21)22(24-27-11-12-28-24)16-3-5-17(26)6-4-16/h3-8,11-12,15,18,29H,2,9-10,13-14H2,1H3,(H,27,28)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 22: 4979-85 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.029
BindingDB Entry DOI: 10.7270/Q2XG9SD4 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50272192
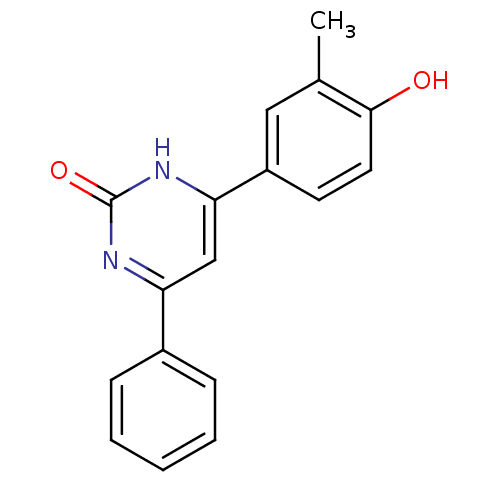
(4-(4-hydroxy-3-methylphenyl)-6-phenylpyrimidin-2(1...)Show InChI InChI=1S/C17H14N2O2/c1-11-9-13(7-8-16(11)20)15-10-14(18-17(21)19-15)12-5-3-2-4-6-12/h2-10,20H,1H3,(H,18,19,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of N-terminus Myc-tagged human CDC7 expressed in Escherichia coli by chemiluminescence assay in presence of ATP |
Bioorg Med Chem Lett 22: 3727-31 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.024
BindingDB Entry DOI: 10.7270/Q20C4WS0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50068561
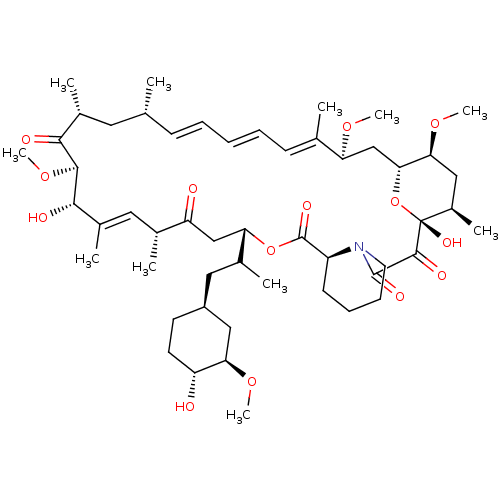
((16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-...)Show SMILES CO[C@@H]1C[C@H](CC(C)[C@@H]2CC(=O)[C@H](C)\C=C(C)\[C@H](O)[C@@H](OC)C(=O)[C@H](C)C[C@H](C)\C=C\C=C\C=C(C)\[C@H](C[C@H]3O[C@](O)([C@H](C)C[C@@H]3OC)C(=O)C(=O)N3CCCC[C@H]3C(=O)O2)OC)CC[C@H]1O |c:14,33,t:29,31| Show InChI InChI=1S/C52H81NO14/c1-30-17-13-12-14-18-31(2)41(62-8)29-45-44(64-10)26-36(7)52(61,67-45)49(58)50(59)53-22-16-15-19-38(53)51(60)66-42(33(4)25-37-20-21-39(54)43(27-37)63-9)28-40(55)32(3)24-35(6)47(57)48(65-11)46(56)34(5)23-30/h12-14,17-18,24,30,32-34,36-39,41-45,47-48,54,57,61H,15-16,19-23,25-29H2,1-11H3/b14-12+,17-13+,31-18+,35-24+/t30-,32-,33?,34-,36-,37+,38+,39-,41+,42+,43-,44+,45-,47+,48+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against FKBP12 |
Bioorg Med Chem Lett 15: 5340-3 (2005)
Article DOI: 10.1016/j.bmcl.2005.06.106
BindingDB Entry DOI: 10.7270/Q2V40TRJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50402413

(CHEMBL2208032)Show SMILES C[C@@H](N)C(=O)Nc1ccc(cc1)-c1ccnc(Nc2ccc(cc2)N2CCOCC2)n1 |r| Show InChI InChI=1S/C23H26N6O2/c1-16(24)22(30)26-18-4-2-17(3-5-18)21-10-11-25-23(28-21)27-19-6-8-20(9-7-19)29-12-14-31-15-13-29/h2-11,16H,12-15,24H2,1H3,(H,26,30)(H,25,27,28)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 |
Bioorg Med Chem Lett 22: 7653-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.007
BindingDB Entry DOI: 10.7270/Q2PZ59ZN |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50391804
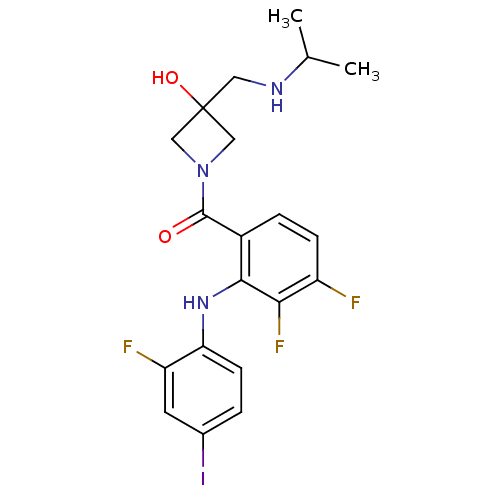
(CHEMBL2146893)Show SMILES CC(C)NCC1(O)CN(C1)C(=O)c1ccc(F)c(F)c1Nc1ccc(I)cc1F Show InChI InChI=1S/C20H21F3IN3O2/c1-11(2)25-8-20(29)9-27(10-20)19(28)13-4-5-14(21)17(23)18(13)26-16-6-3-12(24)7-15(16)22/h3-7,11,25-26,29H,8-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of MEK1-mediated ERK2 T202/Y204 phosphorylation using biotinylated MBP as substrate preincubated for 30 mins measured after 100 mins by cR... |
ACS Med Chem Lett 3: 416-421 (2012)
Article DOI: 10.1021/ml300049d
BindingDB Entry DOI: 10.7270/Q2GT5P80 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data