

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35714 (CHEMBL420762 | Mozavaptan | N-[4-(5-Dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards Vasopressin V1a receptor in rat liver | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513317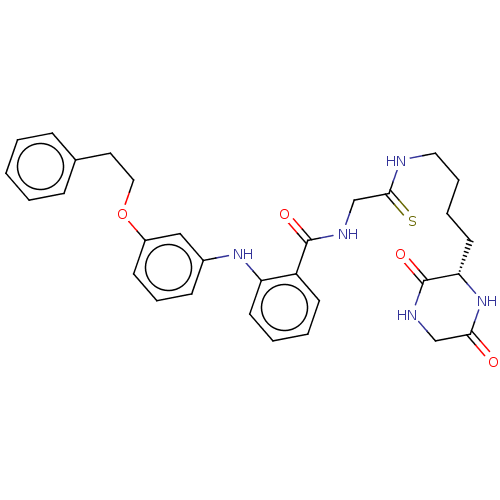 (CHEMBL4465620) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50513318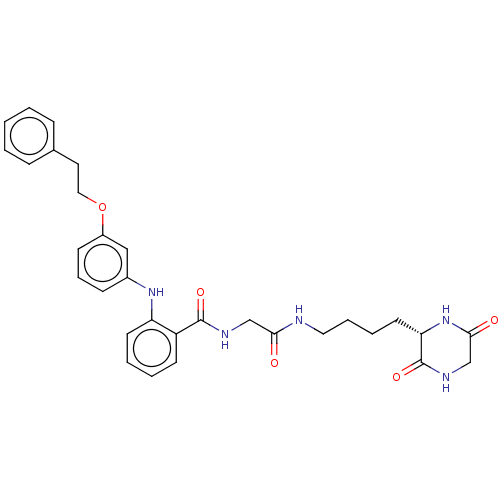 (CHEMBL4516553) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... | J Med Chem 62: 5844-5862 (2019) Article DOI: 10.1021/acs.jmedchem.9b00255 BindingDB Entry DOI: 10.7270/Q2JH3QJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92463 (Imipenem) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50029644 (CHEMBL296908 | N-(3-(4-(4-(2-oxo-3,4-dihydroquinol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity of the compound towards Vasopressin V1a receptor in human liver | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50140672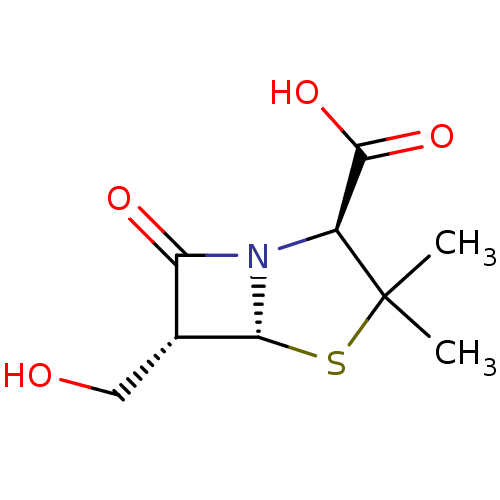 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92461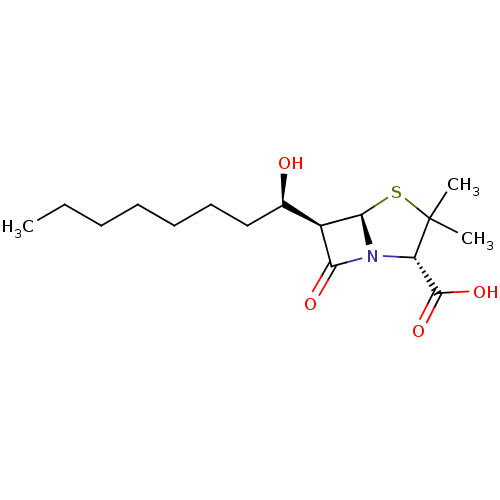 (Beta-lactam compound, 4) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92464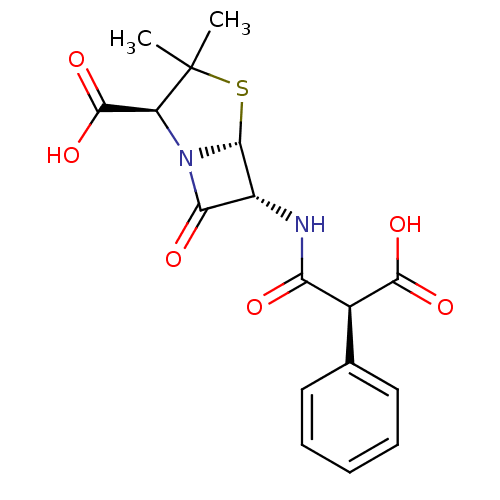 (Carbenicillin) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM50140671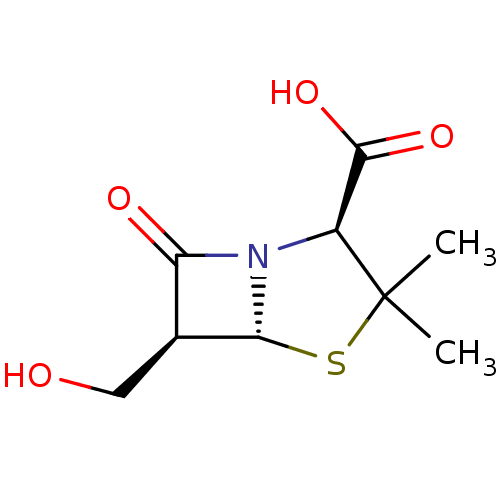 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-7-oxo-4-th...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxa40 (Acinetobacter baumannii) | BDBM92462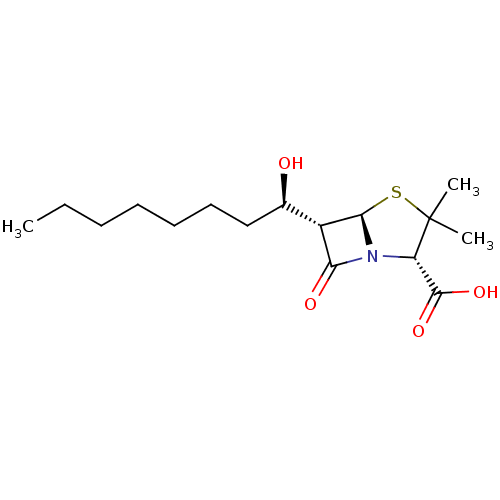 (Beta-lactam compound, 3) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
York University | Assay Description Beta-lactam compounds were assessed as competitive inhibitors using nitrocefin as a reporter substrate. | J Biol Chem 286: 37292-303 (2011) Article DOI: 10.1074/jbc.M111.280115 BindingDB Entry DOI: 10.7270/Q2QV3K38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269429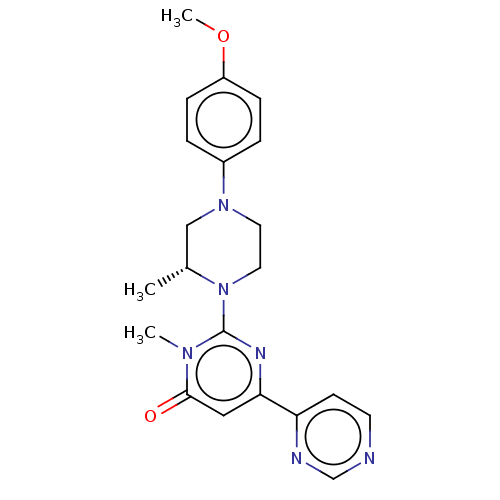 (CHEMBL4084855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269428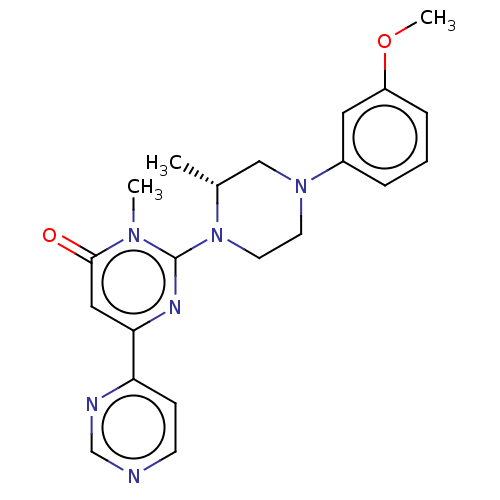 (CHEMBL4063206) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269437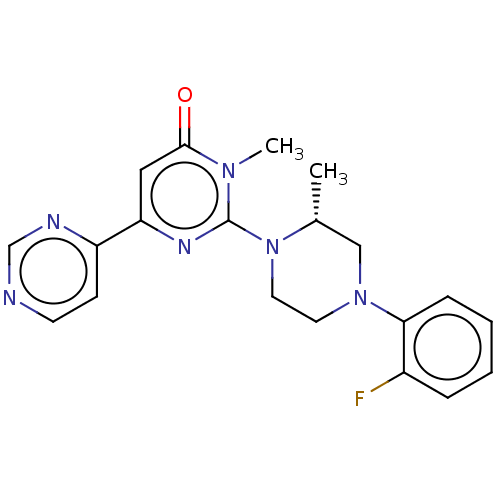 (CHEMBL4077376) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269438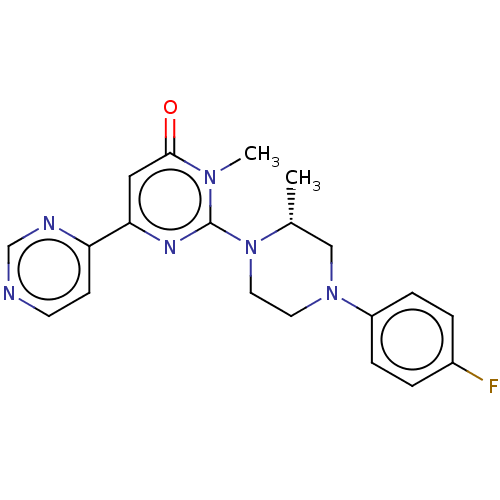 (CHEMBL4076060) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269443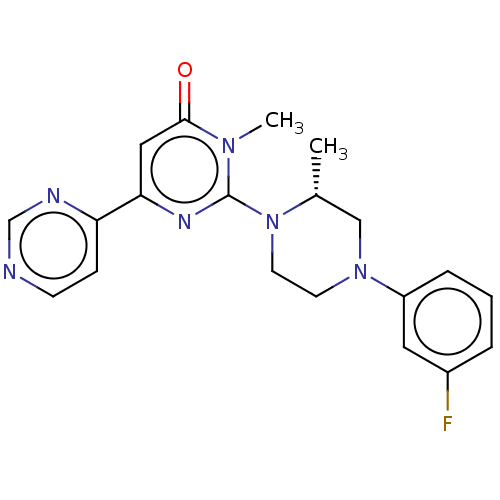 (CHEMBL4095848) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269440 (CHEMBL4065818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094076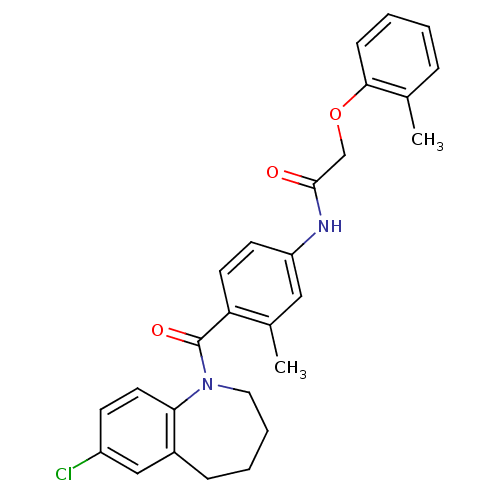 (CHEMBL434654 | N-[4-(7-Chloro-2,3,4,5-tetrahydro-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Concentration of the compound which inhibit [3H]-AVP binding to human Vasopressin V2 receptor coded HeLa cells by 50% | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 21: 1601-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.119 BindingDB Entry DOI: 10.7270/Q2RX9CCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 21: 1601-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.119 BindingDB Entry DOI: 10.7270/Q2RX9CCN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis | Bioorg Med Chem Lett 22: 456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.10.103 BindingDB Entry DOI: 10.7270/Q2NV9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5447 (CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis | Bioorg Med Chem Lett 22: 456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.10.103 BindingDB Entry DOI: 10.7270/Q2NV9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269439 (CHEMBL4077048) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269426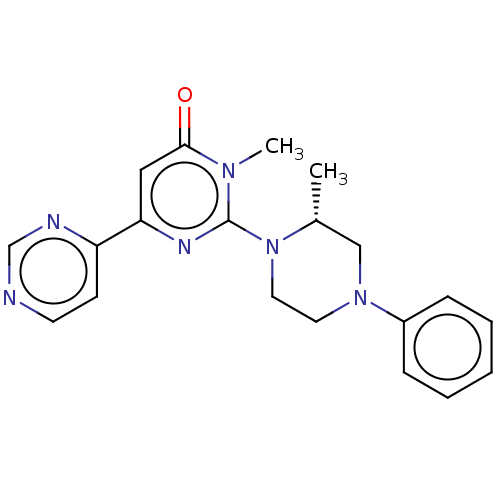 (CHEMBL3719193) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Bos taurus (cattle)) | BDBM155160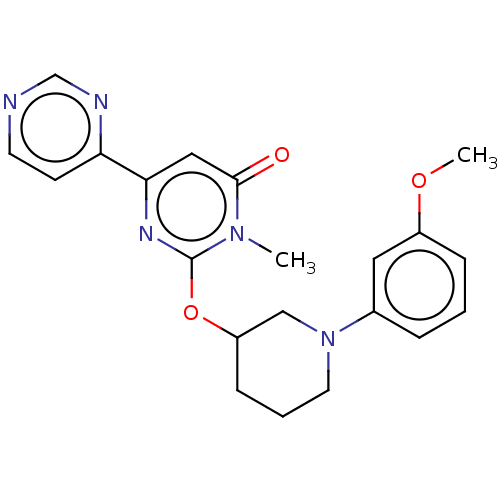 (US9006232, 1.106) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Mitsubishi Tanabe Pharma Corporation US Patent | Assay Description A mixture containing 100 mM MES-sodium hydroxide (pH 6.5), 1 mM magnesium acetate, 0.5 mM EGTA, 5 mM, .beta.-mercaptoethanol, 0.02% Tween 20, 10% gly... | US Patent US9006232 (2015) BindingDB Entry DOI: 10.7270/Q25T3J7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269431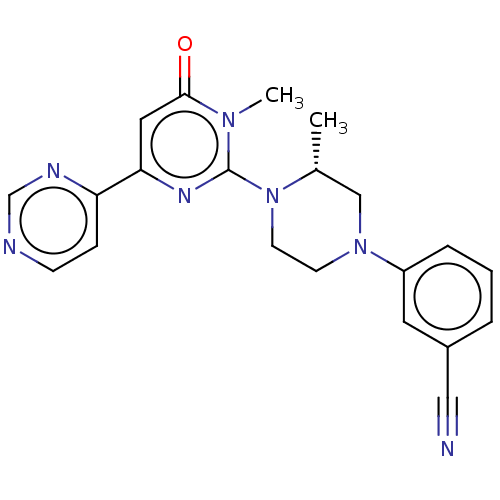 (CHEMBL4074586) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269413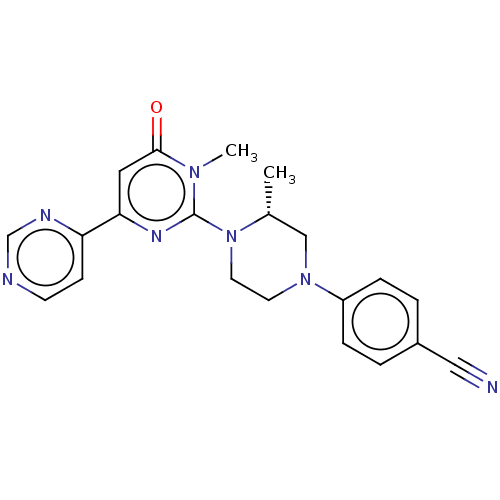 (CHEMBL4093559) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50338593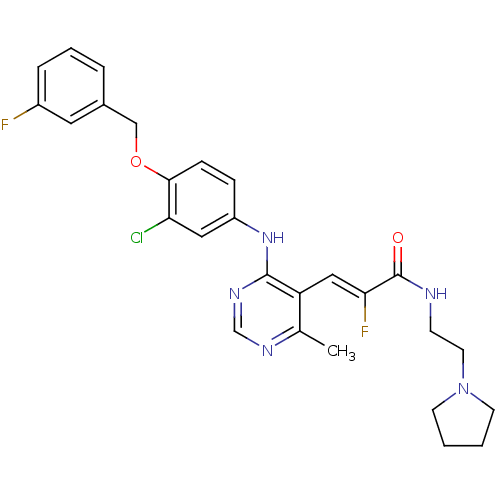 (3-(4-(3-chloro-4-(3-fluorobenzyloxy)phenylamino)-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR by homogeneous time-resolved fluorescence assay | Bioorg Med Chem Lett 21: 1601-6 (2011) Article DOI: 10.1016/j.bmcl.2011.01.119 BindingDB Entry DOI: 10.7270/Q2RX9CCN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50360457 (CHEMBL1934623) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis | Bioorg Med Chem Lett 22: 456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.10.103 BindingDB Entry DOI: 10.7270/Q2NV9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064157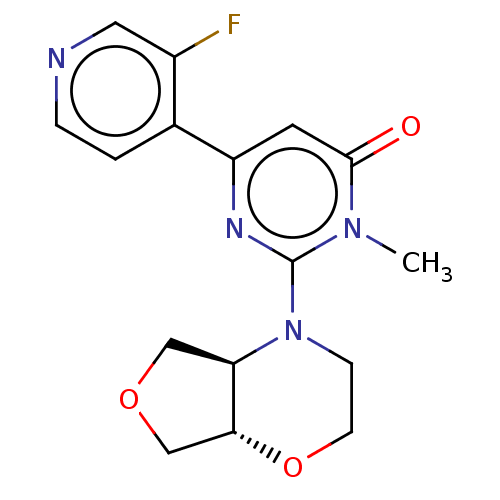 (CHEMBL3401132) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064156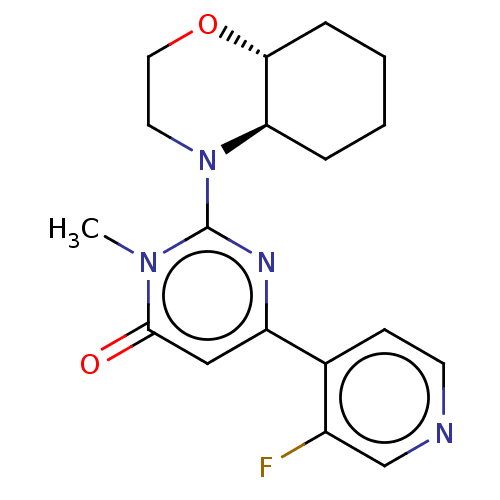 (CHEMBL3401131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269415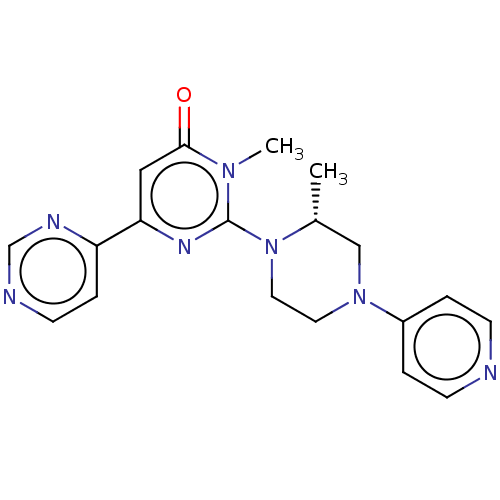 (CHEMBL4065956) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269416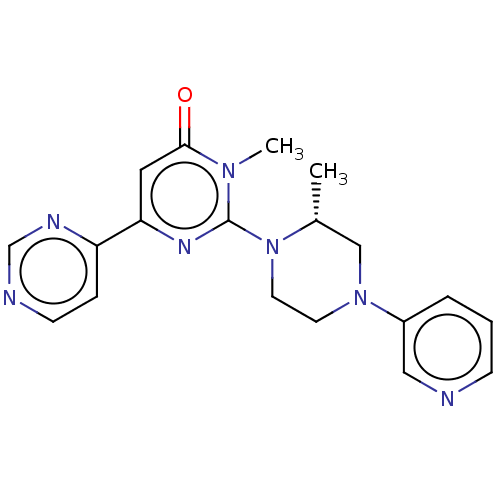 (CHEMBL4067170) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094079 ((4-Amino-2-methyl-phenyl)-(7-chloro-2,3,4,5-tetrah...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269417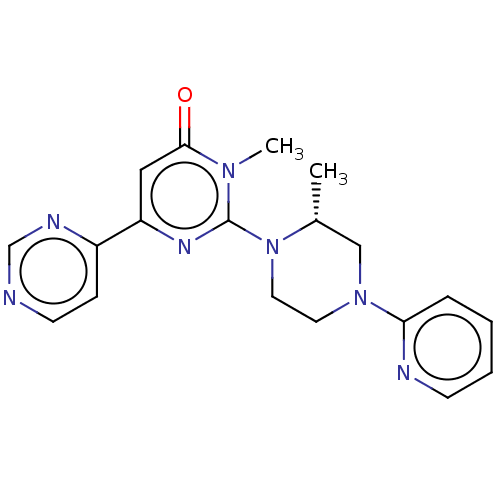 (CHEMBL4093930) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064154 (CHEMBL3401129) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269430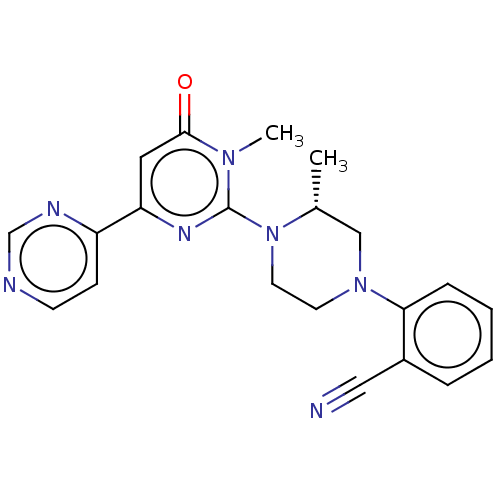 (CHEMBL4101293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064152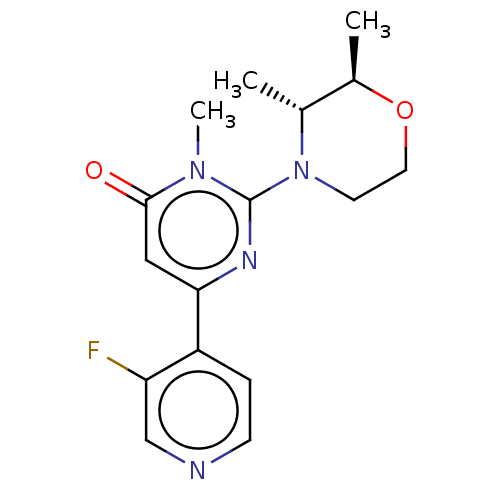 (CHEMBL3401127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094068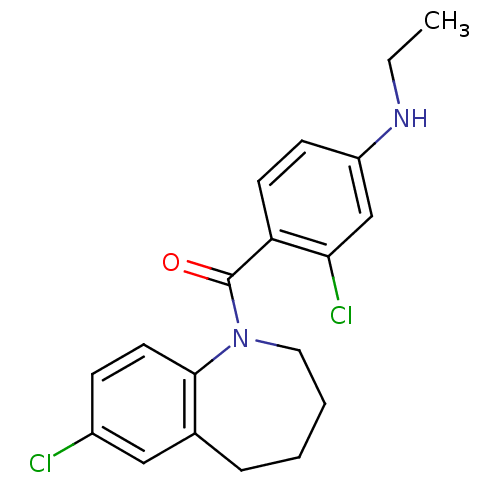 ((2-Chloro-4-ethylamino-phenyl)-(7-chloro-2,3,4,5-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094086 ((4-Allylamino-2-chloro-phenyl)-(2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Bos taurus (cattle)) | BDBM155169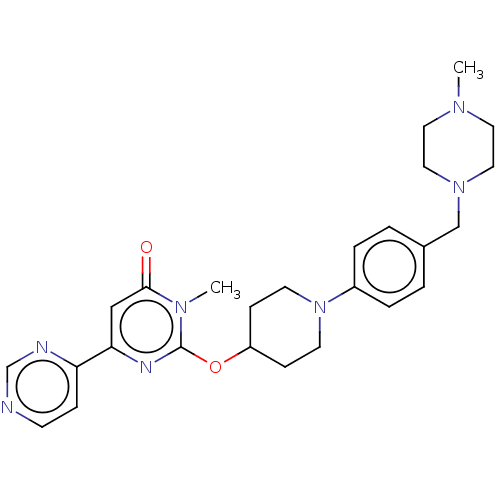 (US9006232, 1.203) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Mitsubishi Tanabe Pharma Corporation US Patent | Assay Description A mixture containing 100 mM MES-sodium hydroxide (pH 6.5), 1 mM magnesium acetate, 0.5 mM EGTA, 5 mM, .beta.-mercaptoethanol, 0.02% Tween 20, 10% gly... | US Patent US9006232 (2015) BindingDB Entry DOI: 10.7270/Q25T3J7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50360459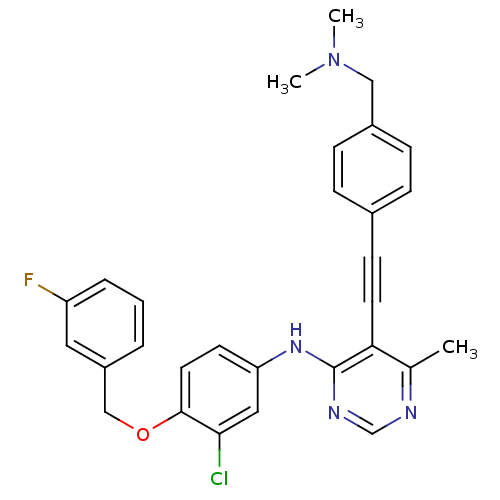 (CHEMBL1934625) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi& Co., Ltd Curated by ChEMBL | Assay Description Inhibition of EGFR phosphorylation in EGF-stimulated human A431 after 2 hrs by Western blot analysis | Bioorg Med Chem Lett 22: 456-60 (2011) Article DOI: 10.1016/j.bmcl.2011.10.103 BindingDB Entry DOI: 10.7270/Q2NV9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50064143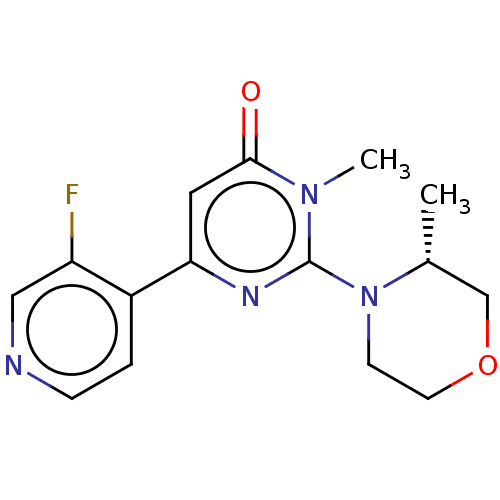 (CHEMBL3401118) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3beta assessed as inhibition of [gamma32P]ATP after 1 hr by liquid scintillation counting in presence of prephosph... | Bioorg Med Chem Lett 25: 1086-91 (2015) Article DOI: 10.1016/j.bmcl.2015.01.005 BindingDB Entry DOI: 10.7270/Q2WD428R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094082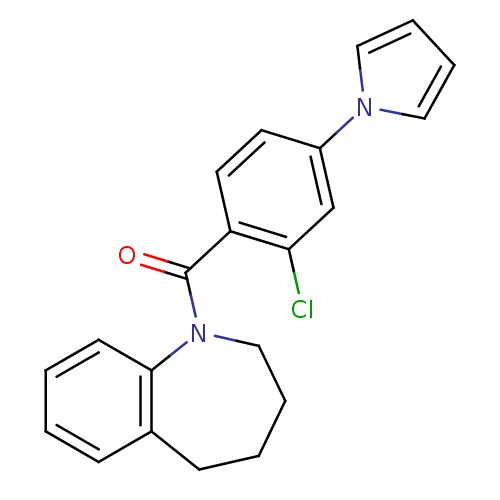 ((2-Chloro-4-pyrrol-1-yl-phenyl)-(2,3,4,5-tetrahydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Bos taurus (cattle)) | BDBM155226 (US9006232, 1.903) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Mitsubishi Tanabe Pharma Corporation US Patent | Assay Description A mixture containing 100 mM MES-sodium hydroxide (pH 6.5), 1 mM magnesium acetate, 0.5 mM EGTA, 5 mM, .beta.-mercaptoethanol, 0.02% Tween 20, 10% gly... | US Patent US9006232 (2015) BindingDB Entry DOI: 10.7270/Q25T3J7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50269427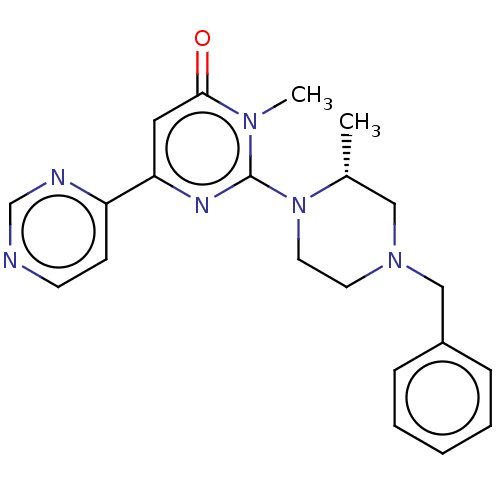 (CHEMBL4082198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sohyaku, Innovative Research Division, Mitsubishi Tanabe Pharma Corporation, 1000, Kamoshida-cho, Aoba-ku, Yokohama 227-0033, Japan. Curated by ChEMBL | Assay Description Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect... | Bioorg Med Chem Lett 27: 3733-3738 (2017) Article DOI: 10.1016/j.bmcl.2017.06.077 BindingDB Entry DOI: 10.7270/Q2XW4N9C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Bos taurus (cattle)) | BDBM155185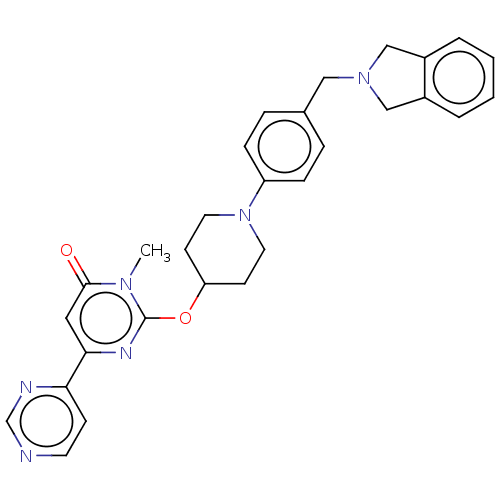 (US9006232, 1.219) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Mitsubishi Tanabe Pharma Corporation US Patent | Assay Description A mixture containing 100 mM MES-sodium hydroxide (pH 6.5), 1 mM magnesium acetate, 0.5 mM EGTA, 5 mM, .beta.-mercaptoethanol, 0.02% Tween 20, 10% gly... | US Patent US9006232 (2015) BindingDB Entry DOI: 10.7270/Q25T3J7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50094066 ((2-Chloro-4-pyrazol-1-yl-phenyl)-(2,3,4,5-tetrahyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Otsuka Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human Vasopressin V2 receptor expressed in HeLa cells | J Med Chem 43: 4388-97 (2000) BindingDB Entry DOI: 10.7270/Q2PG1R04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Bos taurus (cattle)) | BDBM155158 (US9006232, 1.104) | PDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 6.5 | 25 |
Mitsubishi Tanabe Pharma Corporation US Patent | Assay Description A mixture containing 100 mM MES-sodium hydroxide (pH 6.5), 1 mM magnesium acetate, 0.5 mM EGTA, 5 mM, .beta.-mercaptoethanol, 0.02% Tween 20, 10% gly... | US Patent US9006232 (2015) BindingDB Entry DOI: 10.7270/Q25T3J7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 782 total ) | Next | Last >> |