 Found 121 hits with Last Name = 'ojida' and Initial = 'a'
Found 121 hits with Last Name = 'ojida' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591389
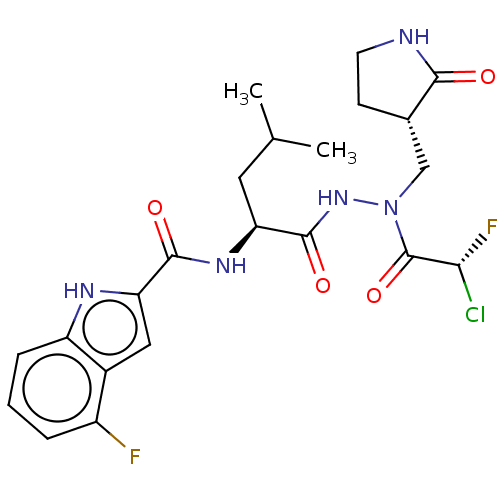
(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591390
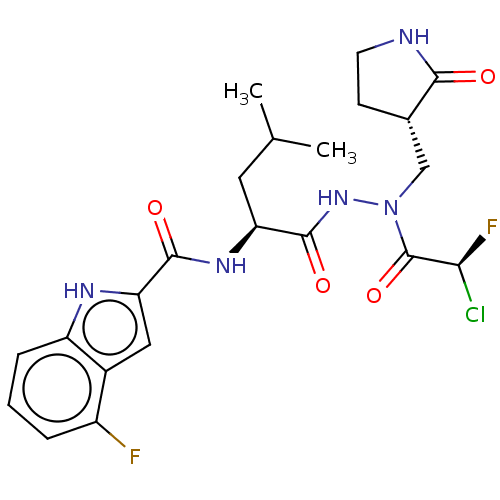
(CHEMBL5190754)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM496902

(CVD-0018409 | PF-07321332 | US11351149, Example 13...)Show SMILES CC(C)(C)[C@H](NC(=O)C(F)(F)F)C(=O)N1C[C@H]2[C@@H]([C@H]1C(=O)N[C@@H](C[C@@H]1CCNC1=O)C#N)C2(C)C Show InChI InChI=1S/C23H32F3N5O4/c1-21(2,3)16(30-20(35)23(24,25)26)19(34)31-10-13-14(22(13,4)5)15(31)18(33)29-12(9-27)8-11-6-7-28-17(11)32/h11-16H,6-8,10H2,1-5H3,(H,28,32)(H,29,33)(H,30,35)/t11-,12-,13-,14-,15-,16+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM420298

(CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C[C@@H]1CCNC1=O)C(=O)CO Show InChI InChI=1S/C24H32N4O6/c1-13(2)9-18(23(32)27-17(20(30)12-29)10-14-7-8-25-22(14)31)28-24(33)19-11-15-16(26-19)5-4-6-21(15)34-3/h4-6,11,13-14,17-18,26,29H,7-10,12H2,1-3H3,(H,25,31)(H,27,32)(H,28,33)/t14-,17-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591389

(CHEMBL5179778)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591391
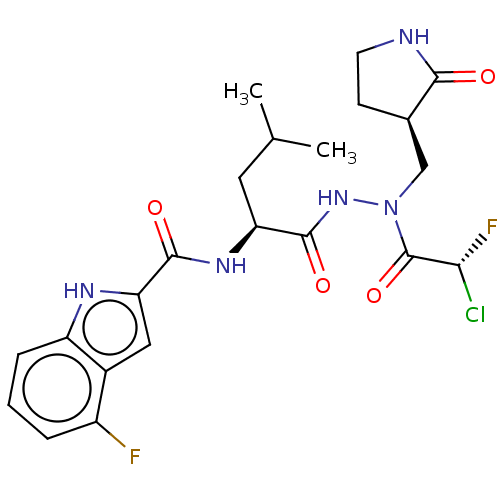
(CHEMBL5179611)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358192
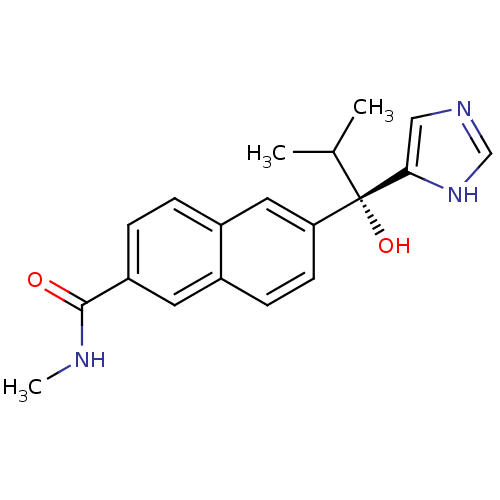
(CHEMBL1921975)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363
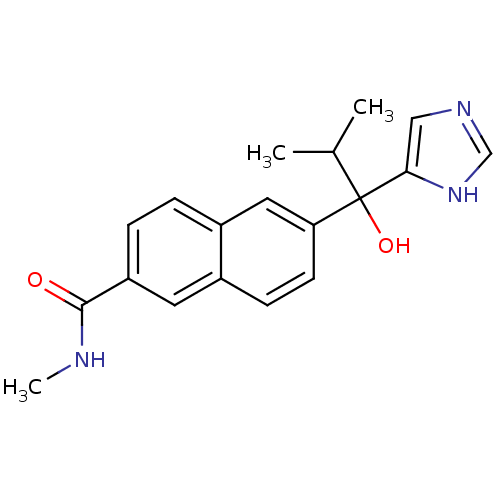
(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591388
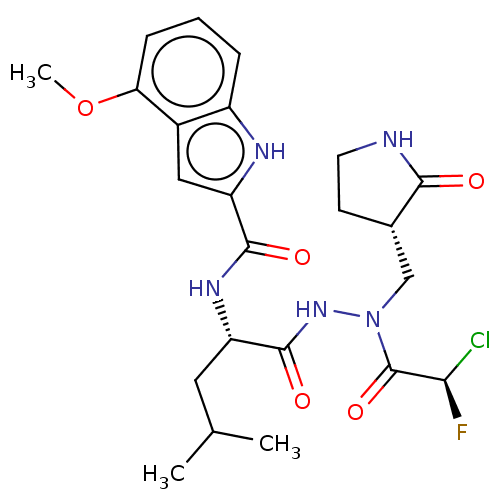
(CHEMBL5198073)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338355
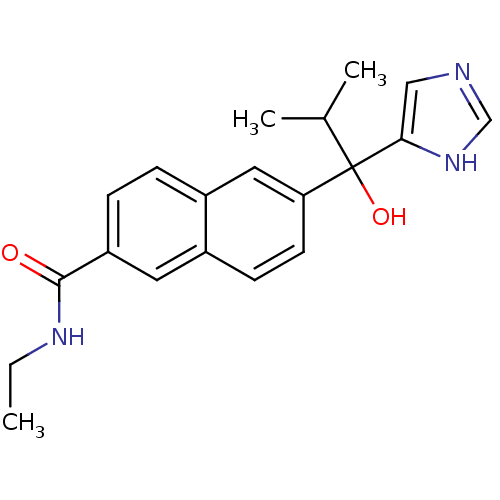
(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338349
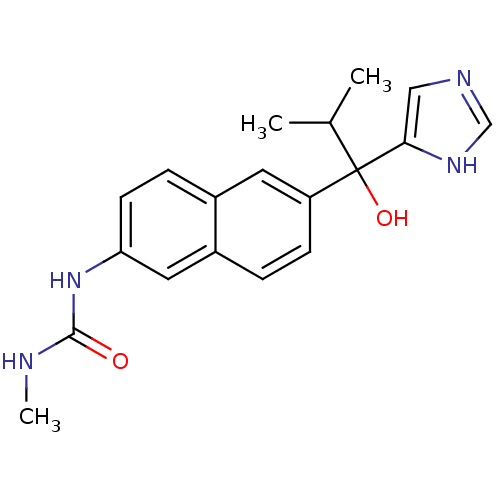
(CHEMBL1682890 | rac-N'-{6-[1-Hydroxy-1-(1H-imidazo...)Show SMILES CNC(=O)Nc1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H22N4O2/c1-12(2)19(25,17-10-21-11-22-17)15-6-4-14-9-16(23-18(24)20-3)7-5-13(14)8-15/h4-12,25H,1-3H3,(H,21,22)(H2,20,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338358
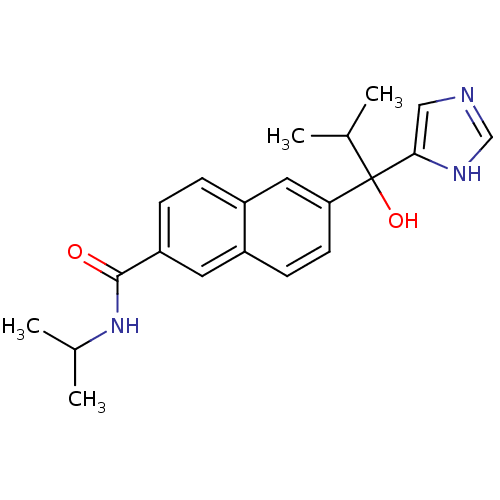
(CHEMBL1682896 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)NC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-13(2)21(26,19-11-22-12-23-19)18-8-7-15-9-17(6-5-16(15)10-18)20(25)24-14(3)4/h5-14,26H,1-4H3,(H,22,23)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338353
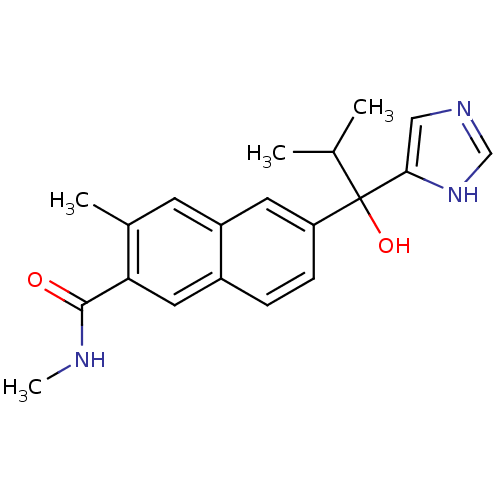
(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338351
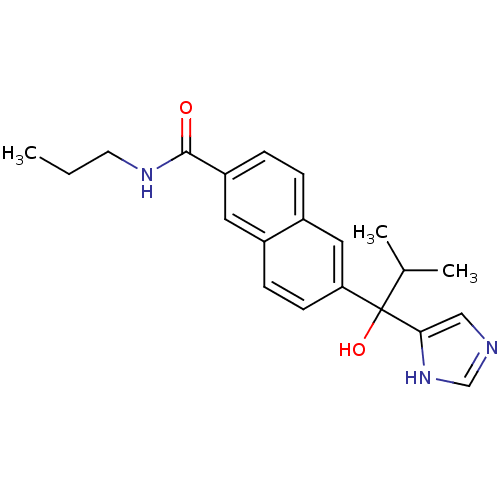
(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358195
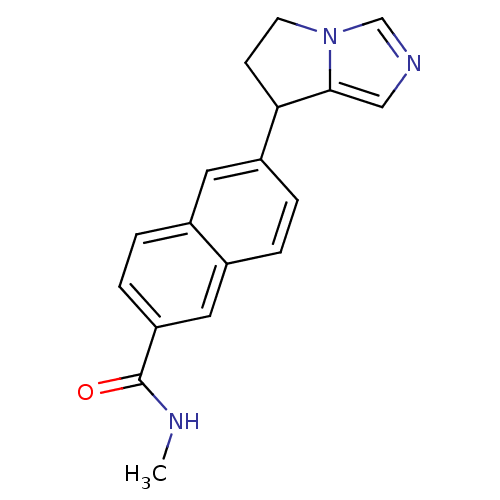
(CHEMBL1921979)Show InChI InChI=1S/C18H17N3O/c1-19-18(22)15-5-3-12-8-14(4-2-13(12)9-15)16-6-7-21-11-20-10-17(16)21/h2-5,8-11,16H,6-7H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338362
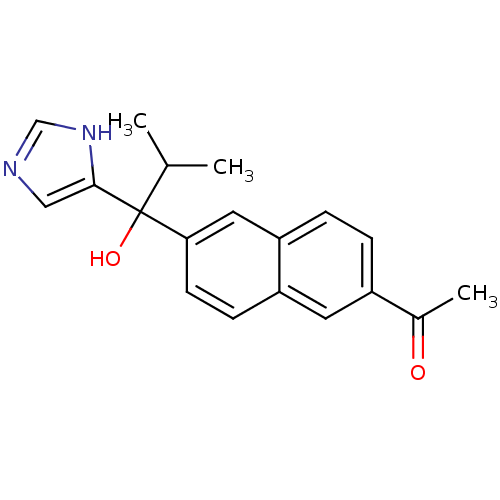
(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50358195

(CHEMBL1921979)Show InChI InChI=1S/C18H17N3O/c1-19-18(22)15-5-3-12-8-14(4-2-13(12)9-15)16-6-7-21-11-20-10-17(16)21/h2-5,8-11,16H,6-7H2,1H3,(H,19,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591392
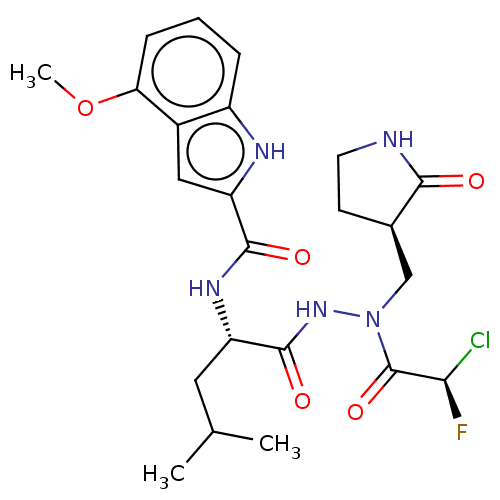
(CHEMBL5195394)Show SMILES COc1cccc2[nH]c(cc12)C(=O)N[C@@H](CC(C)C)C(=O)NN(C[C@H]1CCNC1=O)C(=O)[C@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358201
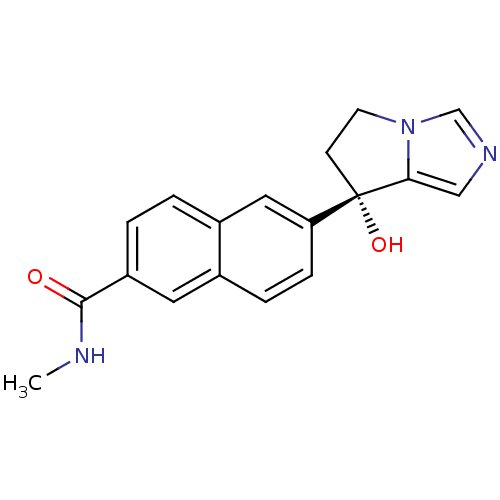
(CHEMBL1921976 | US9611270, orteronel)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@]1(O)CCn2cncc12 |r| Show InChI InChI=1S/C18H17N3O2/c1-19-17(22)14-3-2-13-9-15(5-4-12(13)8-14)18(23)6-7-21-11-20-10-16(18)21/h2-5,8-11,23H,6-7H2,1H3,(H,19,22)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338365
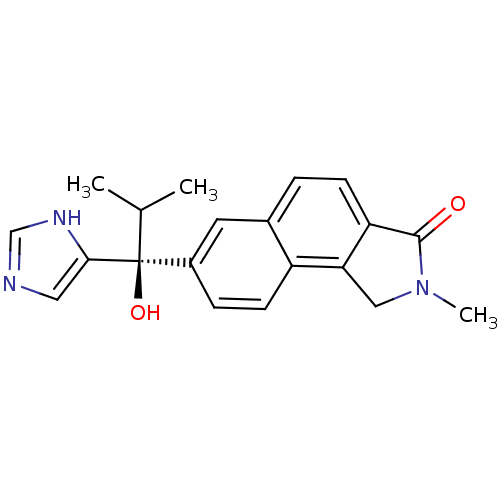
((-)-(S)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338357

(CHEMBL1682903 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)N(C)Cc3cc2c1 Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)16-5-4-13-8-17-15(6-14(13)7-16)10-23(3)19(17)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338366
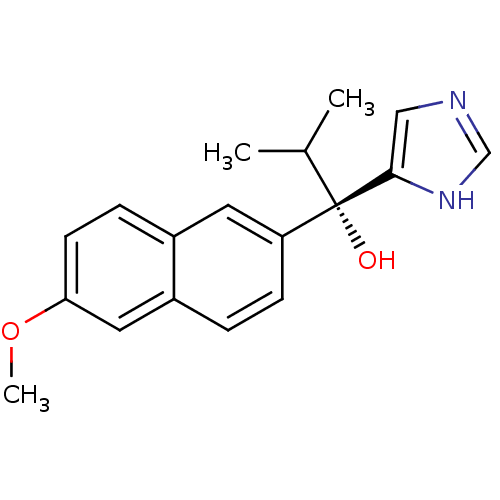
((S)-1-(1H-imidazol-4-yl)-1-(6-methoxynaphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C18H20N2O2/c1-12(2)18(21,17-10-19-11-20-17)15-6-4-14-9-16(22-3)7-5-13(14)8-15/h4-12,21H,1-3H3,(H,19,20)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50358197
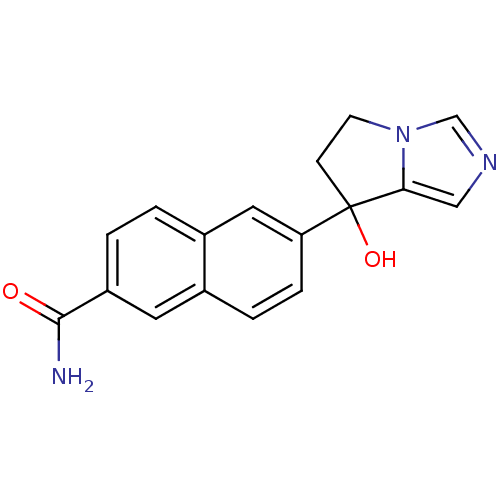
(CHEMBL1921981)Show InChI InChI=1S/C17H15N3O2/c18-16(21)13-2-1-12-8-14(4-3-11(12)7-13)17(22)5-6-20-10-19-9-15(17)20/h1-4,7-10,22H,5-6H2,(H2,18,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338354
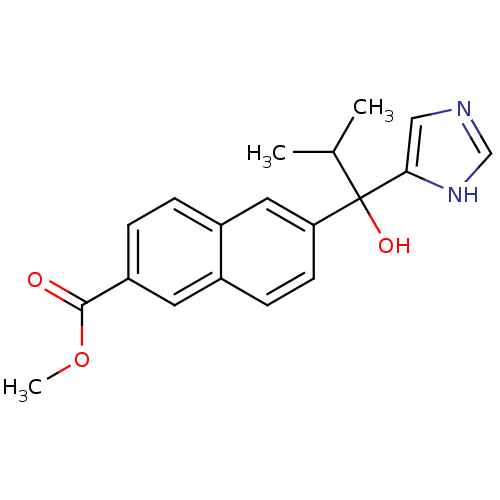
(CHEMBL1682891 | rac-Methyl 6-[1-hydroxy-1-(1H-imid...)Show SMILES COC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20N2O3/c1-12(2)19(23,17-10-20-11-21-17)16-7-6-13-8-15(18(22)24-3)5-4-14(13)9-16/h4-12,23H,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338366

((S)-1-(1H-imidazol-4-yl)-1-(6-methoxynaphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C18H20N2O2/c1-12(2)18(21,17-10-19-11-20-17)15-6-4-14-9-16(22-3)7-5-13(14)8-15/h4-12,21H,1-3H3,(H,19,20)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1ab
(2019-nCoV) | BDBM50591390

(CHEMBL5190754)Show SMILES CC(C)C[C@H](NC(=O)c1cc2c(F)cccc2[nH]1)C(=O)NN(C[C@@H]1CCNC1=O)C(=O)[C@@H](F)Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01081
BindingDB Entry DOI: 10.7270/Q2NK3K0J |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338362

(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358197

(CHEMBL1921981)Show InChI InChI=1S/C17H15N3O2/c18-16(21)13-2-1-12-8-14(4-3-11(12)7-13)17(22)5-6-20-10-19-9-15(17)20/h1-4,7-10,22H,5-6H2,(H2,18,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338352

(CHEMBL1682889 | rac-N-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(NC(C)=O)ccc2c1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,18-10-20-11-21-18)16-6-4-15-9-17(22-13(3)23)7-5-14(15)8-16/h4-12,24H,1-3H3,(H,20,21)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338364
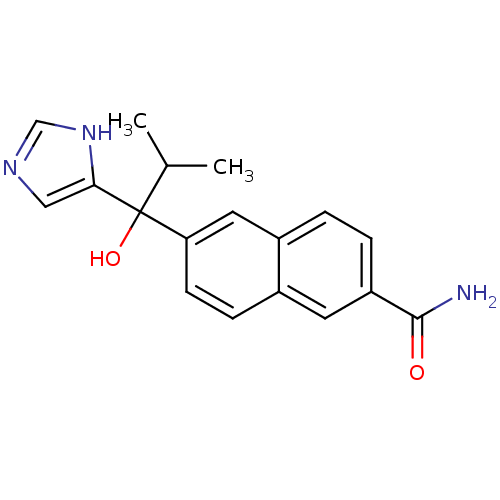
(CHEMBL1682892 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(N)=O Show InChI InChI=1S/C18H19N3O2/c1-11(2)18(23,16-9-20-10-21-16)15-6-5-12-7-14(17(19)22)4-3-13(12)8-15/h3-11,23H,1-2H3,(H2,19,22)(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338350
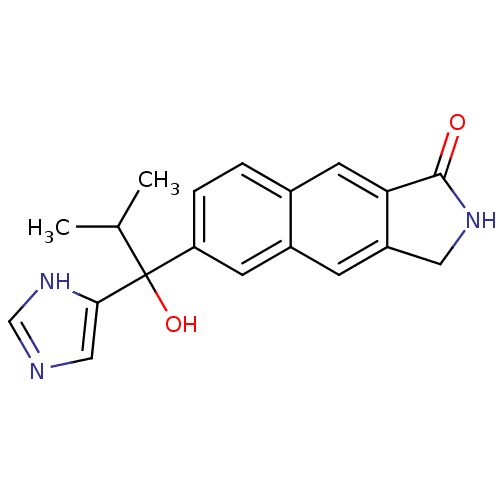
(CHEMBL1682902 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)NCc3cc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)15-4-3-12-7-16-14(5-13(12)6-15)8-21-18(16)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338349

(CHEMBL1682890 | rac-N'-{6-[1-Hydroxy-1-(1H-imidazo...)Show SMILES CNC(=O)Nc1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H22N4O2/c1-12(2)19(25,17-10-21-11-22-17)15-6-4-14-9-16(23-18(24)20-3)7-5-13(14)8-15/h4-12,25H,1-3H3,(H,21,22)(H2,20,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338351

(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50358193
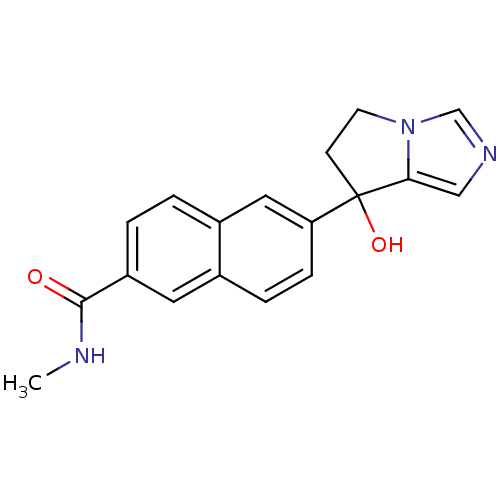
(CHEMBL1921977)Show InChI InChI=1S/C18H17N3O2/c1-19-17(22)14-3-2-13-9-15(5-4-12(13)8-14)18(23)6-7-21-11-20-10-16(18)21/h2-5,8-11,23H,6-7H2,1H3,(H,19,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of human CYP17A1 |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338353

(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338352

(CHEMBL1682889 | rac-N-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(NC(C)=O)ccc2c1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,18-10-20-11-21-18)16-6-4-15-9-17(22-13(3)23)7-5-14(15)8-16/h4-12,24H,1-3H3,(H,20,21)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338350

(CHEMBL1682902 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)NCc3cc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)15-4-3-12-7-16-14(5-13(12)6-15)8-21-18(16)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338354

(CHEMBL1682891 | rac-Methyl 6-[1-hydroxy-1-(1H-imid...)Show SMILES COC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20N2O3/c1-12(2)19(23,17-10-20-11-21-17)16-7-6-13-8-15(18(22)24-3)5-4-14(13)9-16/h4-12,23H,1-3H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338355

(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50358201

(CHEMBL1921976 | US9611270, orteronel)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)[C@@]1(O)CCn2cncc12 |r| Show InChI InChI=1S/C18H17N3O2/c1-19-17(22)14-3-2-13-9-15(5-4-12(13)8-14)18(23)6-7-21-11-20-10-16(18)21/h2-5,8-11,23H,6-7H2,1H3,(H,19,22)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of 17,20-lyase activity of Sprague-Dawley rat testicular microsomal CYP17A1 using [1,2-3H]-17a-hydroxyprogesterone as substrate after 15 m... |
Bioorg Med Chem 19: 6383-99 (2011)
Article DOI: 10.1016/j.bmc.2011.08.066
BindingDB Entry DOI: 10.7270/Q2X63NDF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data