 Found 356 hits with Last Name = 'okamoto' and Initial = 't'
Found 356 hits with Last Name = 'okamoto' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasmepsin II
(Plasmodium falciparum) | BDBM50209559
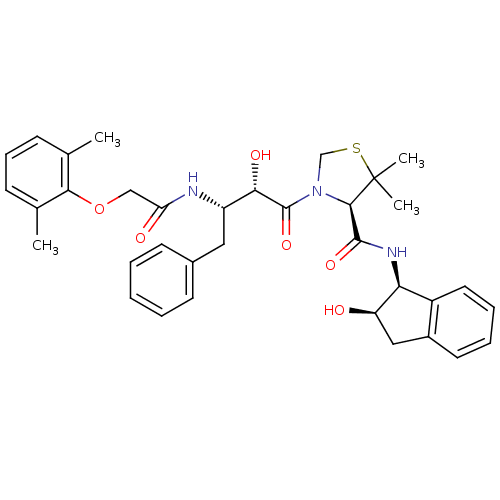
((4R)-3-[(2S,3S)-3-{[(2,6-dimethylphenoxy)acetyl]am...)Show SMILES Cc1cccc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H41N3O6S/c1-21-11-10-12-22(2)31(21)44-19-28(40)36-26(17-23-13-6-5-7-14-23)30(41)34(43)38-20-45-35(3,4)32(38)33(42)37-29-25-16-9-8-15-24(25)18-27(29)39/h5-16,26-27,29-30,32,39,41H,17-20H2,1-4H3,(H,36,40)(H,37,42)/t26-,27+,29-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50065258
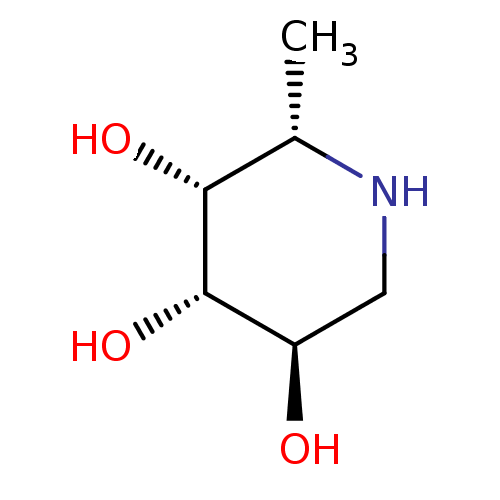
((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...)Show InChI InChI=1S/C6H13NO3/c1-3-5(9)6(10)4(8)2-7-3/h3-10H,2H2,1H3/t3-,4+,5+,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ki value against bovine alpha-L-fucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273723

((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES COc1cccc(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c1 |r| Show InChI InChI=1S/C34H39N3O7S/c1-34(2)31(32(41)36-29-25-15-8-7-12-22(25)17-27(29)38)37(20-45-34)33(42)30(40)26(16-21-10-5-4-6-11-21)35-28(39)19-44-24-14-9-13-23(18-24)43-3/h4-15,18,26-27,29-31,38,40H,16-17,19-20H2,1-3H3,(H,35,39)(H,36,41)/t26-,27+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273742

((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1cc(O)cc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H41N3O7S/c1-20-14-24(39)15-21(2)31(20)45-18-28(41)36-26(16-22-10-6-5-7-11-22)30(42)34(44)38-19-46-35(3,4)32(38)33(43)37-29-25-13-9-8-12-23(25)17-27(29)40/h5-15,26-27,29-30,32,39-40,42H,16-19H2,1-4H3,(H,36,41)(H,37,43)/t26-,27+,29-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50065257

((2R,3R,4R,5R,6S)-2-Hydroxymethyl-6-methyl-piperidi...)Show SMILES C[C@@H]1N[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C7H15NO4/c1-3-5(10)7(12)6(11)4(2-9)8-3/h3-12H,2H2,1H3/t3-,4+,5+,6+,7+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ki value against bovine alpha-L-fucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273751

((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1cc(NCc2ccncc2)cc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C41H47N5O6S/c1-25-18-30(43-22-28-14-16-42-17-15-28)19-26(2)37(25)52-23-34(48)44-32(20-27-10-6-5-7-11-27)36(49)40(51)46-24-53-41(3,4)38(46)39(50)45-35-31-13-9-8-12-29(31)21-33(35)47/h5-19,32-33,35-36,38,43,47,49H,20-24H2,1-4H3,(H,44,48)(H,45,50)/t32-,33+,35-,36-,38+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273746

((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CCNc1cc(C)c(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c(C)c1 |r| Show InChI InChI=1S/C37H46N4O6S/c1-6-38-26-16-22(2)33(23(3)17-26)47-20-30(43)39-28(18-24-12-8-7-9-13-24)32(44)36(46)41-21-48-37(4,5)34(41)35(45)40-31-27-15-11-10-14-25(27)19-29(31)42/h7-17,28-29,31-32,34,38,42,44H,6,18-21H2,1-5H3,(H,39,43)(H,40,45)/t28-,29+,31-,32-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273729
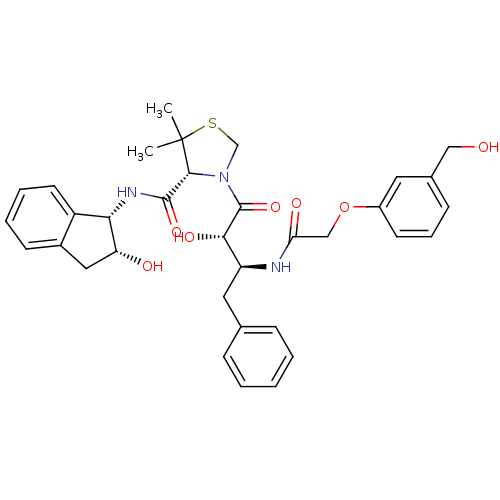
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(CO)c1 |r| Show InChI InChI=1S/C34H39N3O7S/c1-34(2)31(32(42)36-29-25-14-7-6-12-23(25)17-27(29)39)37(20-45-34)33(43)30(41)26(16-21-9-4-3-5-10-21)35-28(40)19-44-24-13-8-11-22(15-24)18-38/h3-15,26-27,29-31,38-39,41H,16-20H2,1-2H3,(H,35,40)(H,36,42)/t26-,27+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273749
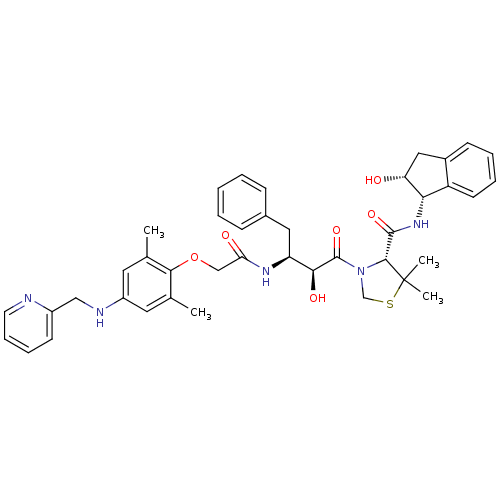
((R)-3-((2S,3S)-3-(2-(2,6-dimethyl-4-(pyridin-2-ylm...)Show SMILES Cc1cc(NCc2ccccn2)cc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C41H47N5O6S/c1-25-18-30(43-22-29-15-10-11-17-42-29)19-26(2)37(25)52-23-34(48)44-32(20-27-12-6-5-7-13-27)36(49)40(51)46-24-53-41(3,4)38(46)39(50)45-35-31-16-9-8-14-28(31)21-33(35)47/h5-19,32-33,35-36,38,43,47,49H,20-24H2,1-4H3,(H,44,48)(H,45,50)/t32-,33+,35-,36-,38+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273730
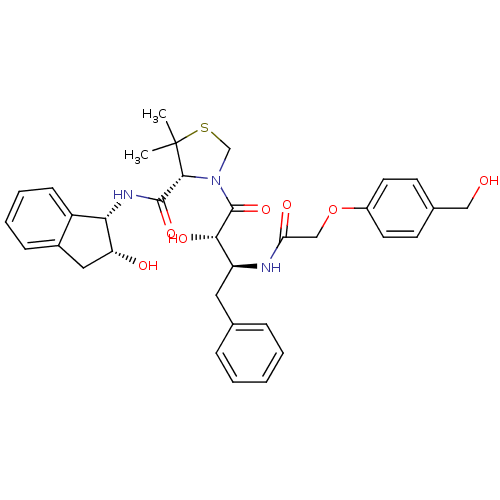
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccc(CO)cc1 |r| Show InChI InChI=1S/C34H39N3O7S/c1-34(2)31(32(42)36-29-25-11-7-6-10-23(25)17-27(29)39)37(20-45-34)33(43)30(41)26(16-21-8-4-3-5-9-21)35-28(40)19-44-24-14-12-22(18-38)13-15-24/h3-15,26-27,29-31,38-39,41H,16-20H2,1-2H3,(H,35,40)(H,36,42)/t26-,27+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273727
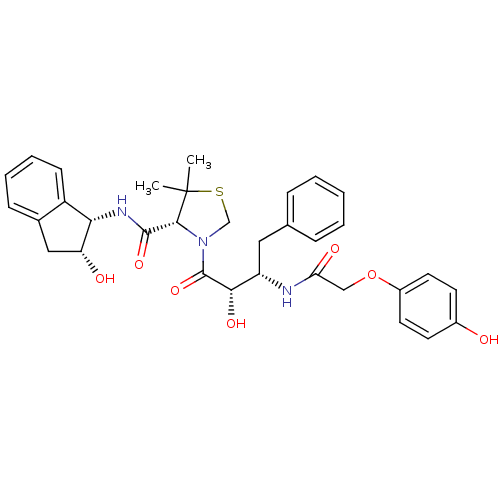
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccc(O)cc1 |r| Show InChI InChI=1S/C33H37N3O7S/c1-33(2)30(31(41)35-28-24-11-7-6-10-21(24)17-26(28)38)36(19-44-33)32(42)29(40)25(16-20-8-4-3-5-9-20)34-27(39)18-43-23-14-12-22(37)13-15-23/h3-15,25-26,28-30,37-38,40H,16-19H2,1-2H3,(H,34,39)(H,35,41)/t25-,26+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273726
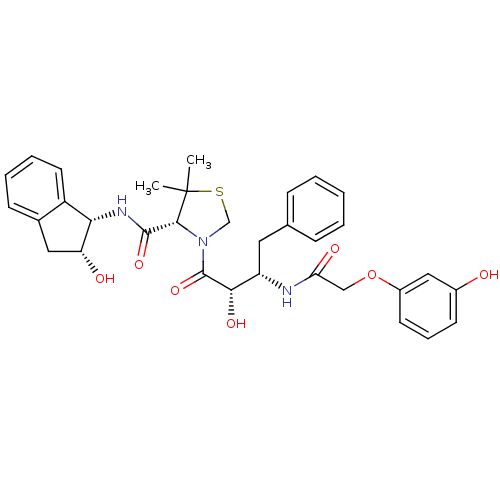
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(O)c1 |r| Show InChI InChI=1S/C33H37N3O7S/c1-33(2)30(31(41)35-28-24-14-7-6-11-21(24)16-26(28)38)36(19-44-33)32(42)29(40)25(15-20-9-4-3-5-10-20)34-27(39)18-43-23-13-8-12-22(37)17-23/h3-14,17,25-26,28-30,37-38,40H,15-16,18-19H2,1-2H3,(H,34,39)(H,35,41)/t25-,26+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273737
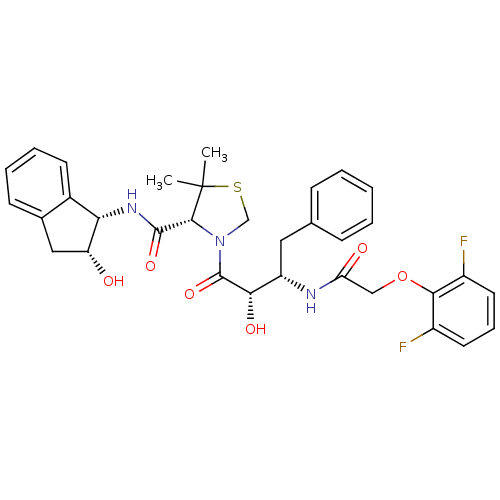
((4R)-3-[(2S,3S)-3-{[(2,6-difluorophenoxy)acetyl]am...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(F)cccc1F |r| Show InChI InChI=1S/C33H35F2N3O6S/c1-33(2)30(31(42)37-27-21-12-7-6-11-20(21)16-25(27)39)38(18-45-33)32(43)28(41)24(15-19-9-4-3-5-10-19)36-26(40)17-44-29-22(34)13-8-14-23(29)35/h3-14,24-25,27-28,30,39,41H,15-18H2,1-2H3,(H,36,40)(H,37,42)/t24-,25+,27-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273741
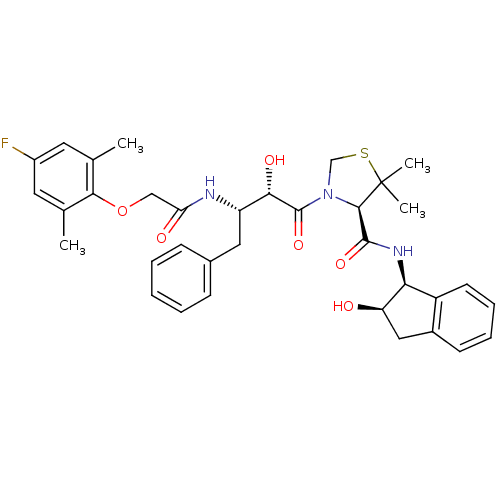
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1cc(F)cc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H40FN3O6S/c1-20-14-24(36)15-21(2)31(20)45-18-28(41)37-26(16-22-10-6-5-7-11-22)30(42)34(44)39-19-46-35(3,4)32(39)33(43)38-29-25-13-9-8-12-23(25)17-27(29)40/h5-15,26-27,29-30,32,40,42H,16-19H2,1-4H3,(H,37,41)(H,38,43)/t26-,27+,29-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273745
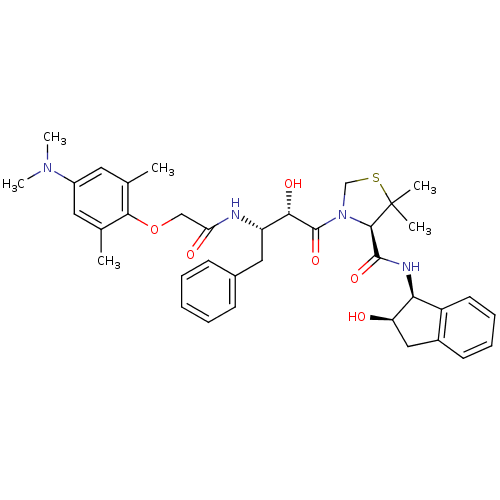
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CN(C)c1cc(C)c(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c(C)c1 |r| Show InChI InChI=1S/C37H46N4O6S/c1-22-16-26(40(5)6)17-23(2)33(22)47-20-30(43)38-28(18-24-12-8-7-9-13-24)32(44)36(46)41-21-48-37(3,4)34(41)35(45)39-31-27-15-11-10-14-25(27)19-29(31)42/h7-17,28-29,31-32,34,42,44H,18-21H2,1-6H3,(H,38,43)(H,39,45)/t28-,29+,31-,32-,34+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273750
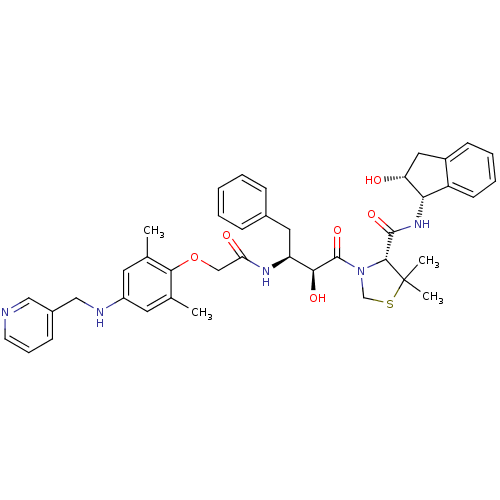
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1cc(NCc2cccnc2)cc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C41H47N5O6S/c1-25-17-30(43-22-28-13-10-16-42-21-28)18-26(2)37(25)52-23-34(48)44-32(19-27-11-6-5-7-12-27)36(49)40(51)46-24-53-41(3,4)38(46)39(50)45-35-31-15-9-8-14-29(31)20-33(35)47/h5-18,21,32-33,35-36,38,43,47,49H,19-20,22-24H2,1-4H3,(H,44,48)(H,45,50)/t32-,33+,35-,36-,38+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273740
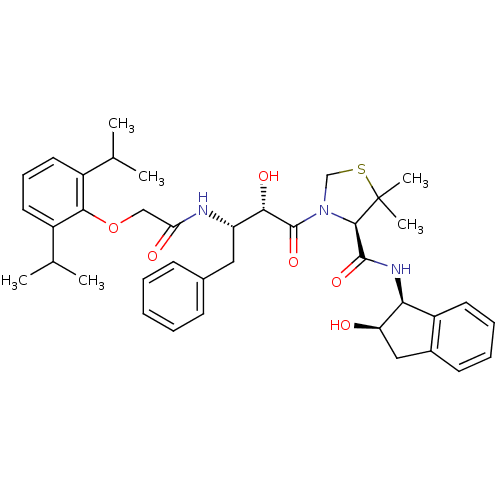
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC(C)c1cccc(C(C)C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C39H49N3O6S/c1-23(2)27-17-12-18-28(24(3)4)35(27)48-21-32(44)40-30(19-25-13-8-7-9-14-25)34(45)38(47)42-22-49-39(5,6)36(42)37(46)41-33-29-16-11-10-15-26(29)20-31(33)43/h7-18,23-24,30-31,33-34,36,43,45H,19-22H2,1-6H3,(H,40,44)(H,41,46)/t30-,31+,33-,34-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273743
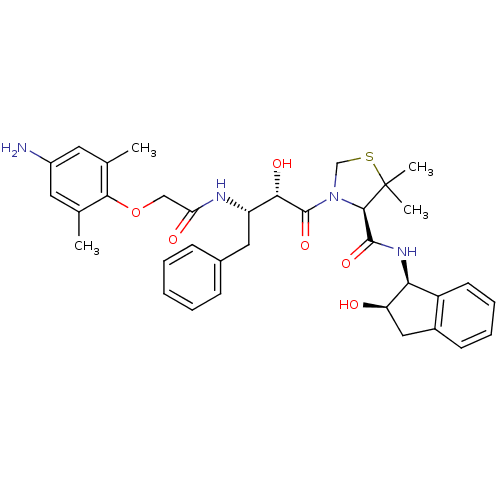
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1cc(N)cc(C)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H42N4O6S/c1-20-14-24(36)15-21(2)31(20)45-18-28(41)37-26(16-22-10-6-5-7-11-22)30(42)34(44)39-19-46-35(3,4)32(39)33(43)38-29-25-13-9-8-12-23(25)17-27(29)40/h5-15,26-27,29-30,32,40,42H,16-19,36H2,1-4H3,(H,37,41)(H,38,43)/t26-,27+,29-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273736
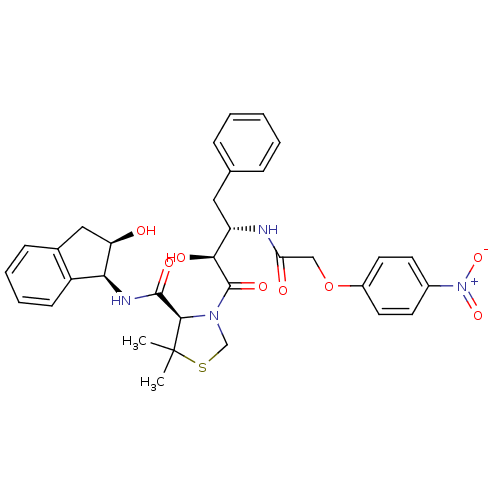
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccc(cc1)[N+]([O-])=O |r| Show InChI InChI=1S/C33H36N4O8S/c1-33(2)30(31(41)35-28-24-11-7-6-10-21(24)17-26(28)38)36(19-46-33)32(42)29(40)25(16-20-8-4-3-5-9-20)34-27(39)18-45-23-14-12-22(13-15-23)37(43)44/h3-15,25-26,28-30,38,40H,16-19H2,1-2H3,(H,34,39)(H,35,41)/t25-,26+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273744
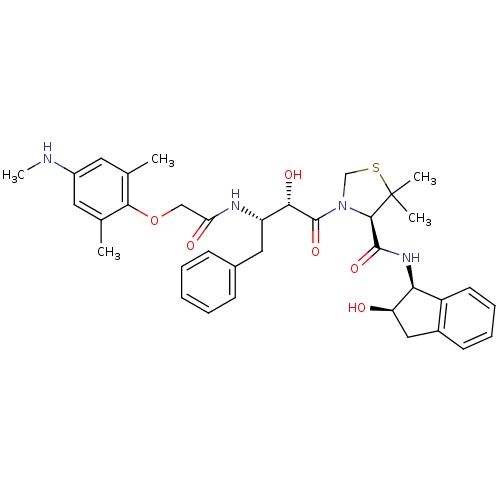
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CNc1cc(C)c(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c(C)c1 |r| Show InChI InChI=1S/C36H44N4O6S/c1-21-15-25(37-5)16-22(2)32(21)46-19-29(42)38-27(17-23-11-7-6-8-12-23)31(43)35(45)40-20-47-36(3,4)33(40)34(44)39-30-26-14-10-9-13-24(26)18-28(30)41/h6-16,27-28,30-31,33,37,41,43H,17-20H2,1-5H3,(H,38,42)(H,39,44)/t27-,28+,30-,31-,33+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273748
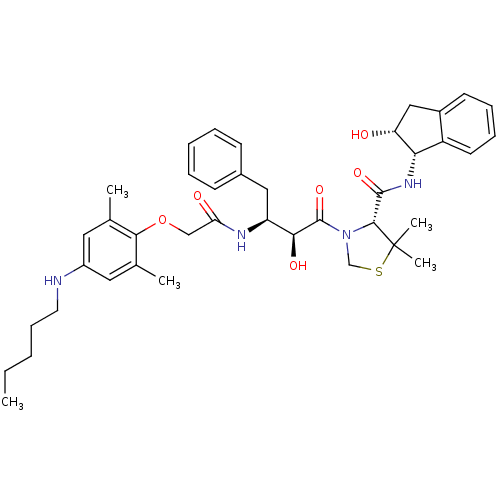
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CCCCCNc1cc(C)c(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c(C)c1 |r| Show InChI InChI=1S/C40H52N4O6S/c1-6-7-13-18-41-29-19-25(2)36(26(3)20-29)50-23-33(46)42-31(21-27-14-9-8-10-15-27)35(47)39(49)44-24-51-40(4,5)37(44)38(48)43-34-30-17-12-11-16-28(30)22-32(34)45/h8-12,14-17,19-20,31-32,34-35,37,41,45,47H,6-7,13,18,21-24H2,1-5H3,(H,42,46)(H,43,48)/t31-,32+,34-,35-,37+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273733
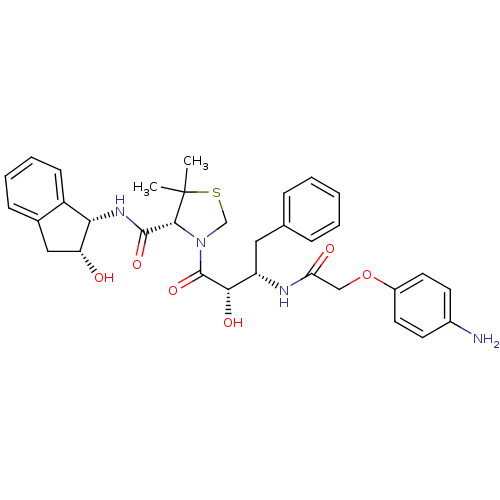
((R)-N-[(1S,2R)-2-Hhydroxyindan-1-yl]-3-[(2S,3S)-3-...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccc(N)cc1 |r| Show InChI InChI=1S/C33H38N4O6S/c1-33(2)30(31(41)36-28-24-11-7-6-10-21(24)17-26(28)38)37(19-44-33)32(42)29(40)25(16-20-8-4-3-5-9-20)35-27(39)18-43-23-14-12-22(34)13-15-23/h3-15,25-26,28-30,38,40H,16-19,34H2,1-2H3,(H,35,39)(H,36,41)/t25-,26+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273732
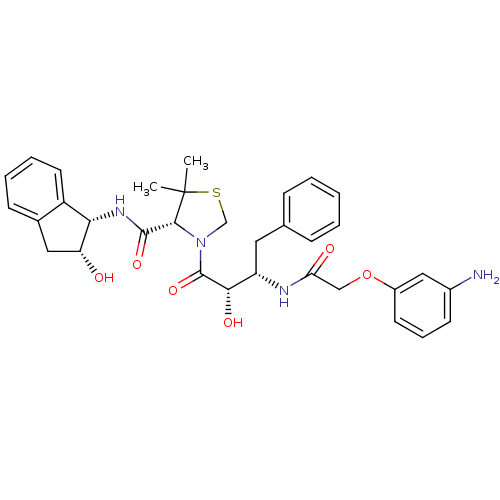
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(N)c1 |r| Show InChI InChI=1S/C33H38N4O6S/c1-33(2)30(31(41)36-28-24-14-7-6-11-21(24)16-26(28)38)37(19-44-33)32(42)29(40)25(15-20-9-4-3-5-10-20)35-27(39)18-43-23-13-8-12-22(34)17-23/h3-14,17,25-26,28-30,38,40H,15-16,18-19,34H2,1-2H3,(H,35,39)(H,36,41)/t25-,26+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273747
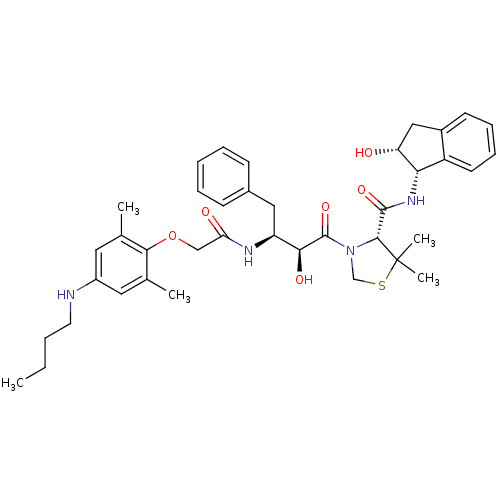
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CCCCNc1cc(C)c(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c(C)c1 |r| Show InChI InChI=1S/C39H50N4O6S/c1-6-7-17-40-28-18-24(2)35(25(3)19-28)49-22-32(45)41-30(20-26-13-9-8-10-14-26)34(46)38(48)43-23-50-39(4,5)36(43)37(47)42-33-29-16-12-11-15-27(29)21-31(33)44/h8-16,18-19,30-31,33-34,36,40,44,46H,6-7,17,20-23H2,1-5H3,(H,41,45)(H,42,47)/t30-,31+,33-,34-,36+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273735

((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1cccc(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C33H36N4O8S/c1-33(2)30(31(41)35-28-24-14-7-6-11-21(24)16-26(28)38)36(19-46-33)32(42)29(40)25(15-20-9-4-3-5-10-20)34-27(39)18-45-23-13-8-12-22(17-23)37(43)44/h3-14,17,25-26,28-30,38,40H,15-16,18-19H2,1-2H3,(H,34,39)(H,35,41)/t25-,26+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273738
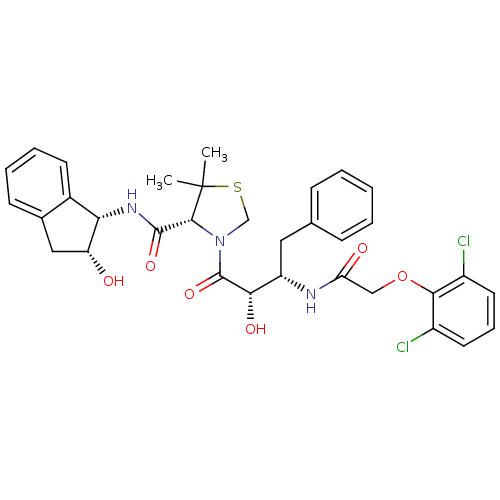
((4R)-3-[(2S,3S)-3-{[(2,6-dichlorophenoxy)acetyl]am...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(Cl)cccc1Cl |r| Show InChI InChI=1S/C33H35Cl2N3O6S/c1-33(2)30(31(42)37-27-21-12-7-6-11-20(21)16-25(27)39)38(18-45-33)32(43)28(41)24(15-19-9-4-3-5-10-19)36-26(40)17-44-29-22(34)13-8-14-23(29)35/h3-14,24-25,27-28,30,39,41H,15-18H2,1-2H3,(H,36,40)(H,37,42)/t24-,25+,27-,28-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273720
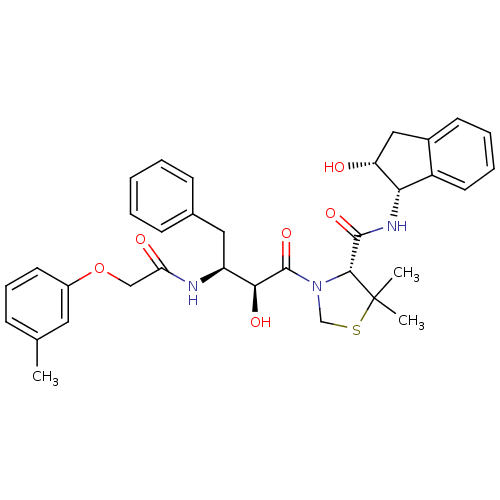
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1cccc(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)c1 |r| Show InChI InChI=1S/C34H39N3O6S/c1-21-10-9-14-24(16-21)43-19-28(39)35-26(17-22-11-5-4-6-12-22)30(40)33(42)37-20-44-34(2,3)31(37)32(41)36-29-25-15-8-7-13-23(25)18-27(29)38/h4-16,26-27,29-31,38,40H,17-20H2,1-3H3,(H,35,39)(H,36,41)/t26-,27+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273734

((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccccc1[N+]([O-])=O |r| Show InChI InChI=1S/C33H36N4O8S/c1-33(2)30(31(41)35-28-22-13-7-6-12-21(22)17-25(28)38)36(19-46-33)32(42)29(40)23(16-20-10-4-3-5-11-20)34-27(39)18-45-26-15-9-8-14-24(26)37(43)44/h3-15,23,25,28-30,38,40H,16-19H2,1-2H3,(H,34,39)(H,35,41)/t23-,25+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273721
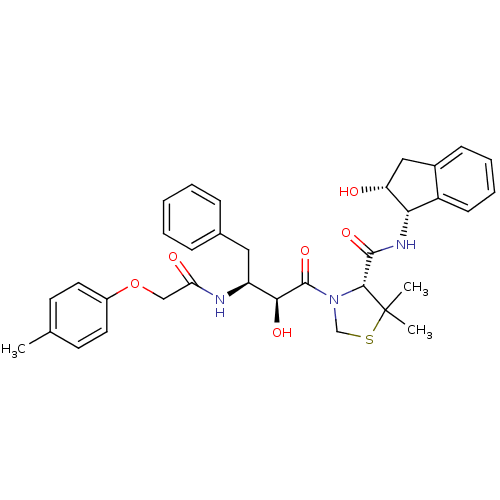
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1ccc(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C34H39N3O6S/c1-21-13-15-24(16-14-21)43-19-28(39)35-26(17-22-9-5-4-6-10-22)30(40)33(42)37-20-44-34(2,3)31(37)32(41)36-29-25-12-8-7-11-23(25)18-27(29)38/h4-16,26-27,29-31,38,40H,17-20H2,1-3H3,(H,35,39)(H,36,41)/t26-,27+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50163439
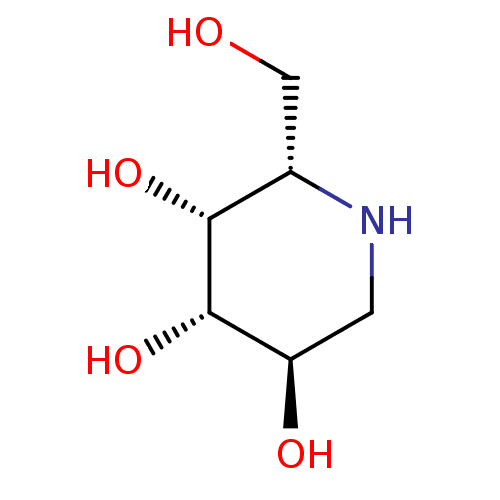
((2S,3R,4S,5R)-2-Hydroxymethyl-piperidine-3,4,5-tri...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5+,6-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ki value against bovine alpha-L-fucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273725
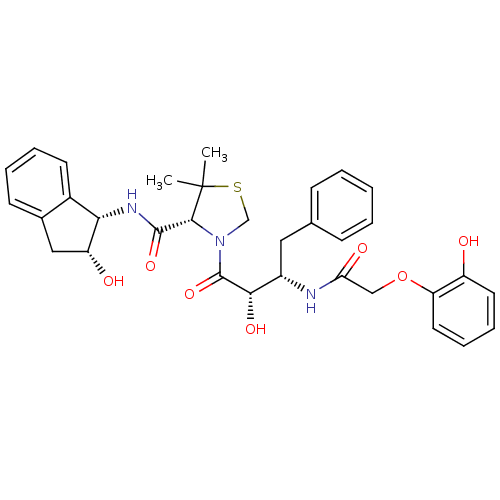
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccccc1O |r| Show InChI InChI=1S/C33H37N3O7S/c1-33(2)30(31(41)35-28-22-13-7-6-12-21(22)17-25(28)38)36(19-44-33)32(42)29(40)23(16-20-10-4-3-5-11-20)34-27(39)18-43-26-15-9-8-14-24(26)37/h3-15,23,25,28-30,37-38,40H,16-19H2,1-2H3,(H,34,39)(H,35,41)/t23-,25+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273731
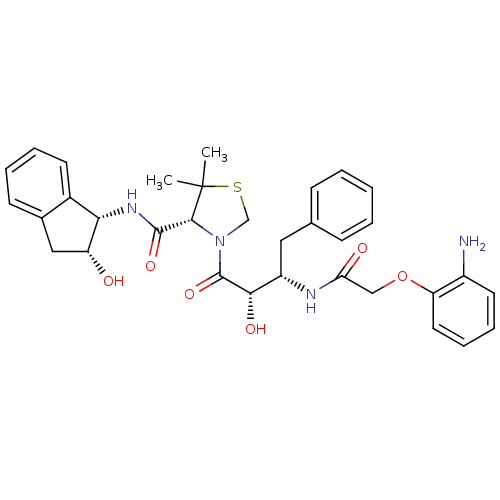
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccccc1N |r| Show InChI InChI=1S/C33H38N4O6S/c1-33(2)30(31(41)36-28-22-13-7-6-12-21(22)17-25(28)38)37(19-44-33)32(42)29(40)24(16-20-10-4-3-5-11-20)35-27(39)18-43-26-15-9-8-14-23(26)34/h3-15,24-25,28-30,38,40H,16-19,34H2,1-2H3,(H,35,39)(H,36,41)/t24-,25+,28-,29-,30+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273728
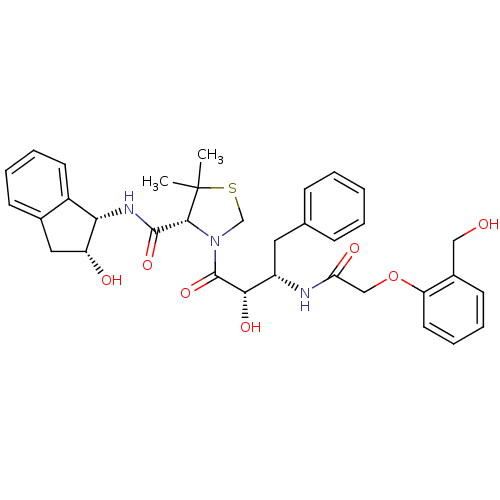
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES CC1(C)SCN([C@@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1ccccc1CO |r| Show InChI InChI=1S/C34H39N3O7S/c1-34(2)31(32(42)36-29-24-14-8-6-12-22(24)17-26(29)39)37(20-45-34)33(43)30(41)25(16-21-10-4-3-5-11-21)35-28(40)19-44-27-15-9-7-13-23(27)18-38/h3-15,25-26,29-31,38-39,41H,16-20H2,1-2H3,(H,35,40)(H,36,42)/t25-,26+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273719
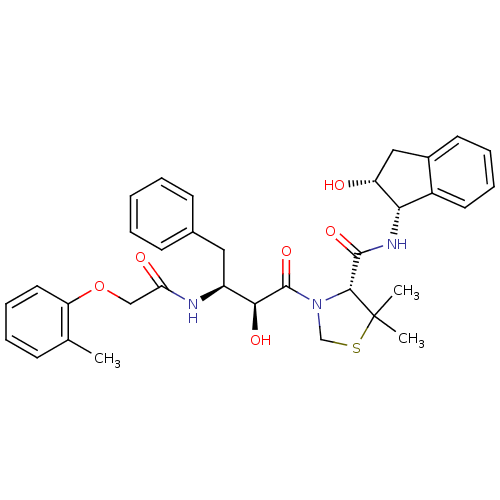
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES Cc1ccccc1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C34H39N3O6S/c1-21-11-7-10-16-27(21)43-19-28(39)35-25(17-22-12-5-4-6-13-22)30(40)33(42)37-20-44-34(2,3)31(37)32(41)36-29-24-15-9-8-14-23(24)18-26(29)38/h4-16,25-26,29-31,38,40H,17-20H2,1-3H3,(H,35,39)(H,36,41)/t25-,26+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273722
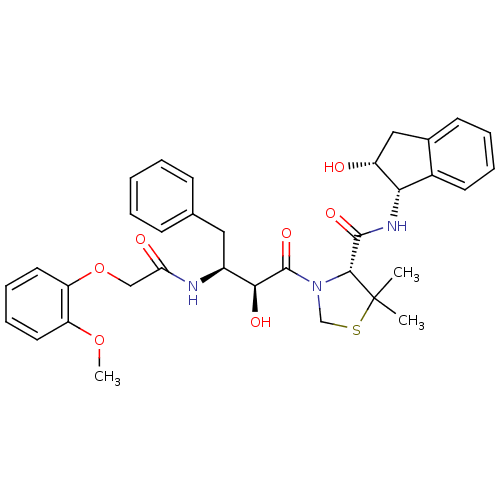
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES COc1ccccc1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C34H39N3O7S/c1-34(2)31(32(41)36-29-23-14-8-7-13-22(23)18-25(29)38)37(20-45-34)33(42)30(40)24(17-21-11-5-4-6-12-21)35-28(39)19-44-27-16-10-9-15-26(27)43-3/h4-16,24-25,29-31,38,40H,17-20H2,1-3H3,(H,35,39)(H,36,41)/t24-,25+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50369362
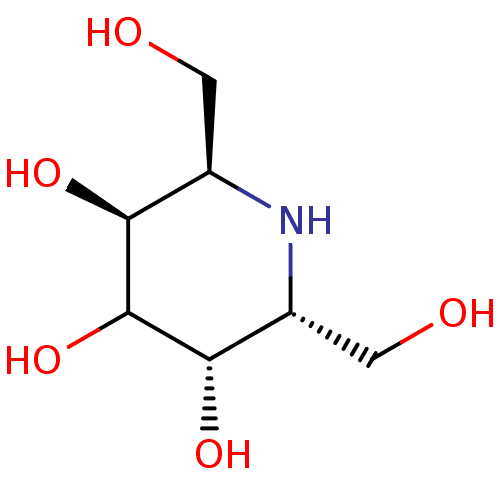
(CHEMBL1169500)Show InChI InChI=1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5+,6+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ki value against bovine alpha-L-fucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273724
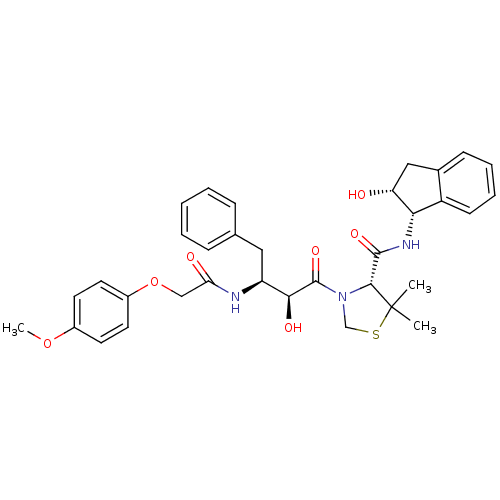
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES COc1ccc(OCC(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)N[C@@H]2[C@H](O)Cc3ccccc23)cc1 |r| Show InChI InChI=1S/C34H39N3O7S/c1-34(2)31(32(41)36-29-25-12-8-7-11-22(25)18-27(29)38)37(20-45-34)33(42)30(40)26(17-21-9-5-4-6-10-21)35-28(39)19-44-24-15-13-23(43-3)14-16-24/h4-16,26-27,29-31,38,40H,17-20H2,1-3H3,(H,35,39)(H,36,41)/t26-,27+,29-,30-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 479 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Plasmepsin II
(Plasmodium falciparum) | BDBM50273739
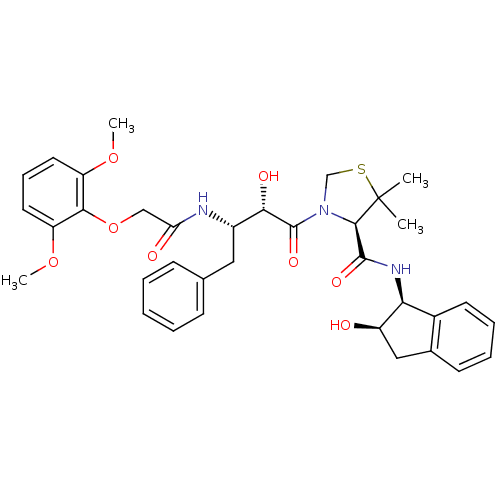
((R)-N-[(1S,2R)-2-Hydroxyindan-1-yl]-3-[(2S,3S)-3-(...)Show SMILES COc1cccc(OC)c1OCC(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H41N3O8S/c1-35(2)32(33(42)37-29-23-14-9-8-13-22(23)18-25(29)39)38(20-47-35)34(43)30(41)24(17-21-11-6-5-7-12-21)36-28(40)19-46-31-26(44-3)15-10-16-27(31)45-4/h5-16,24-25,29-30,32,39,41H,17-20H2,1-4H3,(H,36,40)(H,37,42)/t24-,25+,29-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 782 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum plasmepsin 2 |
Bioorg Med Chem 16: 10049-60 (2008)
Article DOI: 10.1016/j.bmc.2008.10.011
BindingDB Entry DOI: 10.7270/Q2G160PJ |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50065259

((2R,3R,4R,5R)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4-,5-,6-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ki value against bovine alpha-L-fucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Bos taurus) | BDBM50163446
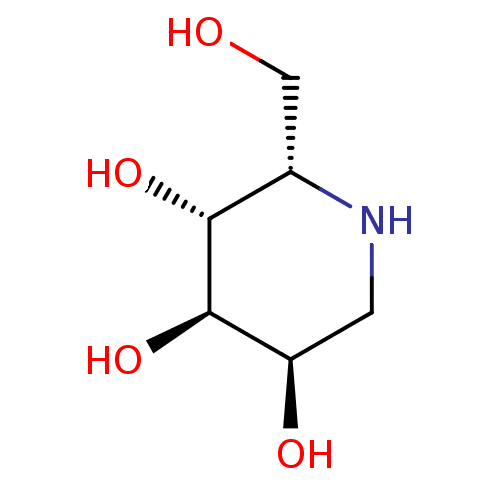
((2S,3R,4R,5R)-2-Hydroxymethyl-piperidine-3,4,5-tri...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5+,6+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Ki value against bovine alpha-L-fucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Putative alpha-glucosidase
(Oryza sativa subsp. japonica) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against rice alpha-glucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Maltase-glucoamylase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human alpha-glucosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50375510

(CHEMBL405957)Show InChI InChI=1S/C6H13NO4/c8-1-3-5(10)6(11)4(2-9)7-3/h3-11H,1-2H2/t3-,4-,5-,6-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat isomaltase |
Bioorg Med Chem 16: 2734-40 (2008)
Article DOI: 10.1016/j.bmc.2008.01.032
BindingDB Entry DOI: 10.7270/Q2FB53T0 |
More data for this
Ligand-Target Pair | |
Lysosomal acid glucosylceramidase
(Homo sapiens (Human)) | BDBM50182801
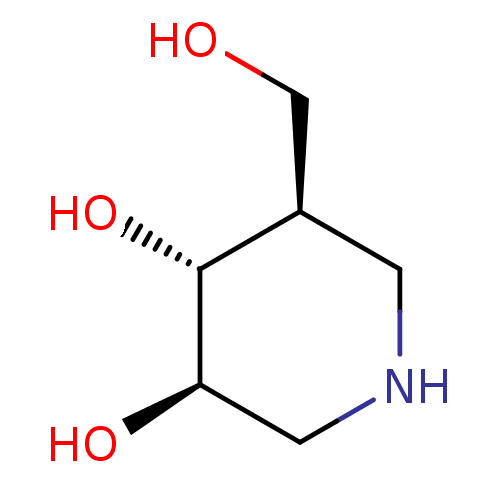
((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...)Show InChI InChI=1S/C6H13NO3/c8-3-4-1-7-2-5(9)6(4)10/h4-10H,1-3H2/t4-,5-,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of human lysosomal beta-glucosidase |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM18351

((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5-,6-/m1/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of maltase in human Caco-2 cell model system after 2 hrs |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Homo sapiens (Human)) | BDBM50263044
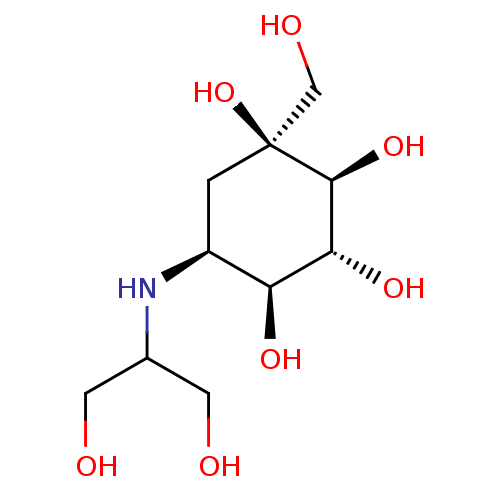
(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of maltase in human Caco-2 cell model system after 2 hrs |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | |
Alpha-galactosidase A
(Homo sapiens (Human)) | BDBM50163440
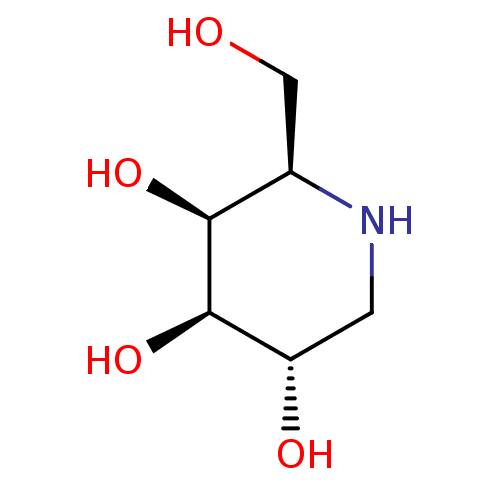
((2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-tr...)Show InChI InChI=1S/C6H13NO4/c8-2-3-5(10)6(11)4(9)1-7-3/h3-11H,1-2H2/t3-,4+,5+,6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Toyama Medical and Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human alpha-galactosidase |
J Med Chem 48: 2036-44 (2005)
Article DOI: 10.1021/jm0495881
BindingDB Entry DOI: 10.7270/Q2DF6S0M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50263044

(CHEMBL476960 | Voglibose)Show SMILES OCC(CO)N[C@H]1C[C@](O)(CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H21NO7/c12-2-5(3-13)11-6-1-10(18,4-14)9(17)8(16)7(6)15/h5-9,11-18H,1-4H2/t6-,7-,8+,9-,10-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal brush border membrane sucrase |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | |
Sucrase-isomaltase, intestinal
(Rattus norvegicus (Rat)) | BDBM50375511
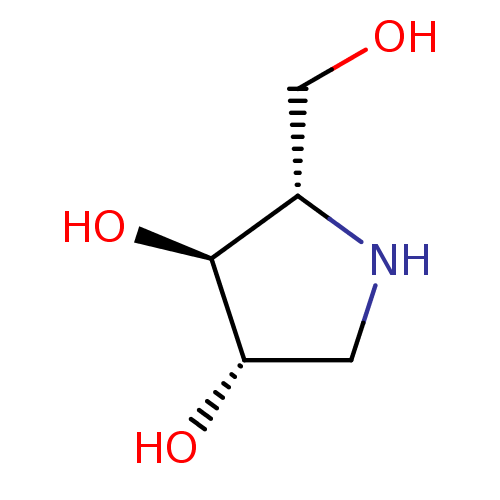
(CHEMBL406973)Show InChI InChI=1S/C5H11NO3/c7-2-3-5(9)4(8)1-6-3/h3-9H,1-2H2/t3-,4-,5-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat isomaltase |
Bioorg Med Chem 16: 2734-40 (2008)
Article DOI: 10.1016/j.bmc.2008.01.032
BindingDB Entry DOI: 10.7270/Q2FB53T0 |
More data for this
Ligand-Target Pair | |
Lysosomal alpha-glucosidase
(Rattus norvegicus) | BDBM50259956
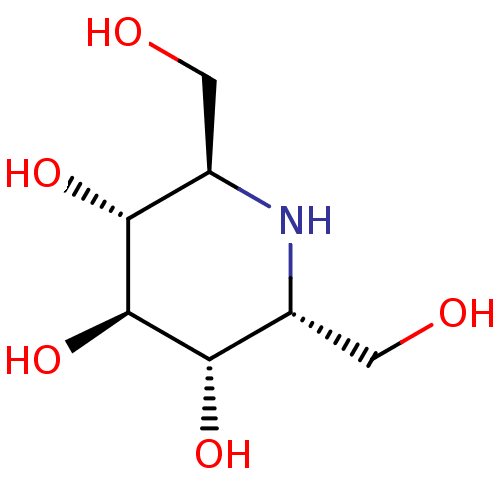
(2,6-Bis-hydroxymethyl-piperidine-3,4,5-triol | CHE...)Show SMILES OC[C@H]1N[C@H](CO)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C7H15NO5/c9-1-3-5(11)7(13)6(12)4(2-10)8-3/h3-13H,1-2H2/t3-,4-,5-,6+,7+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University
Curated by ChEMBL
| Assay Description
Inhibition of rat intestinal brush border membrane maltase |
Bioorg Med Chem 16: 7330-6 (2008)
Article DOI: 10.1016/j.bmc.2008.06.026
BindingDB Entry DOI: 10.7270/Q27D2W2B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data