

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122995 ((S)-3-(2-(1-methoxypropan-2-ylamino)pyridin-4-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122996 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyrimidin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil nucleotide/cysteinyl leukotriene receptor (Homo sapiens (Human)) | BDBM50318031 (CHEMBL1097279 | cangrelor) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at human GPR17 expressed in human 1321N1 cells assessed as inhibition of UDP-glucose-induced [35S]GTPgammaS binding after 30 mins... | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50318031 (CHEMBL1097279 | cangrelor) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor by [35S]GTPgammaS binding assay | J Med Chem 53: 3489-501 (2010) Article DOI: 10.1021/jm901691y BindingDB Entry DOI: 10.7270/Q28C9WFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50122997 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyridin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50122995 ((S)-3-(2-(1-methoxypropan-2-ylamino)pyridin-4-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224496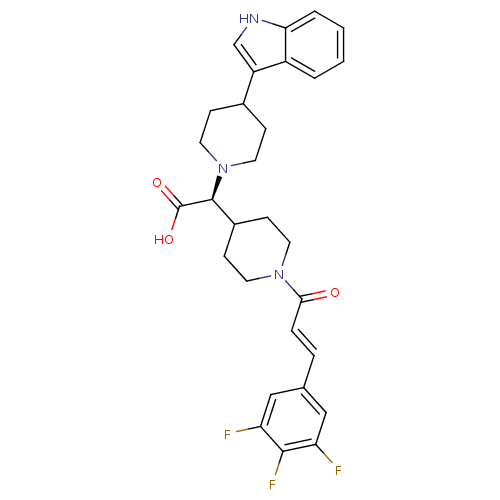 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium flux | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122997 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyridin-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50123004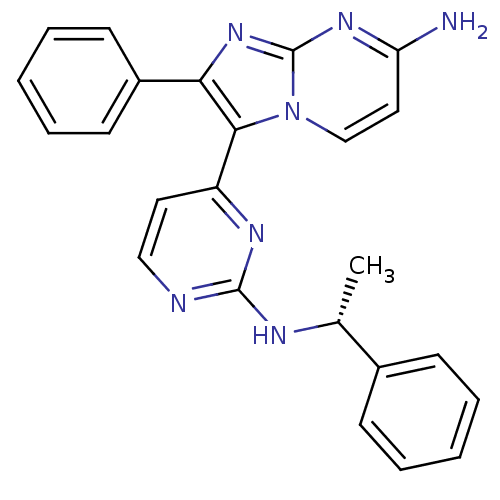 (2-Phenyl-3-[2-((R)-1-phenyl-ethylamino)-pyrimidin-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224501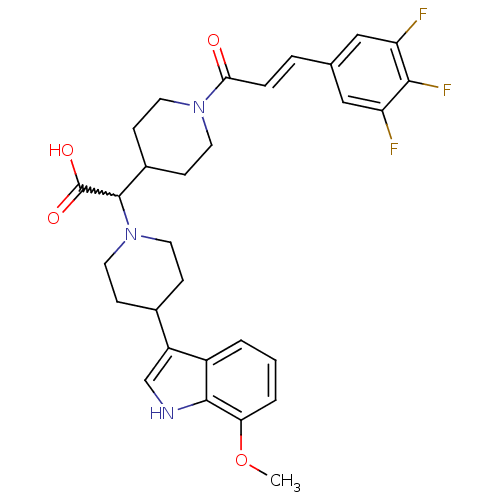 ((E)-2-(4-(7-methoxy-1H-indol-3-yl)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50123000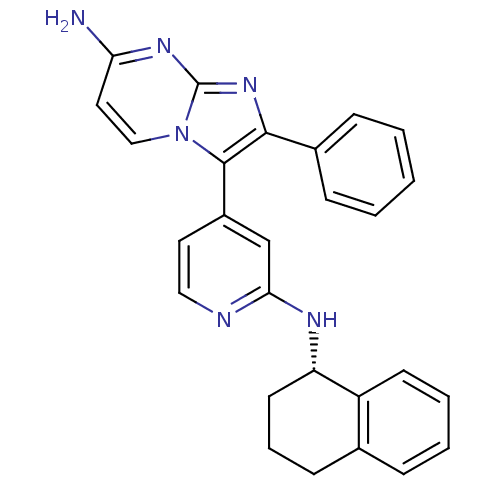 (2-Phenyl-3-{2-[(S)-(1,2,3,4-tetrahydro-naphthalen-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224523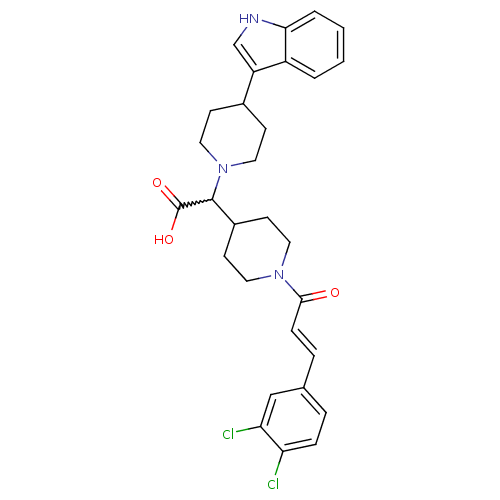 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224500 ((E)-2-(4-(5-amino-1H-indol-3-yl)piperidin-1-yl)-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224502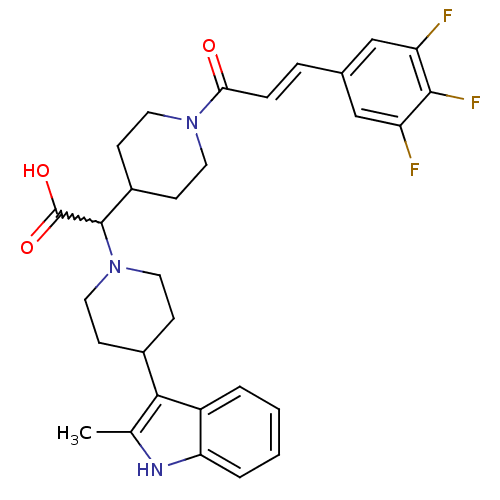 ((E)-2-(4-(2-methyl-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224496 ((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224511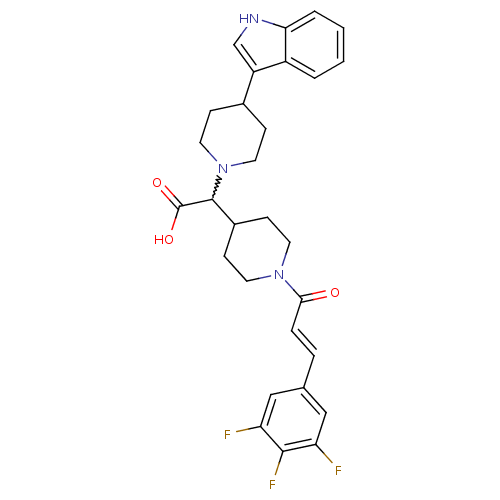 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50123003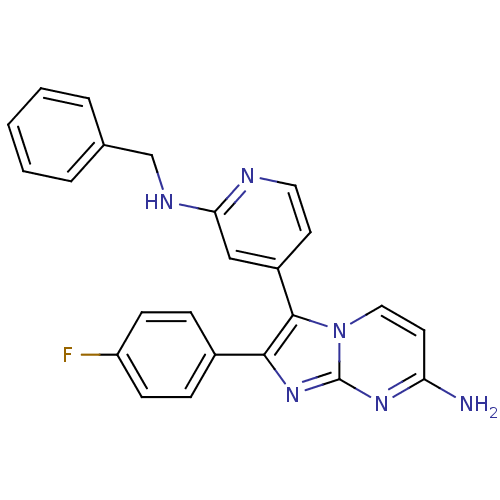 (3-(2-(benzylamino)pyridin-4-yl)-2-(4-fluorophenyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123003 (3-(2-(benzylamino)pyridin-4-yl)-2-(4-fluorophenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224524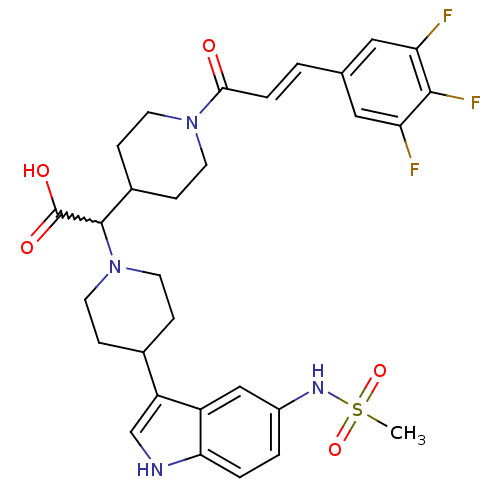 ((E)-2-(4-(5-(methylsulfonamido)-1H-indol-3-yl)pipe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM15457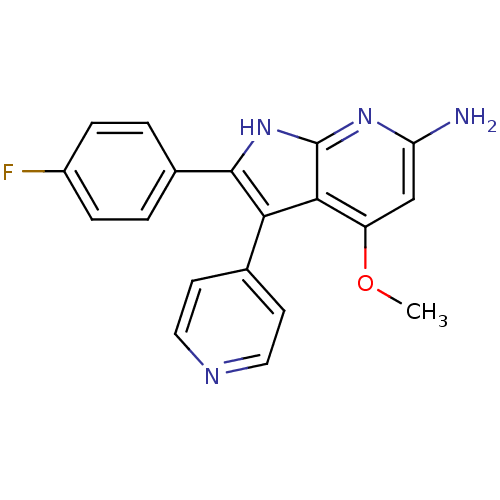 (2-(4-fluorophenyl)-4-methoxy-3-(pyridin-4-yl)-1H-p...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224519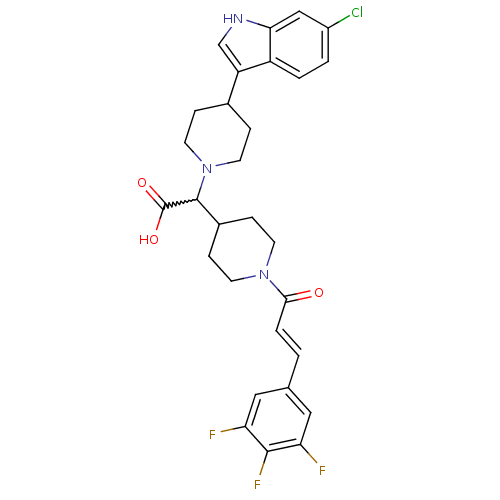 ((E)-2-(4-(6-chloro-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123000 (2-Phenyl-3-{2-[(S)-(1,2,3,4-tetrahydro-naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012060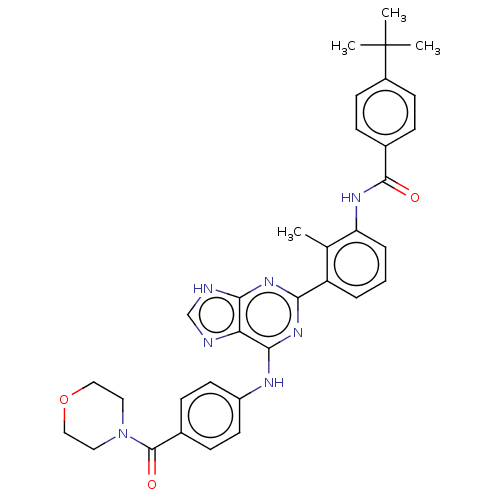 (CHEMBL3263640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK-mediated proliferation in human tonsilar B cells assessed as [3H]thymidine incorporation after 1 hr by liquid scintillation countin... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50122996 (2-Phenyl-3-[2-((S)-1-phenyl-ethylamino)-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012060 (CHEMBL3263640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012060 (CHEMBL3263640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK-induced calcium flux in human Ramos cells | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50216434 (CHEMBL245686 | dimethyl-(tetrahydro-pyran-4-yl)-{4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells | Bioorg Med Chem Lett 17: 4382-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.115 BindingDB Entry DOI: 10.7270/Q2F47NVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50216453 (CHEMBL247537 | dimethyl-{4-[(E)-3-(4'-methyl-biphe...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells | Bioorg Med Chem Lett 17: 4382-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.115 BindingDB Entry DOI: 10.7270/Q2F47NVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224512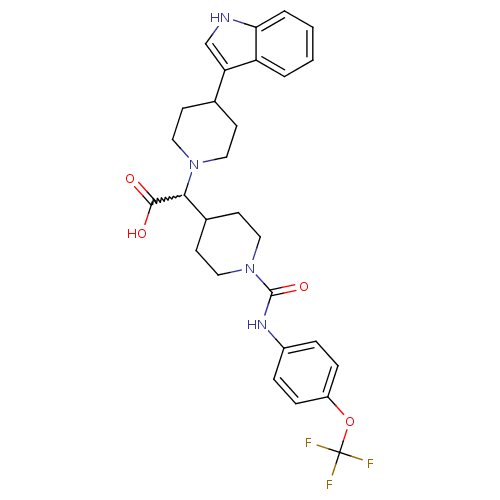 (2-(1-((4-(trifluoromethoxy)phenyl)carbamoyl)piperi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Tec (Homo sapiens (Human)) | BDBM50012060 (CHEMBL3263640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of TEC (unknown origin) | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224508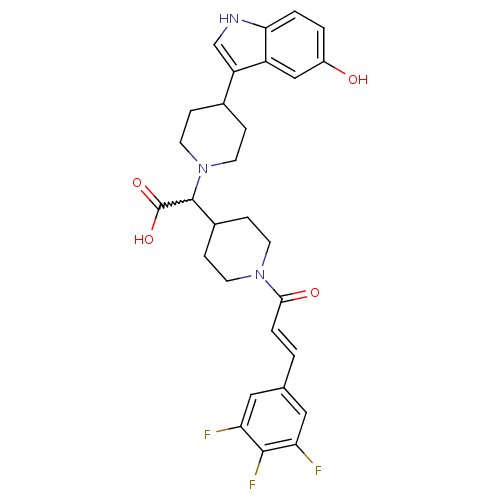 ((E)-2-(4-(5-hydroxy-1H-indol-3-yl)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224510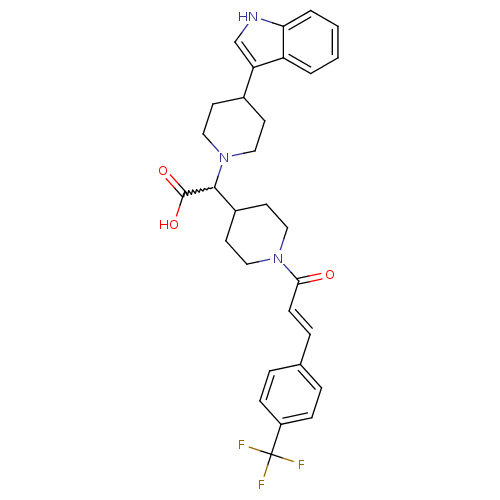 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224521 ((E)-2-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012073 (CHEMBL3263634) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012095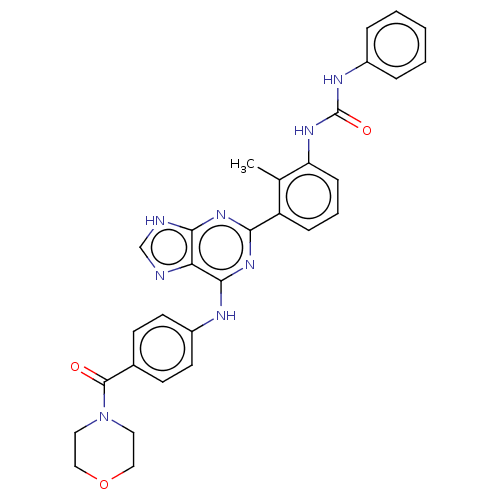 (CHEMBL3263656) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012079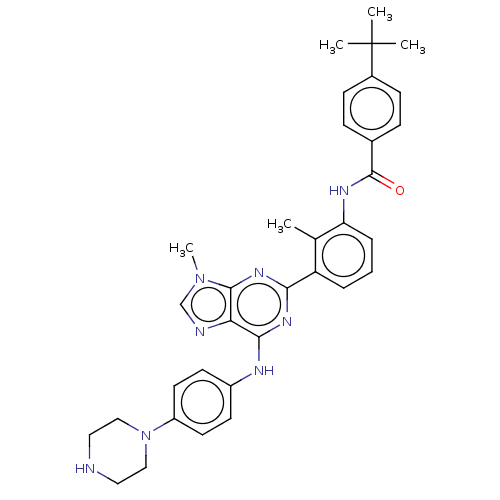 (CHEMBL3263639) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224506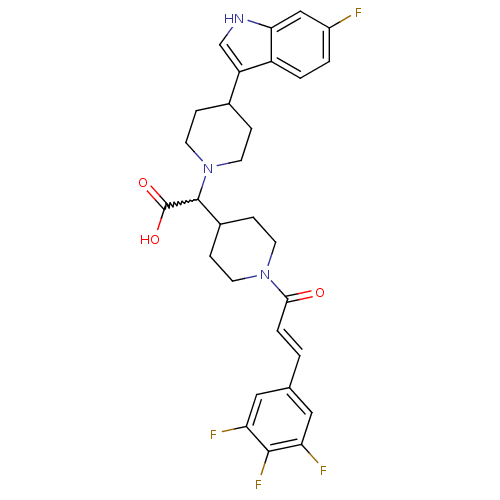 ((E)-2-(4-(6-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224499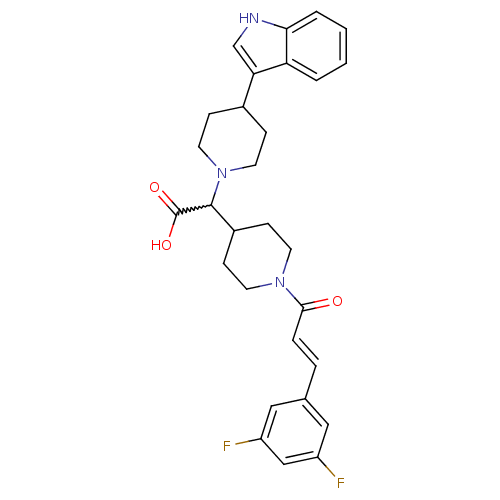 ((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50216435 (CHEMBL397647 | N-(4-(3,4-dichlorobenzamido)benzyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells | Bioorg Med Chem Lett 17: 4382-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.115 BindingDB Entry DOI: 10.7270/Q2F47NVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Mus musculus (mouse)) | BDBM50123002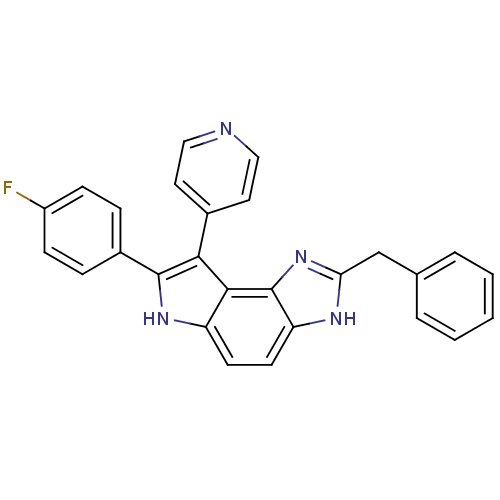 (2-Benzyl-7-(4-fluoro-phenyl)-8-pyridin-4-yl-3,6-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of murine p38 alpha kinase | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012071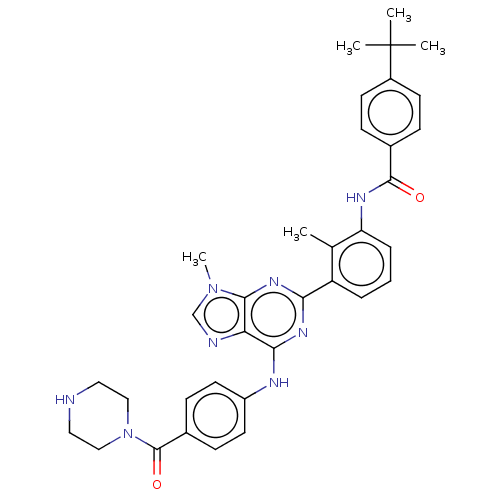 (CHEMBL3263632) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50224516 (2-(1-((3,4-dichlorophenyl)carbamoyl)piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of human CCR2 | J Med Chem 50: 5561-3 (2007) Article DOI: 10.1021/jm070902b BindingDB Entry DOI: 10.7270/Q24M948Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012087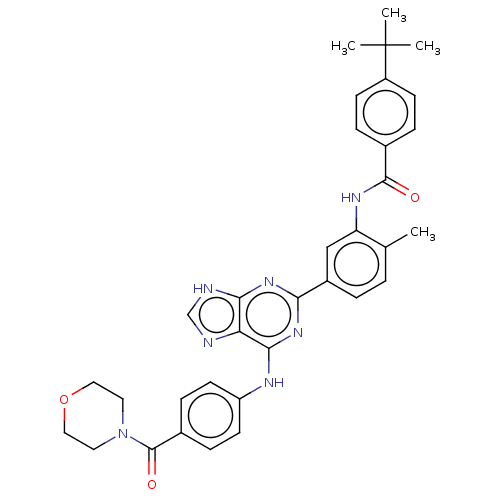 (CHEMBL3263648) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012097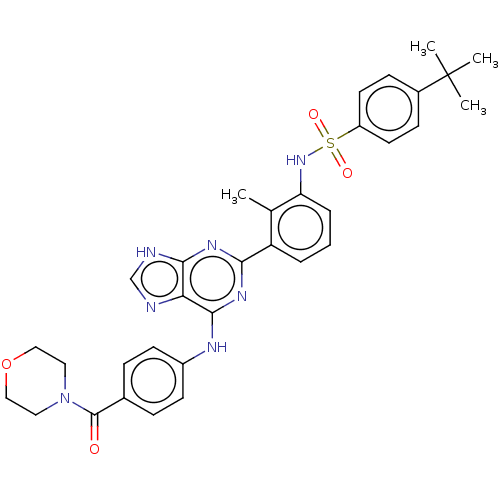 (CHEMBL3263658) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM50122998 (3-(2-Methylsulfanyl-pyrimidin-4-yl)-2-phenyl-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of LPS-induced p38-related TNF-alpha production in human peripheral blood mononuclear cells | Bioorg Med Chem Lett 13: 347-50 (2003) BindingDB Entry DOI: 10.7270/Q2G73D2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012072 (CHEMBL3263633) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50216442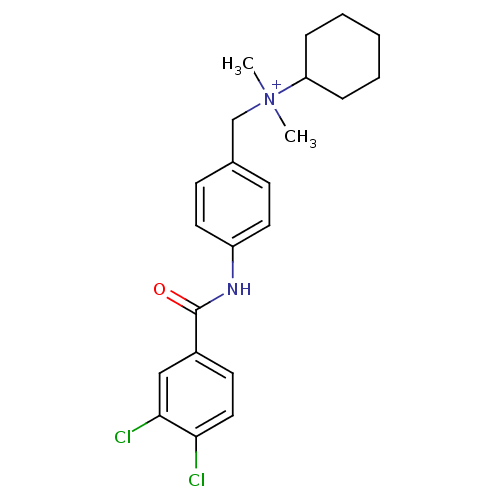 (CHEMBL393631 | N-(4-(3,4-dichlorobenzamido)benzyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 receptor expressed in THP1 cells | Bioorg Med Chem Lett 17: 4382-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.115 BindingDB Entry DOI: 10.7270/Q2F47NVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012060 (CHEMBL3263640) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of BTK in human tonsilar B cells assessed as inhibition of IL6 expression after 1 hr by EIA | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012088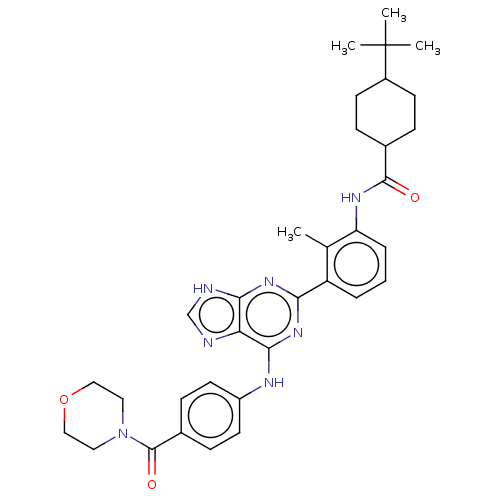 (CHEMBL3263649) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50012085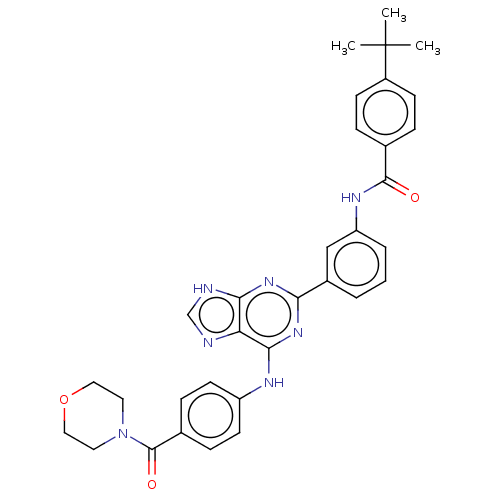 (CHEMBL3263646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of full length human wild type his-tagged BTK expressed in Sf9 cells using biotinylated peptide as substrate after 1 hr by Fluorescence as... | Bioorg Med Chem Lett 24: 2206-11 (2014) Article DOI: 10.1016/j.bmcl.2014.02.075 BindingDB Entry DOI: 10.7270/Q2K075TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 267 total ) | Next | Last >> |