

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316153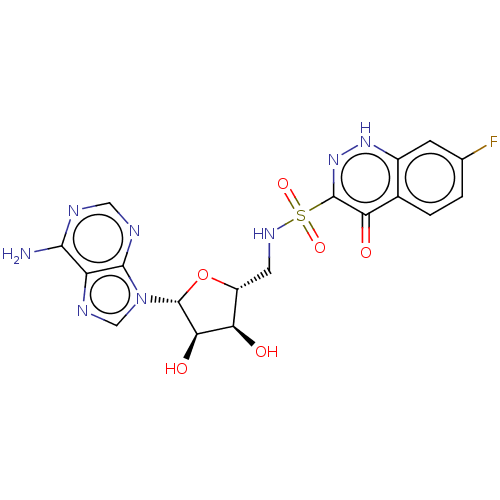 (CHEMBL4299745) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316152 (CHEMBL4299749) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316151 (CHEMBL4299747) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316154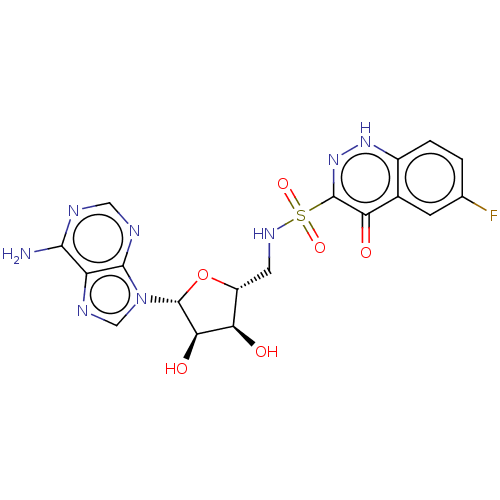 (CHEMBL4299748) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316149 (CHEMBL4168282) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Salicyl-AMP ligase / salicyl-S-ArCP synthetase (Mycobacterium tuberculosis) | BDBM50316150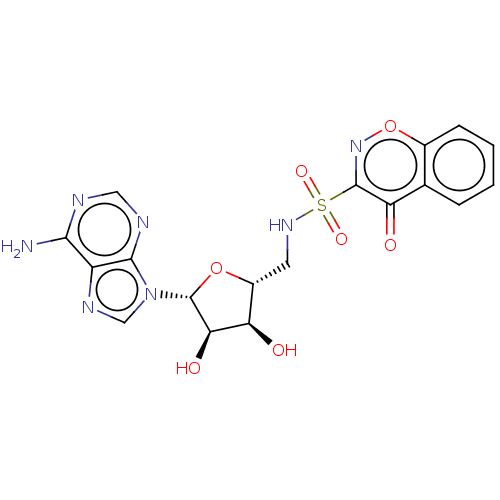 (CHEMBL4160378) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis recombinant salicylate ligase MbtA expressed in Escherichia coli using salicylic acid as substrate after 20 ... | ACS Med Chem Lett 9: 386-391 (2018) Article DOI: 10.1021/acsmedchemlett.8b00090 BindingDB Entry DOI: 10.7270/Q2RR21SH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50052059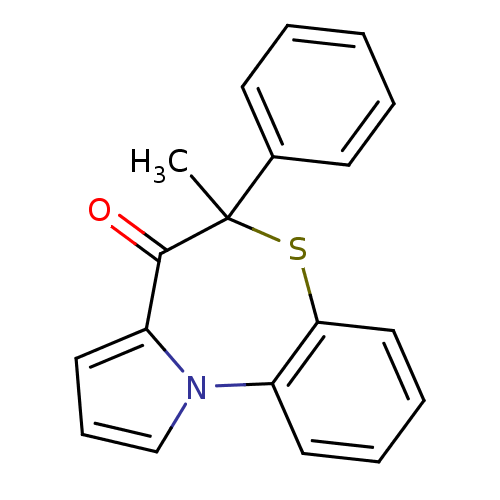 (5-Methyl-5-phenyl-6-thia-10b-aza-benzo[e]azulen-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50052043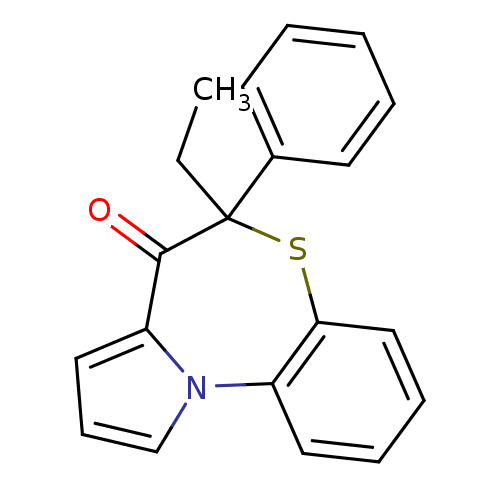 (5-Ethyl-5-phenyl-6-thia-10b-aza-benzo[e]azulen-4-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2473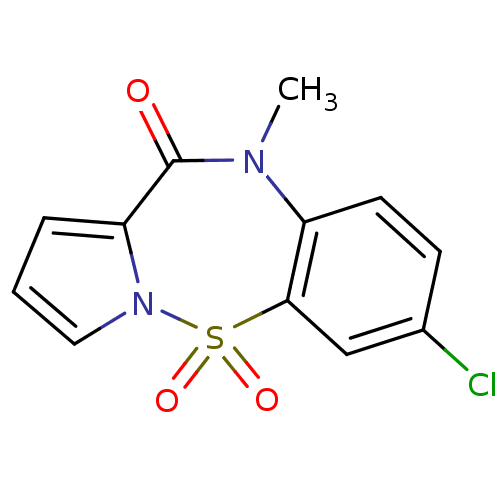 (13-chloro-9-methyl-2-thia-3,9-diazatricyclo[8.4.0....) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2472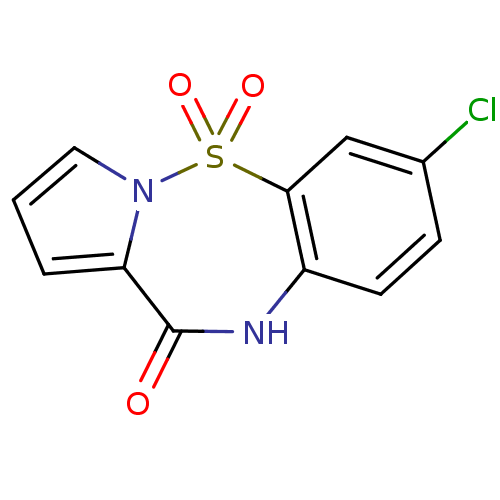 (13-chloro-2-thia-3,9-diazatricyclo[8.4.0.0^{3,7}]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50498540 (CHEMBL3609278) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50498543 (CHEMBL3609279) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50498542 (CHEMBL3608739) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2474 (13-chloro-9-ethyl-2-thia-3,9-diazatricyclo[8.4.0.0...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2475 (13-chloro-9-(prop-2-en-1-yl)-2-thia-3,9-diazatricy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50498539 (CHEMBL3608735) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2465 (10-methyi-5H- pyrrolo1,2 -b] [ 1,2,5] benzothiadia...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2466 (9-ethyl-2-thia-3,9-diazatricyclo[8.4.0.0^{3,7}]tet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2464 (2-thia-3,9-diazatricyclo[8.4.0.0^{3,7}]tetradeca-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2468 (9-(propan-2-yl)-2-thia-3,9-diazatricyclo[8.4.0.0^{...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2469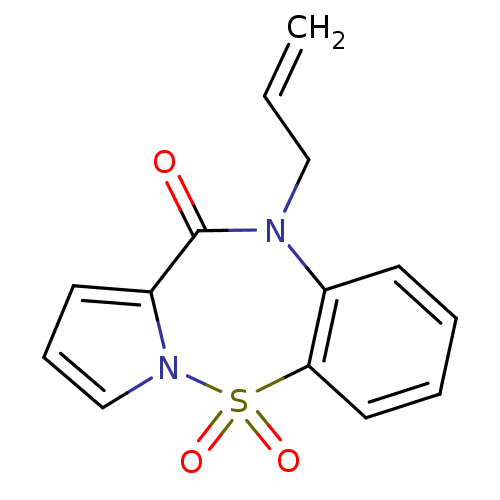 (9-(prop-2-en-1-yl)-2-thia-3,9-diazatricyclo[8.4.0....) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50498541 (CHEMBL3608732) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106768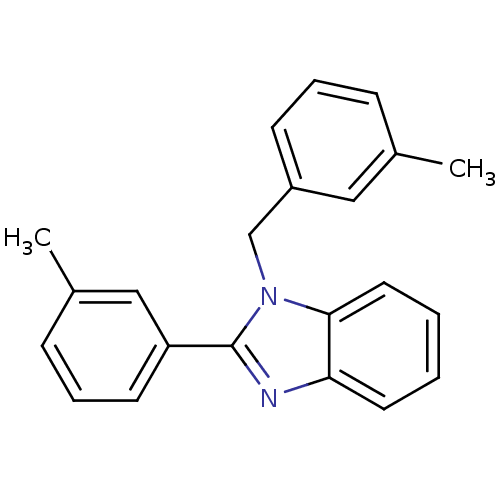 (2‐(3‐methylphenyl)‐1‐[(3&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106762 (2‐(2‐chlorophenyl)‐1‐[(2&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106765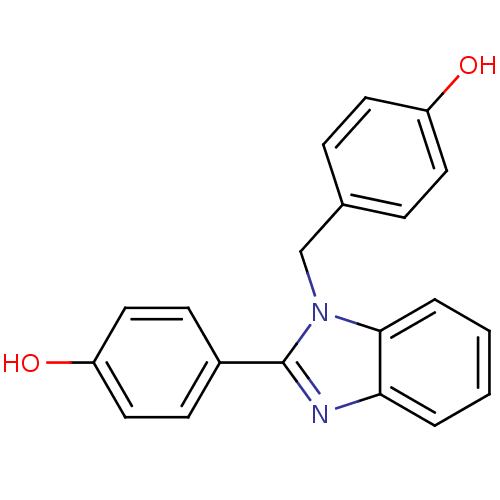 (4‐{1‐[(4‐hydroxyphenyl)methyl]&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106772 (2‐(3‐methoxyphenyl)‐1‐[(3&...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106770 (1‐{1‐[(1‐hydroxynaphthalen‐...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM108032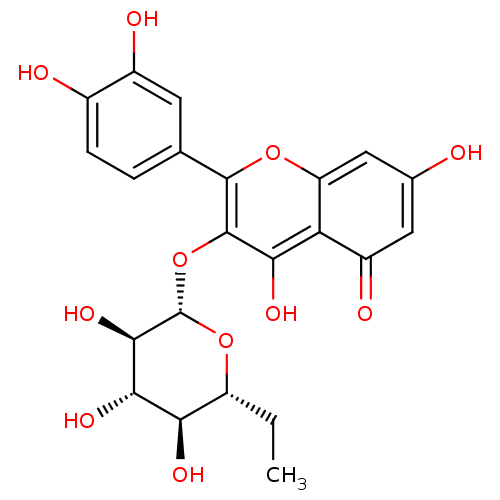 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-[(2S,3R,4S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM35440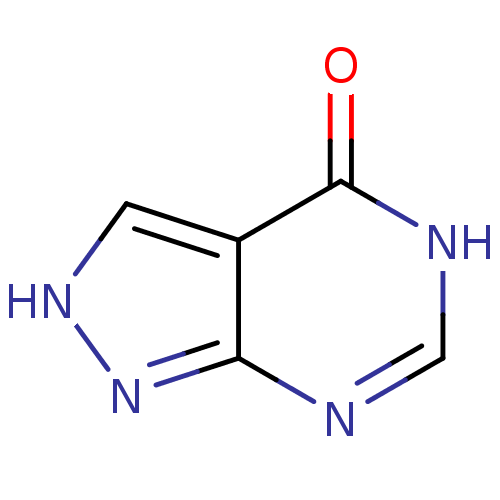 (ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106763 (2‐(2‐methylphenyl)‐1‐[(2&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50100861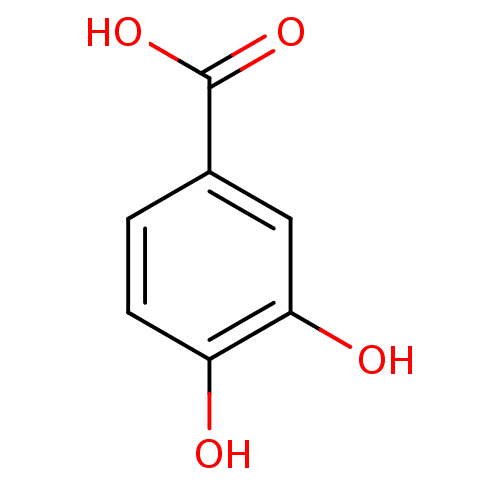 (3,4-Dihydroxybenzoate, VIII | 3,4-dihydroxybenzoic...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106766 (3‐{1‐[(3‐hydroxyphenyl)methyl]&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106761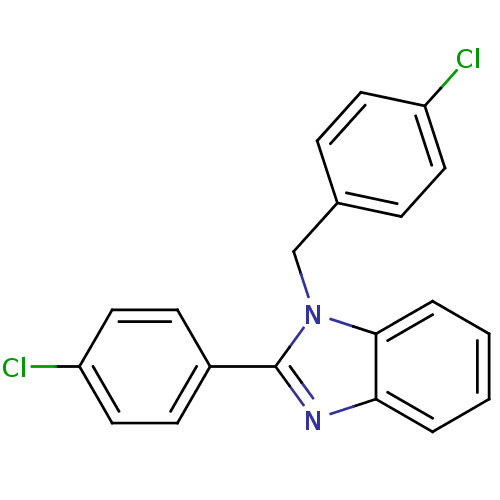 (2‐(4‐chlorophenyl)‐1‐[(4&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM108031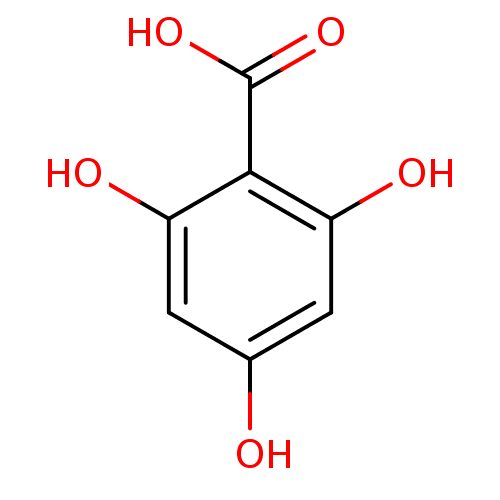 (2,4,6-trihydroxybenzoic acid (M3)) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM4374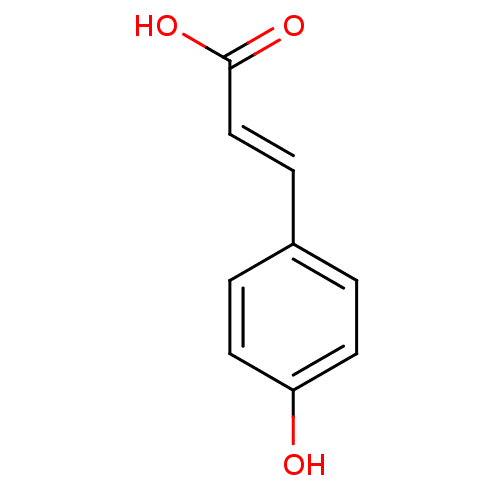 ((2E)-3-(4-hydroxyphenyl)prop-2-enoic acid | (2E)-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM108034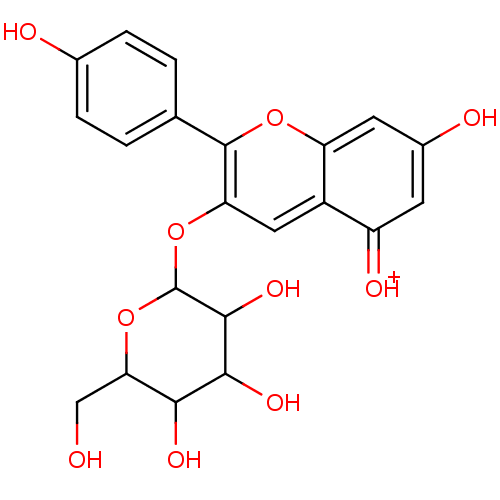 (5,7‐dihydroxy‐2‐(4‐hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106771 (2‐(pyridin‐3‐yl)‐1‐(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50347135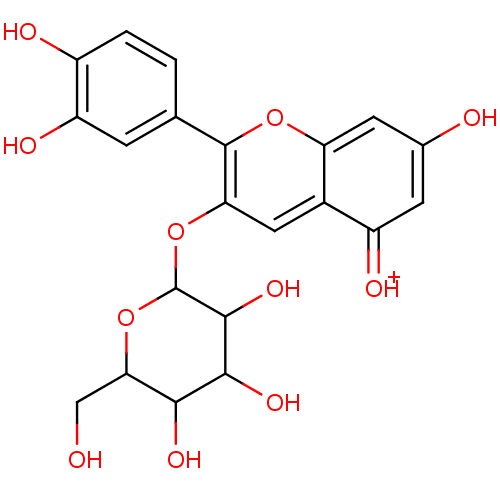 (Cyanidin-3-glucoside (M8) | KUROMANIN) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106764 (2‐(4‐methylphenyl)‐1‐[(4&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM35440 (ALLOPURINOL | MLS000069453 | SMR000059083 | cid_20...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106767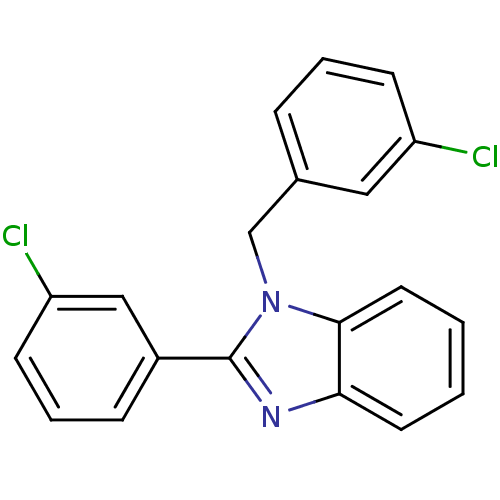 (2‐(3‐chlorophenyl)‐1‐[(3&#...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50214744 ((2E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM50337364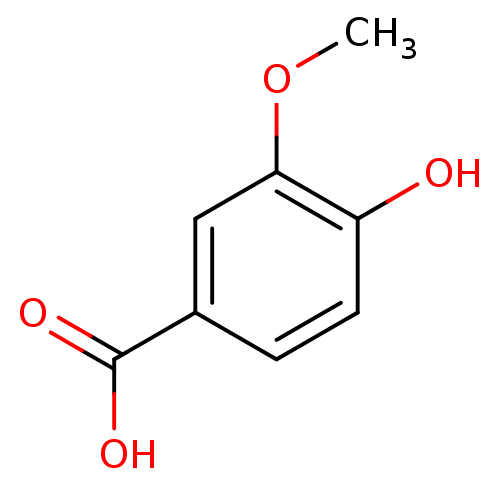 (3-methoxy-4-hydroxybenzoic acid | 4-Hydroxy-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106773 (2‐(4‐methoxyphenyl)‐1‐[(4&...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM108033 (3'-methoxyhirsutrin (M7) | 3‐{[(2S,3R,4S...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM108035 (5,7‐dihydroxy‐2‐(4‐hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
SRTM University, Nanded, Maharashtra, 431606, India; Department of Molecular Biotechnology, College of Life and Environmental Sciences, Konkuk University, Seoul, 143-701, South Korea. | Assay Description Inhibition of xanthine oxidase (XO) by each isolated phenolics was measured by following the decrease in the uric acid formation at 293nm at 25°... | Chem Biol Drug Des 83: 119-25 (2014) Article DOI: 10.1111/cbdd.12205 BindingDB Entry DOI: 10.7270/Q2Z89B2H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM61780 ((6E)-6-(3-salicyl-1H-benzimidazol-2-ylidene)cycloh...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Bos taurus (Bovine)) | BDBM106769 (1‐benzyl‐2‐phenyl‐1,3̴...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
Konkuk University | Assay Description Bovine milk XO activity was assayed spectrophotometrically by measuring the uric acid formation at 293 nm using a UV-visible spectrophotometer at 25&... | Chem Biol Drug Des 82: 290-5 (2013) Article DOI: 10.1111/cbdd.12141 BindingDB Entry DOI: 10.7270/Q2TM78SJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50102264 (CHEMBL322698 | Pyrazine-2-carboxylic acid N'-pyrro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50494046 (CHEMBL2440486) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Experimental Botany ASCR & Palack£ University Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase assessed as inhibition of biotin-dUTP incorporation | Bioorg Med Chem 23: 5247-63 (2015) Article DOI: 10.1016/j.bmc.2015.06.016 BindingDB Entry DOI: 10.7270/Q20P131K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 62 total ) | Next | Last >> |