 Found 304 hits with Last Name = 'patel' and Initial = 'sr'
Found 304 hits with Last Name = 'patel' and Initial = 'sr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50162083
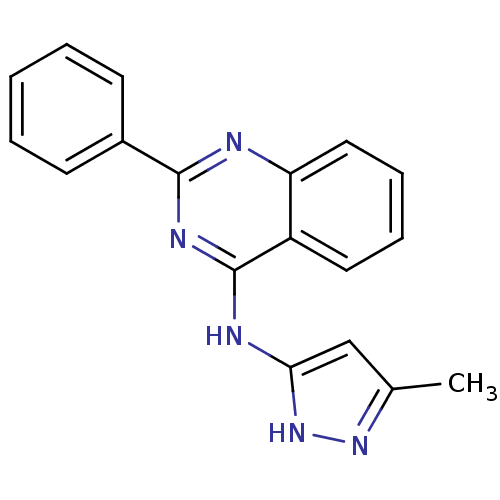
((5-Methyl-1H-pyrazol-3-yl)-(2-phenyl-quinazolin-4-...)Show InChI InChI=1S/C18H15N5/c1-12-11-16(23-22-12)20-18-14-9-5-6-10-15(14)19-17(21-18)13-7-3-2-4-8-13/h2-11H,1H3,(H2,19,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475257
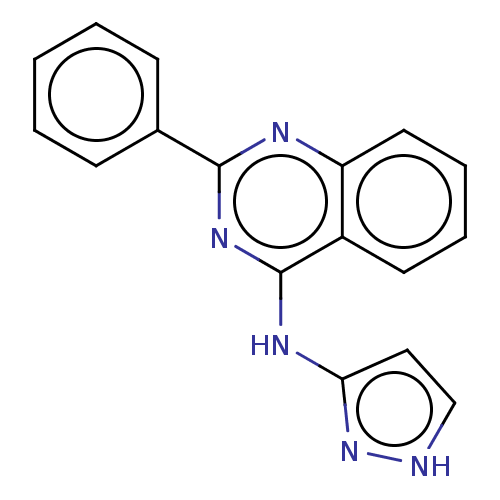
(CHEMBL179420)Show InChI InChI=1S/C17H13N5/c1-2-6-12(7-3-1)16-19-14-9-5-4-8-13(14)17(21-16)20-15-10-11-18-22-15/h1-11H,(H2,18,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475255
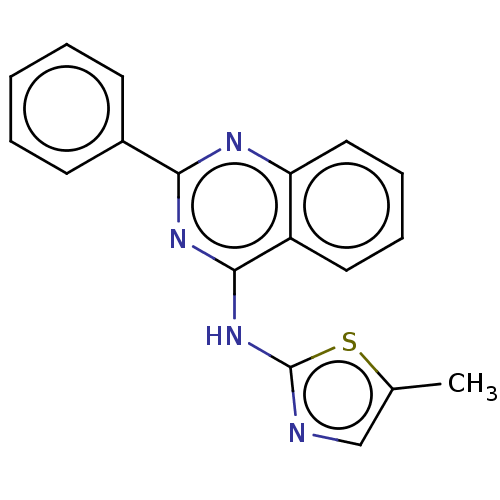
(CHEMBL179341)Show InChI InChI=1S/C18H14N4S/c1-12-11-19-18(23-12)22-17-14-9-5-6-10-15(14)20-16(21-17)13-7-3-2-4-8-13/h2-11H,1H3,(H,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475254
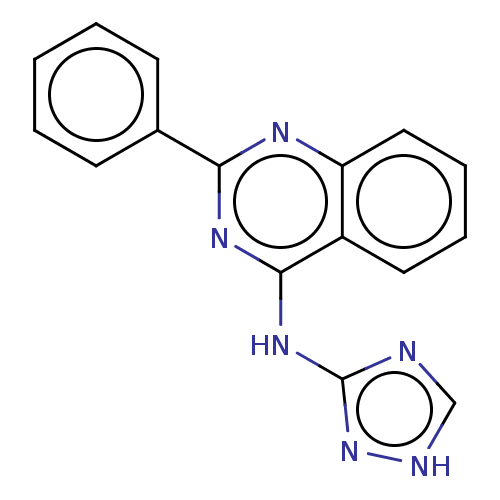
(CHEMBL361127)Show InChI InChI=1S/C16H12N6/c1-2-6-11(7-3-1)14-19-13-9-5-4-8-12(13)15(20-14)21-16-17-10-18-22-16/h1-10H,(H2,17,18,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50033345
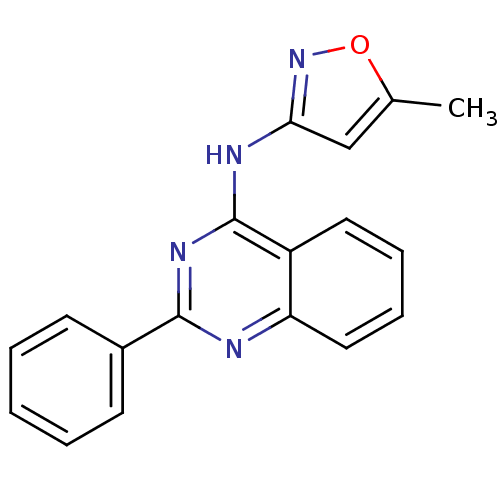
((5-Methyl-isoxazol-3-yl)-(2-phenyl-quinazolin-4-yl...)Show InChI InChI=1S/C18H14N4O/c1-12-11-16(22-23-12)20-18-14-9-5-6-10-15(14)19-17(21-18)13-7-3-2-4-8-13/h2-11H,1H3,(H,19,20,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475256
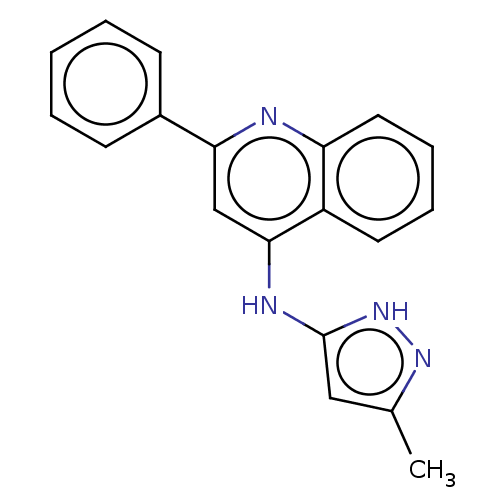
(CHEMBL176248)Show InChI InChI=1S/C19H16N4/c1-13-11-19(23-22-13)21-18-12-17(14-7-3-2-4-8-14)20-16-10-6-5-9-15(16)18/h2-12H,1H3,(H2,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475253
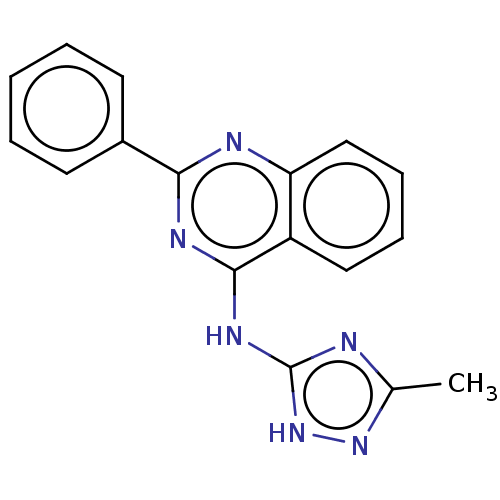
(CHEMBL178264)Show InChI InChI=1S/C17H14N6/c1-11-18-17(23-22-11)21-16-13-9-5-6-10-14(13)19-15(20-16)12-7-3-2-4-8-12/h2-10H,1H3,(H2,18,19,20,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 |
J Med Chem 48: 1278-81 (2005)
Article DOI: 10.1021/jm0492249
BindingDB Entry DOI: 10.7270/Q2X92F1P |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436256
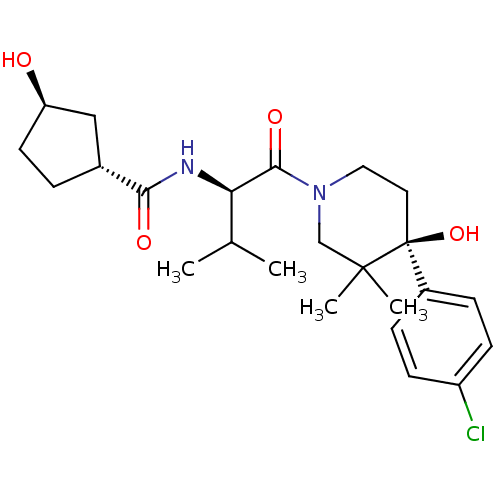
(CHEMBL2398726)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CC[C@@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19-,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436283

(CHEMBL2398729)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CC[C@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436260

(CHEMBL2398717)Show SMILES CC(C)[C@@H](NC(=O)CC1CC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H33ClN2O3/c1-15(2)20(25-19(27)13-16-5-6-16)21(28)26-12-11-23(29,22(3,4)14-26)17-7-9-18(24)10-8-17/h7-10,15-16,20,29H,5-6,11-14H2,1-4H3,(H,25,27)/t20-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056498

(CHEMBL3334818)Show SMILES CC(C)[C@@H](NC(=O)NCc1ccccc1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C26H34ClN3O3/c1-18(2)22(29-24(32)28-16-19-8-6-5-7-9-19)23(31)30-15-14-26(33,25(3,4)17-30)20-10-12-21(27)13-11-20/h5-13,18,22,33H,14-17H2,1-4H3,(H2,28,29,32)/t22-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056504

(CHEMBL3334824)Show SMILES CC(C)[C@@H](NC(=O)NCC(C)(C)O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H36ClN3O4/c1-15(2)18(26-20(29)25-13-22(5,6)30)19(28)27-12-11-23(31,21(3,4)14-27)16-7-9-17(24)10-8-16/h7-10,15,18,30-31H,11-14H2,1-6H3,(H2,25,26,29)/t18-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436256

(CHEMBL2398726)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CC[C@@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19-,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of RANTES-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436288

(CHEMBL2398728)Show SMILES CC(C)[C@@H](NC(=O)[C@H]1CC[C@@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19+,20+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436282
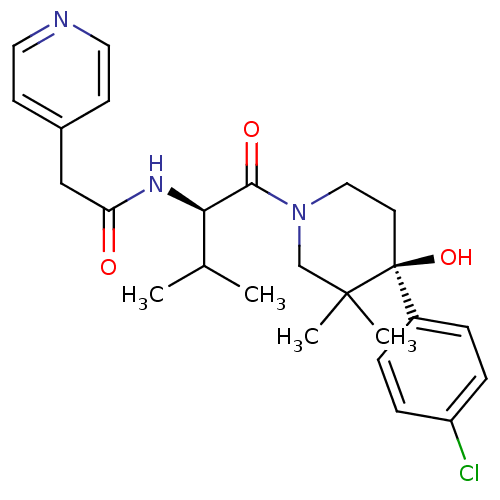
(CHEMBL2398769)Show SMILES CC(C)[C@@H](NC(=O)Cc1ccncc1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C25H32ClN3O3/c1-17(2)22(28-21(30)15-18-9-12-27-13-10-18)23(31)29-14-11-25(32,24(3,4)16-29)19-5-7-20(26)8-6-19/h5-10,12-13,17,22,32H,11,14-16H2,1-4H3,(H,28,30)/t22-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436287

(CHEMBL2398744)Show SMILES CC(C)CCC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H37ClN2O3/c1-16(2)7-12-20(28)26-21(17(3)4)22(29)27-14-13-24(30,23(5,6)15-27)18-8-10-19(25)11-9-18/h8-11,16-17,21,30H,7,12-15H2,1-6H3,(H,26,28)/t21-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436284
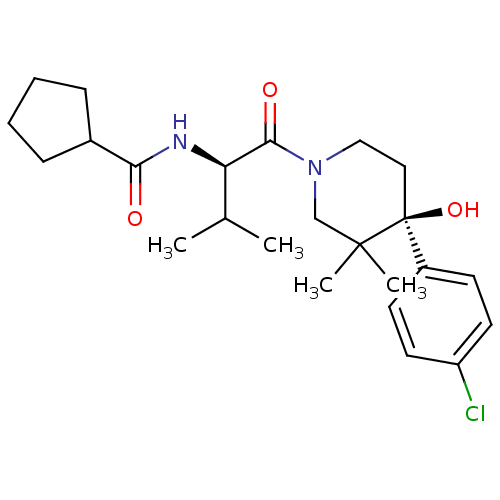
(CHEMBL2398747 | US8633226, 460)Show SMILES CC(C)[C@@H](NC(=O)C1CCCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O3/c1-16(2)20(26-21(28)17-7-5-6-8-17)22(29)27-14-13-24(30,23(3,4)15-27)18-9-11-19(25)12-10-18/h9-12,16-17,20,30H,5-8,13-15H2,1-4H3,(H,26,28)/t20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436288

(CHEMBL2398728)Show SMILES CC(C)[C@@H](NC(=O)[C@H]1CC[C@@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19+,20+,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436308
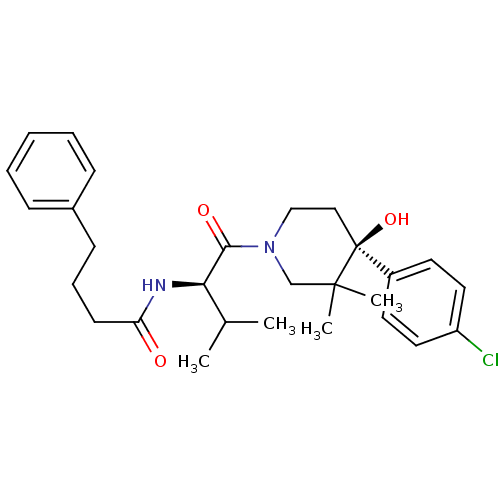
(CHEMBL2398739)Show SMILES CC(C)[C@@H](NC(=O)CCCc1ccccc1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C28H37ClN2O3/c1-20(2)25(30-24(32)12-8-11-21-9-6-5-7-10-21)26(33)31-18-17-28(34,27(3,4)19-31)22-13-15-23(29)16-14-22/h5-7,9-10,13-16,20,25,34H,8,11-12,17-19H2,1-4H3,(H,30,32)/t25-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436287

(CHEMBL2398744)Show SMILES CC(C)CCC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H37ClN2O3/c1-16(2)7-12-20(28)26-21(17(3)4)22(29)27-14-13-24(30,23(5,6)15-27)18-8-10-19(25)11-9-18/h8-11,16-17,21,30H,7,12-15H2,1-6H3,(H,26,28)/t21-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436286

(CHEMBL2398737)Show SMILES CC(C)[C@@H](NC(=O)Cc1ccccc1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C26H33ClN2O3/c1-18(2)23(28-22(30)16-19-8-6-5-7-9-19)24(31)29-15-14-26(32,25(3,4)17-29)20-10-12-21(27)13-11-20/h5-13,18,23,32H,14-17H2,1-4H3,(H,28,30)/t23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436307
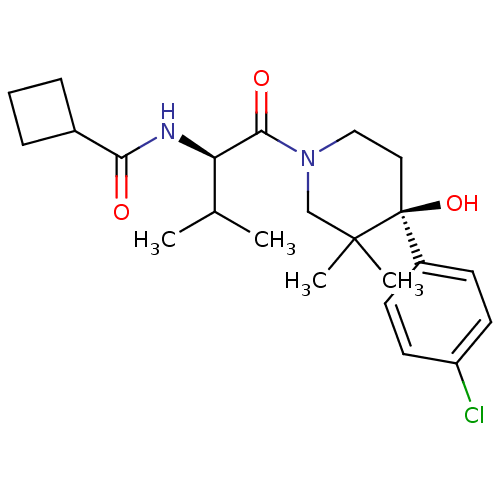
(CHEMBL2398746)Show SMILES CC(C)[C@@H](NC(=O)C1CCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H33ClN2O3/c1-15(2)19(25-20(27)16-6-5-7-16)21(28)26-13-12-23(29,22(3,4)14-26)17-8-10-18(24)11-9-17/h8-11,15-16,19,29H,5-7,12-14H2,1-4H3,(H,25,27)/t19-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436285
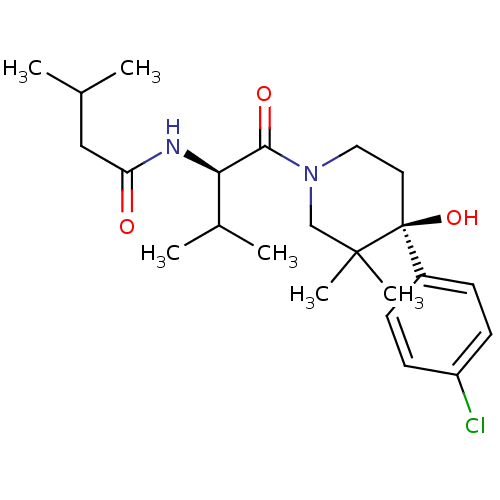
(CHEMBL2398743 | US8633226, 466)Show SMILES CC(C)CC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H35ClN2O3/c1-15(2)13-19(27)25-20(16(3)4)21(28)26-12-11-23(29,22(5,6)14-26)17-7-9-18(24)10-8-17/h7-10,15-16,20,29H,11-14H2,1-6H3,(H,25,27)/t20-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056505
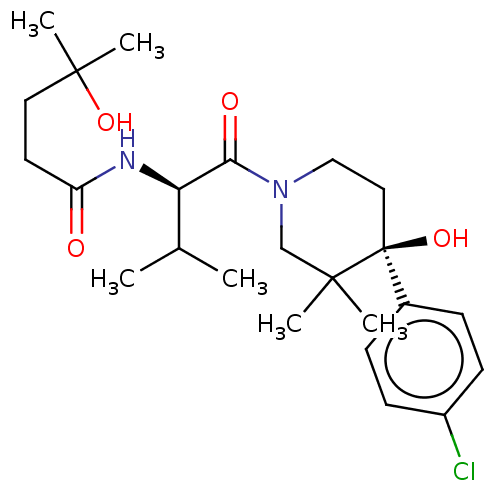
(CHEMBL3334825)Show SMILES CC(C)[C@@H](NC(=O)CCC(C)(C)O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H37ClN2O4/c1-16(2)20(26-19(28)11-12-23(5,6)30)21(29)27-14-13-24(31,22(3,4)15-27)17-7-9-18(25)10-8-17/h7-10,16,20,30-31H,11-15H2,1-6H3,(H,26,28)/t20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056497

(CHEMBL3334817)Show SMILES CC[C@H](C)NC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H36ClN3O3/c1-7-16(4)25-21(29)26-19(15(2)3)20(28)27-13-12-23(30,22(5,6)14-27)17-8-10-18(24)11-9-17/h8-11,15-16,19,30H,7,12-14H2,1-6H3,(H2,25,26,29)/t16-,19+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR1 receptor in human THP1 cells assessed as inhibition of MIP-1alpha induced chemotaxis after 60 mins |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056503

(CHEMBL3334823)Show SMILES C[C@@H](O)CNC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C22H34ClN3O4/c1-14(2)18(25-20(29)24-12-15(3)27)19(28)26-11-10-22(30,21(4,5)13-26)16-6-8-17(23)9-7-16/h6-9,14-15,18,27,30H,10-13H2,1-5H3,(H2,24,25,29)/t15-,18-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of Coll-3 MMP-13 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50081851

((2R,3R)-3-(cyclopentylmethyl)-2-((1-(dimethylamino...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C29H42N4O5S/c1-31(2)26-15-9-14-23-22(26)13-10-16-27(23)39(37,38)32(3)20-25(28(34)30-36)24(19-21-11-5-6-12-21)29(35)33-17-7-4-8-18-33/h9-10,13-16,21,24-25,36H,4-8,11-12,17-20H2,1-3H3,(H,30,34)/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HFC MMP-1 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056441

(CHEMBL3334725)Show SMILES CC(C)[C@@H](NC(=O)NC1CCCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H36ClN3O3/c1-16(2)20(27-22(30)26-19-7-5-6-8-19)21(29)28-14-13-24(31,23(3,4)15-28)17-9-11-18(25)12-10-17/h9-12,16,19-20,31H,5-8,13-15H2,1-4H3,(H2,26,27,30)/t20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056476

(CHEMBL3334728)Show SMILES CC(C)[C@@H](NC(=O)N[C@@H]1CC[C@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H36ClN3O4/c1-15(2)20(27-22(31)26-18-9-10-19(29)13-18)21(30)28-12-11-24(32,23(3,4)14-28)16-5-7-17(25)8-6-16/h5-8,15,18-20,29,32H,9-14H2,1-4H3,(H2,26,27,31)/t18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056497

(CHEMBL3334817)Show SMILES CC[C@H](C)NC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H36ClN3O3/c1-7-16(4)25-21(29)26-19(15(2)3)20(28)27-13-12-23(30,22(5,6)14-27)17-8-10-18(24)11-9-17/h8-11,15-16,19,30H,7,12-14H2,1-6H3,(H2,25,26,29)/t16-,19+,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056501
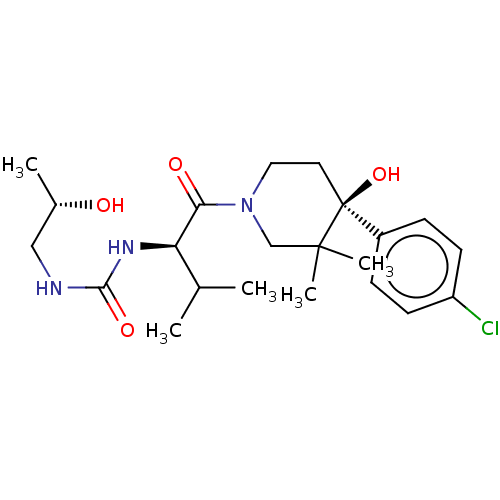
(CHEMBL3334821)Show SMILES C[C@H](O)CNC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C22H34ClN3O4/c1-14(2)18(25-20(29)24-12-15(3)27)19(28)26-11-10-22(30,21(4,5)13-26)16-6-8-17(23)9-7-16/h6-9,14-15,18,27,30H,10-13H2,1-5H3,(H2,24,25,29)/t15-,18+,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50056502

(CHEMBL3334822)Show SMILES C[C@@H](O)CC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C22H33ClN2O4/c1-14(2)19(24-18(27)12-15(3)26)20(28)25-11-10-22(29,21(4,5)13-25)16-6-8-17(23)9-7-16/h6-9,14-15,19,26,29H,10-13H2,1-5H3,(H,24,27)/t15-,19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Displacement of [125I]MIP-1alpha from CCR1 in human THP1 cells |
J Med Chem 57: 7550-64 (2014)
Article DOI: 10.1021/jm5003167
BindingDB Entry DOI: 10.7270/Q2TB18JC |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436256

(CHEMBL2398726)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CC[C@@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19-,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436257
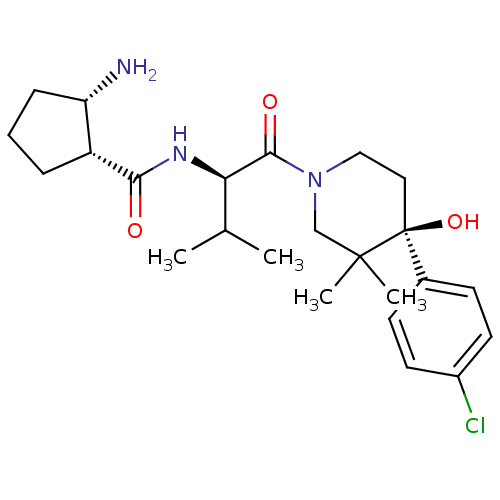
(CHEMBL2398724)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CCC[C@@H]1N)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H36ClN3O3/c1-15(2)20(27-21(29)18-6-5-7-19(18)26)22(30)28-13-12-24(31,23(3,4)14-28)16-8-10-17(25)11-9-16/h8-11,15,18-20,31H,5-7,12-14,26H2,1-4H3,(H,27,29)/t18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436306
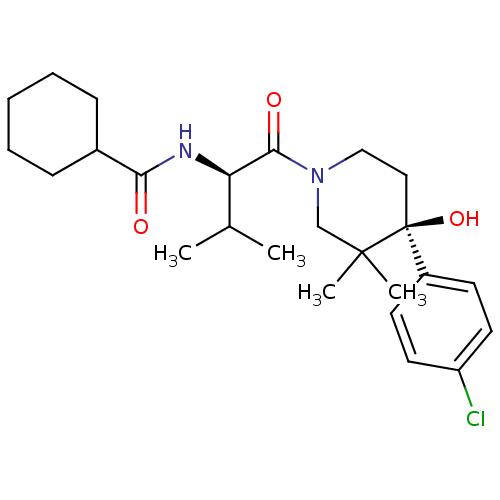
(CHEMBL2398748)Show SMILES CC(C)[C@@H](NC(=O)C1CCCCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C25H37ClN2O3/c1-17(2)21(27-22(29)18-8-6-5-7-9-18)23(30)28-15-14-25(31,24(3,4)16-28)19-10-12-20(26)13-11-19/h10-13,17-18,21,31H,5-9,14-16H2,1-4H3,(H,27,29)/t21-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436258
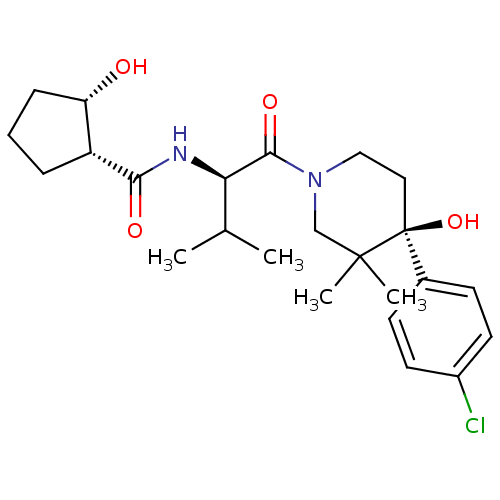
(CHEMBL2398722)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CCC[C@@H]1O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)18-6-5-7-19(18)28)22(30)27-13-12-24(31,23(3,4)14-27)16-8-10-17(25)11-9-16/h8-11,15,18-20,28,31H,5-7,12-14H2,1-4H3,(H,26,29)/t18-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436276
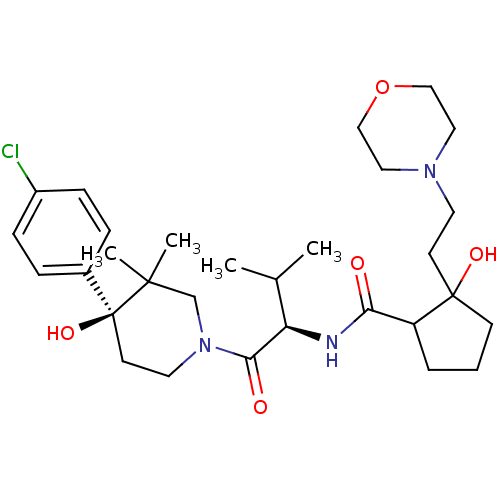
(CHEMBL2398750)Show SMILES CC(C)[C@@H](NC(=O)C1CCCC1(O)CCN1CCOCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C30H46ClN3O5/c1-21(2)25(32-26(35)24-6-5-11-29(24,37)12-14-33-16-18-39-19-17-33)27(36)34-15-13-30(38,28(3,4)20-34)22-7-9-23(31)10-8-22/h7-10,21,24-25,37-38H,5-6,11-20H2,1-4H3,(H,32,35)/t24?,25-,29?,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436284

(CHEMBL2398747 | US8633226, 460)Show SMILES CC(C)[C@@H](NC(=O)C1CCCC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O3/c1-16(2)20(26-21(28)17-7-5-6-8-17)22(29)27-14-13-24(30,23(3,4)15-27)18-9-11-19(25)12-10-18/h9-12,16-17,20,30H,5-8,13-15H2,1-4H3,(H,26,28)/t20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436305

(CHEMBL2398749)Show SMILES CC(C)[C@@H](NC(=O)CC12CC3CC(CC(C3)C1)C2)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r,TLB:7:8:11:15.14.13,THB:9:10:13:17.8.16,9:8:11.10.15:13,16:8:11:15.14.13,16:14:11:17.9.8| Show InChI InChI=1S/C30H43ClN2O3/c1-19(2)26(32-25(34)17-29-14-20-11-21(15-29)13-22(12-20)16-29)27(35)33-10-9-30(36,28(3,4)18-33)23-5-7-24(31)8-6-23/h5-8,19-22,26,36H,9-18H2,1-4H3,(H,32,34)/t20?,21?,22?,26-,29?,30+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436256

(CHEMBL2398726)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CC[C@@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19-,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MPIF-1-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436283

(CHEMBL2398729)Show SMILES CC(C)[C@@H](NC(=O)[C@@H]1CC[C@H](O)C1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H35ClN2O4/c1-15(2)20(26-21(29)16-5-10-19(28)13-16)22(30)27-12-11-24(31,23(3,4)14-27)17-6-8-18(25)9-7-17/h6-9,15-16,19-20,28,31H,5,10-14H2,1-4H3,(H,26,29)/t16-,19+,20-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human CCR1 assessed as inhibition of MIP1alpha-induced chemotaxis |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436285

(CHEMBL2398743 | US8633226, 466)Show SMILES CC(C)CC(=O)N[C@H](C(C)C)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C23H35ClN2O3/c1-15(2)13-19(27)25-20(16(3)4)21(28)26-12-11-23(29,22(5,6)14-26)17-7-9-18(24)10-8-17/h7-10,15-16,20,29H,11-14H2,1-6H3,(H,25,27)/t20-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436270
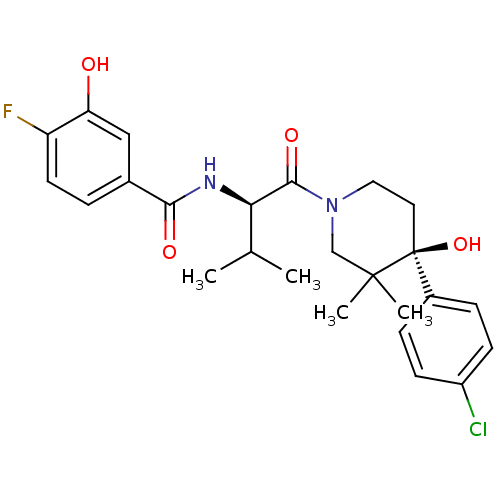
(CHEMBL2398763)Show SMILES CC(C)[C@@H](NC(=O)c1ccc(F)c(O)c1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C25H30ClFN2O4/c1-15(2)21(28-22(31)16-5-10-19(27)20(30)13-16)23(32)29-12-11-25(33,24(3,4)14-29)17-6-8-18(26)9-7-17/h5-10,13,15,21,30,33H,11-12,14H2,1-4H3,(H,28,31)/t21-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436304
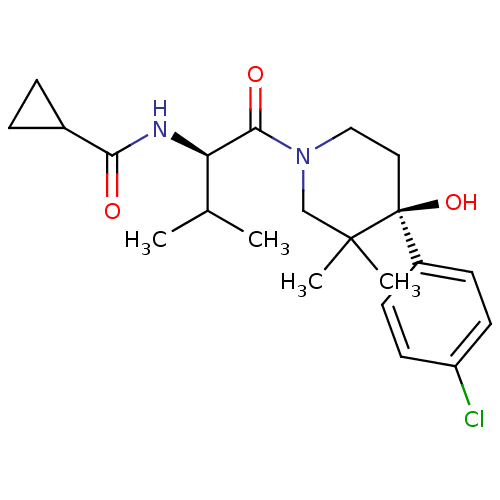
(CHEMBL2398745)Show SMILES CC(C)[C@@H](NC(=O)C1CC1)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C22H31ClN2O3/c1-14(2)18(24-19(26)15-5-6-15)20(27)25-12-11-22(28,21(3,4)13-25)16-7-9-17(23)10-8-16/h7-10,14-15,18,28H,5-6,11-13H2,1-4H3,(H,24,26)/t18-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50436274
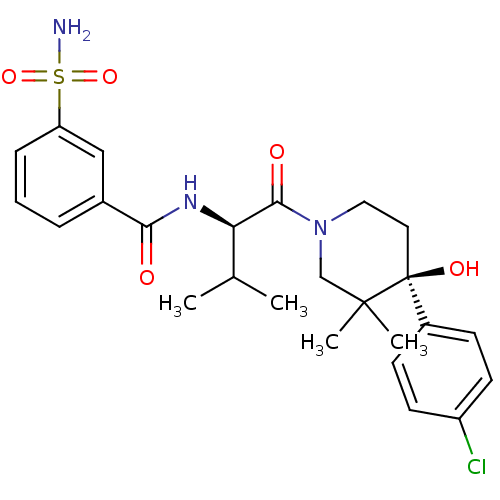
(CHEMBL2398767)Show SMILES CC(C)[C@@H](NC(=O)c1cccc(c1)S(N)(=O)=O)C(=O)N1CC[C@](O)(c2ccc(Cl)cc2)C(C)(C)C1 |r| Show InChI InChI=1S/C25H32ClN3O5S/c1-16(2)21(28-22(30)17-6-5-7-20(14-17)35(27,33)34)23(31)29-13-12-25(32,24(3,4)15-29)18-8-10-19(26)11-9-18/h5-11,14,16,21,32H,12-13,15H2,1-4H3,(H,28,30)(H2,27,33,34)/t21-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human CCR1 |
Bioorg Med Chem Lett 23: 3833-40 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.079
BindingDB Entry DOI: 10.7270/Q24X596G |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081866

((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCOCC1)C(=O)NO Show InChI InChI=1S/C23H35N3O7S/c1-25(34(30,31)19-9-7-18(32-2)8-10-19)16-21(22(27)24-29)20(15-17-5-3-4-6-17)23(28)26-11-13-33-14-12-26/h7-10,17,20-21,29H,3-6,11-16H2,1-2H3,(H,24,27)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50081869
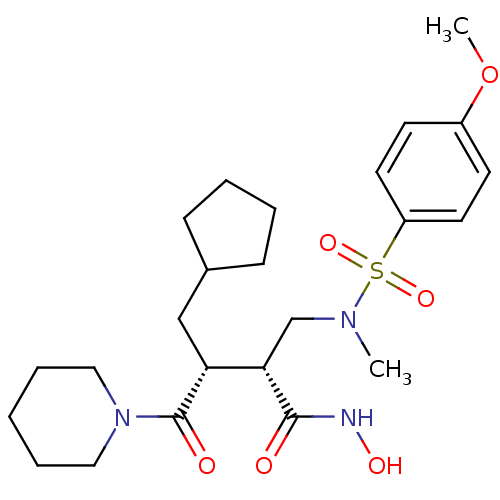
((2R,3R)-3-(cyclopentylmethyl)-N-hydroxy-2-((4-meth...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)C[C@@H]([C@@H](CC1CCCC1)C(=O)N1CCCCC1)C(=O)NO Show InChI InChI=1S/C24H37N3O6S/c1-26(34(31,32)20-12-10-19(33-2)11-13-20)17-22(23(28)25-30)21(16-18-8-4-5-9-18)24(29)27-14-6-3-7-15-27/h10-13,18,21-22,30H,3-9,14-17H2,1-2H3,(H,25,28)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Biotech Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of HNC MMP-8 |
Bioorg Med Chem Lett 9: 2887-92 (1999)
BindingDB Entry DOI: 10.7270/Q25H7FGN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data