 Found 5201 hits with Last Name = 'poss' and Initial = 'ma'
Found 5201 hits with Last Name = 'poss' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150431
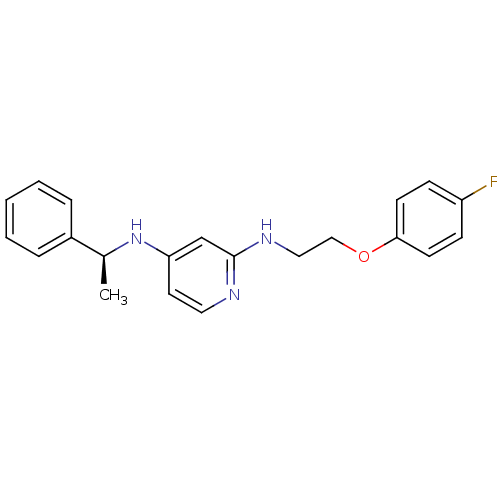
((S)-N2-(2-(4-fluorophenoxy)ethyl)-N4-(1-phenylethy...)Show InChI InChI=1S/C21H22FN3O/c1-16(17-5-3-2-4-6-17)25-19-11-12-23-21(15-19)24-13-14-26-20-9-7-18(22)8-10-20/h2-12,15-16H,13-14H2,1H3,(H2,23,24,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150439
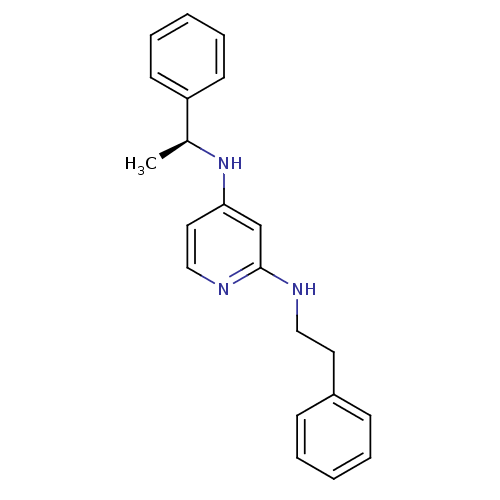
(CHEMBL182923 | N*2*-Phenethyl-N*4*-((S)-1-phenyl-e...)Show InChI InChI=1S/C21H23N3/c1-17(19-10-6-3-7-11-19)24-20-13-15-23-21(16-20)22-14-12-18-8-4-2-5-9-18/h2-11,13,15-17H,12,14H2,1H3,(H2,22,23,24)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150430

(CHEMBL183277 | N*2*-[2-(4-Fluoro-phenoxy)-ethyl]-N...)Show InChI InChI=1S/C20H21FN4O/c1-15(16-5-3-2-4-6-16)24-19-11-12-22-20(25-19)23-13-14-26-18-9-7-17(21)8-10-18/h2-12,15H,13-14H2,1H3,(H2,22,23,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150425
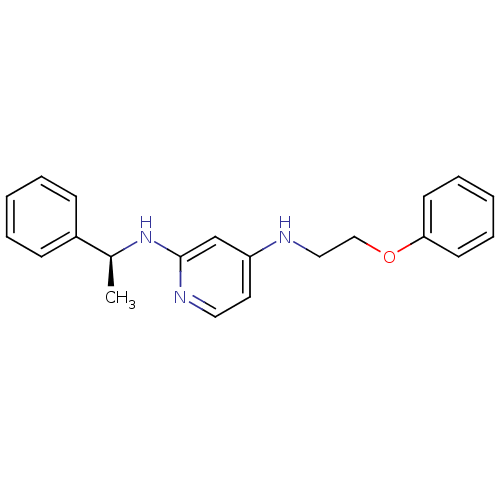
(CHEMBL184607 | N*4*-(2-Phenoxy-ethyl)-N*2*-((S)-1-...)Show InChI InChI=1S/C21H23N3O/c1-17(18-8-4-2-5-9-18)24-21-16-19(12-13-23-21)22-14-15-25-20-10-6-3-7-11-20/h2-13,16-17H,14-15H2,1H3,(H2,22,23,24)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150441

(CHEMBL181605 | N*2*-[2-(4-Fluoro-phenyl)-ethyl]-N*...)Show InChI InChI=1S/C21H22FN3/c1-16(18-5-3-2-4-6-18)25-20-12-14-24-21(15-20)23-13-11-17-7-9-19(22)10-8-17/h2-10,12,14-16H,11,13H2,1H3,(H2,23,24,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150428

(CHEMBL183423 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...)Show InChI InChI=1S/C21H22FN3O/c1-16(17-5-3-2-4-6-17)25-21-15-19(11-12-24-21)23-13-14-26-20-9-7-18(22)8-10-20/h2-12,15-16H,13-14H2,1H3,(H2,23,24,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150446

(CHEMBL181356 | N*2*-(2-Phenoxy-ethyl)-N*4*-((S)-1-...)Show InChI InChI=1S/C21H23N3O/c1-17(18-8-4-2-5-9-18)24-19-12-13-22-21(16-19)23-14-15-25-20-10-6-3-7-11-20/h2-13,16-17H,14-15H2,1H3,(H2,22,23,24)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150422
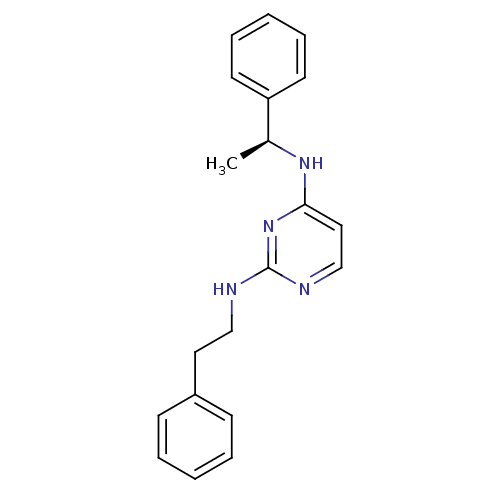
(CHEMBL185384 | N*2*-Phenethyl-N*4*-((S)-1-phenyl-e...)Show InChI InChI=1S/C20H22N4/c1-16(18-10-6-3-7-11-18)23-19-13-15-22-20(24-19)21-14-12-17-8-4-2-5-9-17/h2-11,13,15-16H,12,14H2,1H3,(H2,21,22,23,24)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449917
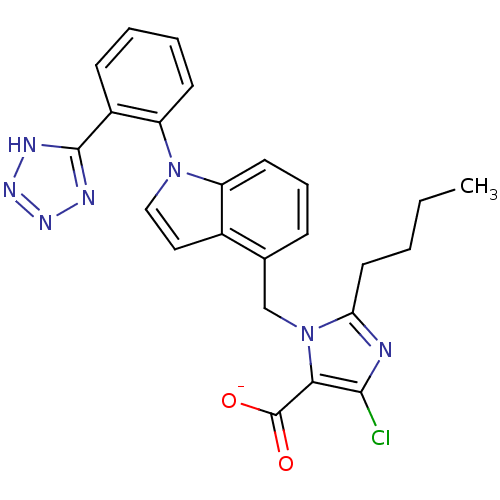
(BMS-180560 | CHEMBL2021417)Show SMILES [Li+].[Li]O.CCCCc1nc(Cl)c(C([O-])=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[nH]n1 Show InChI InChI=1S/C24H22ClN7O2.2Li.H2O/c1-2-3-11-20-26-22(25)21(24(33)34)32(20)14-15-7-6-10-18-16(15)12-13-31(18)19-9-5-4-8-17(19)23-27-29-30-28-23;;;/h4-10,12-13H,2-3,11,14H2,1H3,(H,33,34)(H,27,28,29,30);;;1H2/q;2*+1;/p-2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150438
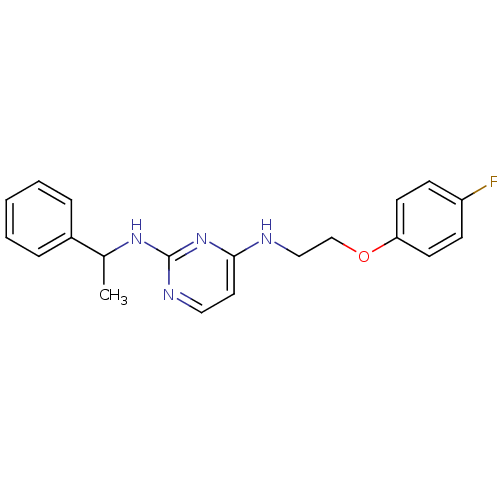
(CHEMBL182071 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...)Show InChI InChI=1S/C20H21FN4O/c1-15(16-5-3-2-4-6-16)24-20-23-12-11-19(25-20)22-13-14-26-18-9-7-17(21)8-10-18/h2-12,15H,13-14H2,1H3,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150408
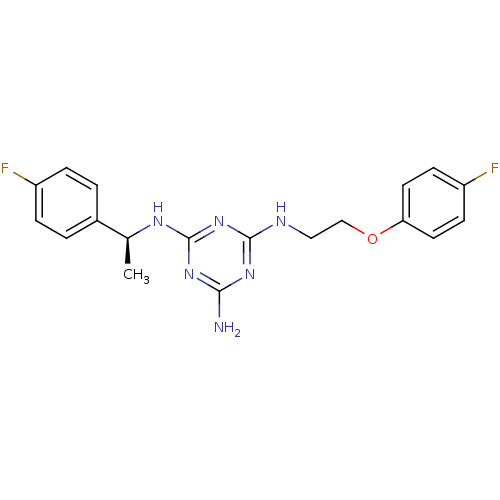
(CHEMBL182937 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...)Show SMILES C[C@H](Nc1nc(N)nc(NCCOc2ccc(F)cc2)n1)c1ccc(F)cc1 Show InChI InChI=1S/C19H20F2N6O/c1-12(13-2-4-14(20)5-3-13)24-19-26-17(22)25-18(27-19)23-10-11-28-16-8-6-15(21)7-9-16/h2-9,12H,10-11H2,1H3,(H4,22,23,24,25,26,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150413
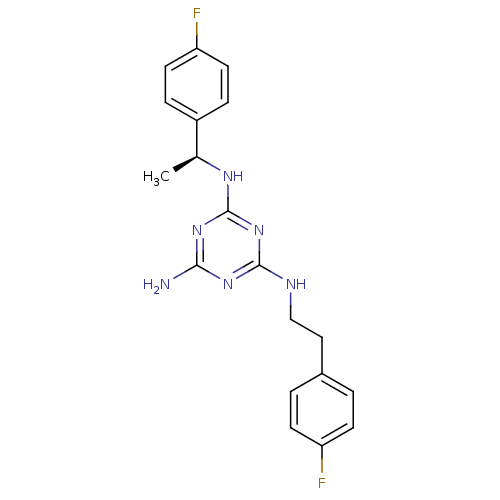
(CHEMBL413049 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-[...)Show SMILES C[C@H](Nc1nc(N)nc(NCCc2ccc(F)cc2)n1)c1ccc(F)cc1 Show InChI InChI=1S/C19H20F2N6/c1-12(14-4-8-16(21)9-5-14)24-19-26-17(22)25-18(27-19)23-11-10-13-2-6-15(20)7-3-13/h2-9,12H,10-11H2,1H3,(H4,22,23,24,25,26,27)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150433
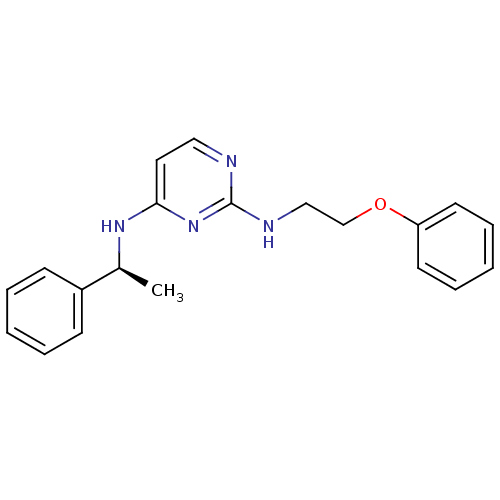
(CHEMBL185511 | N*2*-(2-Phenoxy-ethyl)-N*4*-((S)-1-...)Show InChI InChI=1S/C20H22N4O/c1-16(17-8-4-2-5-9-17)23-19-12-13-21-20(24-19)22-14-15-25-18-10-6-3-7-11-18/h2-13,16H,14-15H2,1H3,(H2,21,22,23,24)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150407
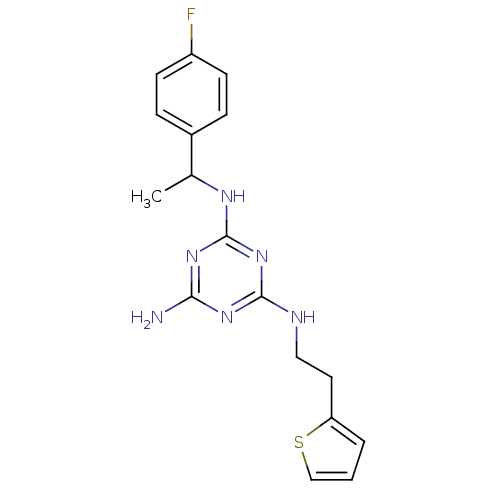
(CHEMBL180086 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...)Show InChI InChI=1S/C17H19FN6S/c1-11(12-4-6-13(18)7-5-12)21-17-23-15(19)22-16(24-17)20-9-8-14-3-2-10-25-14/h2-7,10-11H,8-9H2,1H3,(H4,19,20,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50049201

(2-Cyclopropyl-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-...)Show SMILES OC(=O)c1c(OCc2ccc(cc2)-c2ccccc2-c2nnn[nH]2)c(nc2ccccc12)C1CC1 Show InChI InChI=1S/C27H21N5O3/c33-27(34)23-21-7-3-4-8-22(21)28-24(18-13-14-18)25(23)35-15-16-9-11-17(12-10-16)19-5-1-2-6-20(19)26-29-31-32-30-26/h1-12,18H,13-15H2,(H,33,34)(H,29,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Receptor binding affinity determined from competitive binding assay using 1251 labelled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 201-206 (1994)
Article DOI: 10.1016/S0960-894X(01)81147-1
BindingDB Entry DOI: 10.7270/Q2TH8MMS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150410
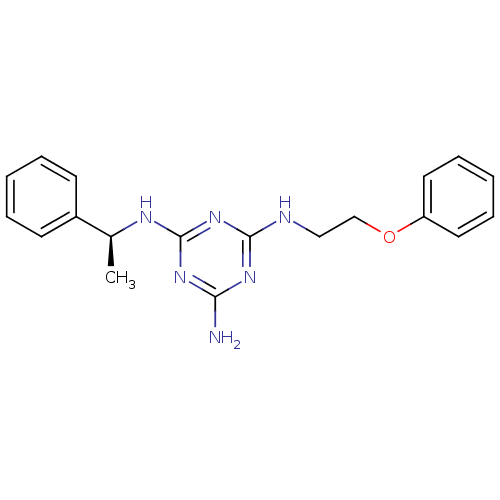
(CHEMBL182174 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...)Show InChI InChI=1S/C19H22N6O/c1-14(15-8-4-2-5-9-15)22-19-24-17(20)23-18(25-19)21-12-13-26-16-10-6-3-7-11-16/h2-11,14H,12-13H2,1H3,(H4,20,21,22,23,24,25)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150436
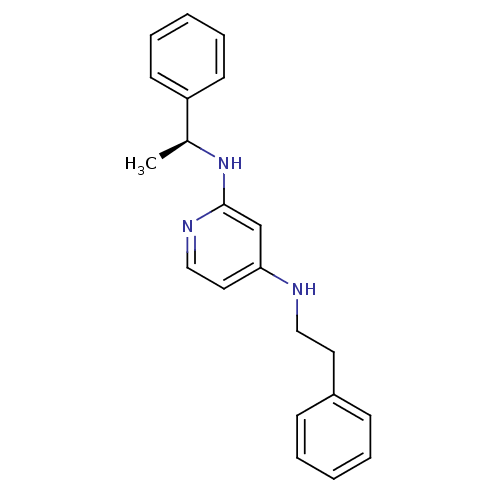
(CHEMBL181950 | N*4*-Phenethyl-N*2*-((S)-1-phenyl-e...)Show InChI InChI=1S/C21H23N3/c1-17(19-10-6-3-7-11-19)24-21-16-20(13-15-23-21)22-14-12-18-8-4-2-5-9-18/h2-11,13,15-17H,12,14H2,1H3,(H2,22,23,24)/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150412
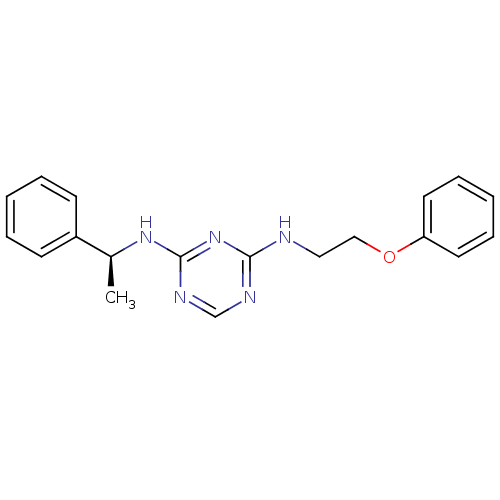
(CHEMBL183862 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...)Show InChI InChI=1S/C19H21N5O/c1-15(16-8-4-2-5-9-16)23-19-22-14-21-18(24-19)20-12-13-25-17-10-6-3-7-11-17/h2-11,14-15H,12-13H2,1H3,(H2,20,21,22,23,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150432
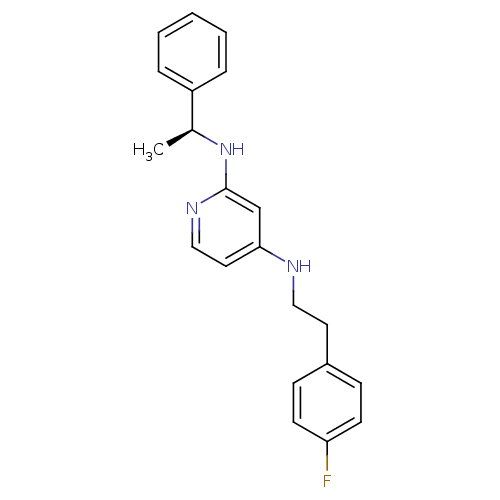
(CHEMBL185512 | N*4*-[2-(4-Fluoro-phenyl)-ethyl]-N*...)Show InChI InChI=1S/C21H22FN3/c1-16(18-5-3-2-4-6-18)25-21-15-20(12-14-24-21)23-13-11-17-7-9-19(22)10-8-17/h2-10,12,14-16H,11,13H2,1H3,(H2,23,24,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398864
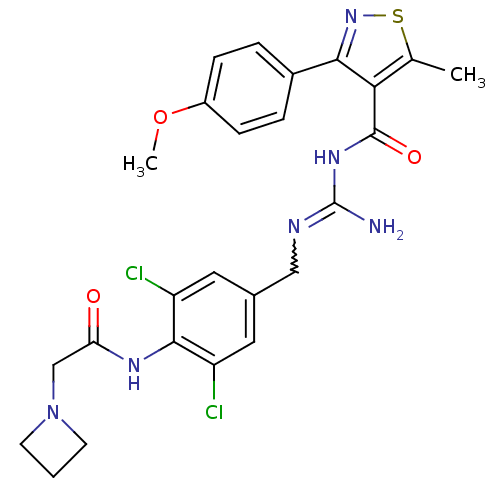
(CHEMBL2178164)Show SMILES COc1ccc(cc1)-c1nsc(C)c1C(=O)NC(N)=NCc1cc(Cl)c(NC(=O)CN2CCC2)c(Cl)c1 |w:19.21| Show InChI InChI=1S/C25H26Cl2N6O3S/c1-14-21(22(32-37-14)16-4-6-17(36-2)7-5-16)24(35)31-25(28)29-12-15-10-18(26)23(19(27)11-15)30-20(34)13-33-8-3-9-33/h4-7,10-11H,3,8-9,12-13H2,1-2H3,(H,30,34)(H3,28,29,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150437
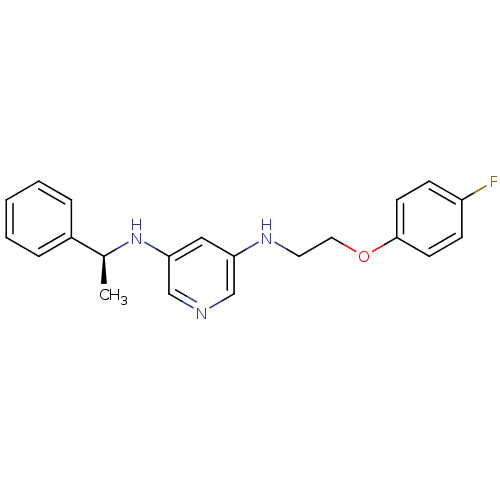
(CHEMBL184789 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...)Show InChI InChI=1S/C21H22FN3O/c1-16(17-5-3-2-4-6-17)25-20-13-19(14-23-15-20)24-11-12-26-21-9-7-18(22)8-10-21/h2-10,13-16,24-25H,11-12H2,1H3/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150440

(CHEMBL359725 | N-(2-Phenoxy-ethyl)-N''-((S)-1-phen...)Show InChI InChI=1S/C21H23N3O/c1-17(18-8-4-2-5-9-18)24-20-14-19(15-22-16-20)23-12-13-25-21-10-6-3-7-11-21/h2-11,14-17,23-24H,12-13H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449914
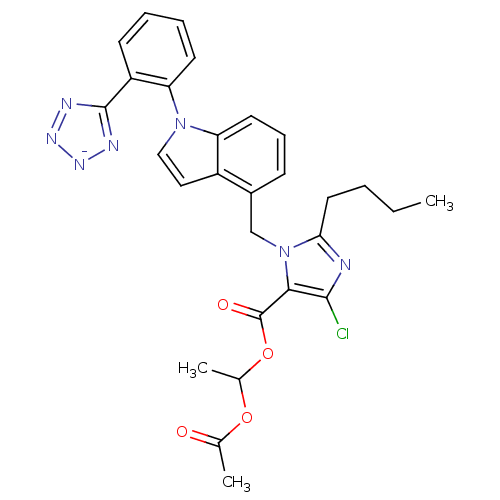
(CHEMBL2079784)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(C)OC(C)=O)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C28H27ClN7O4.K/c1-4-5-13-24-30-26(29)25(28(38)40-18(3)39-17(2)37)36(24)16-19-9-8-12-22-20(19)14-15-35(22)23-11-7-6-10-21(23)27-31-33-34-32-27;/h6-12,14-15,18H,4-5,13,16H2,1-3H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150423

(CHEMBL182680 | N-[2-(4-Fluoro-phenyl)-ethyl]-N''-(...)Show InChI InChI=1S/C20H21FN4/c1-15(17-5-3-2-4-6-17)25-20-13-19(23-14-24-20)22-12-11-16-7-9-18(21)10-8-16/h2-10,13-15H,11-12H2,1H3,(H2,22,23,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150417
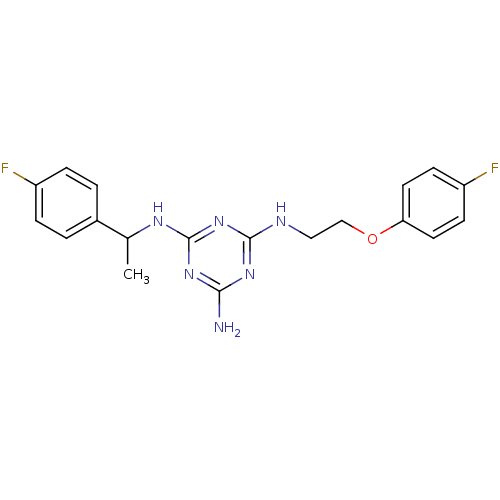
(CHEMBL182322 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...)Show SMILES CC(Nc1nc(N)nc(NCCOc2ccc(F)cc2)n1)c1ccc(F)cc1 Show InChI InChI=1S/C19H20F2N6O/c1-12(13-2-4-14(20)5-3-13)24-19-26-17(22)25-18(27-19)23-10-11-28-16-8-6-15(21)7-9-16/h2-9,12H,10-11H2,1H3,(H4,22,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398882
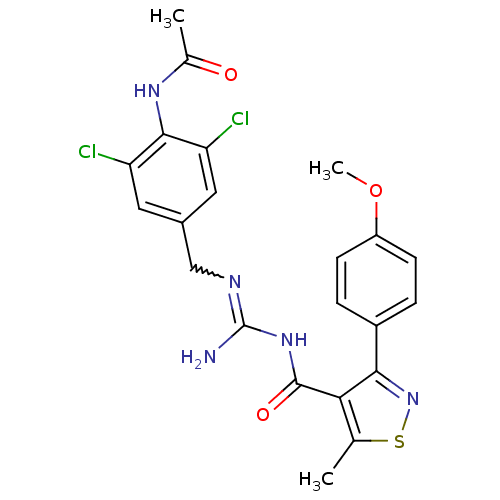
(CHEMBL2178180)Show SMILES COc1ccc(cc1)-c1nsc(C)c1C(=O)NC(N)=NCc1cc(Cl)c(NC(C)=O)c(Cl)c1 |w:19.21| Show InChI InChI=1S/C22H21Cl2N5O3S/c1-11-18(19(29-33-11)14-4-6-15(32-3)7-5-14)21(31)28-22(25)26-10-13-8-16(23)20(17(24)9-13)27-12(2)30/h4-9H,10H2,1-3H3,(H,27,30)(H3,25,26,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150424

(CHEMBL182943 | N-[2-(4-Fluoro-phenoxy)-ethyl]-N''-...)Show InChI InChI=1S/C20H21FN4O/c1-15(16-5-3-2-4-6-16)25-20-13-19(23-14-24-20)22-11-12-26-18-9-7-17(21)8-10-18/h2-10,13-15H,11-12H2,1H3,(H2,22,23,24,25)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398863
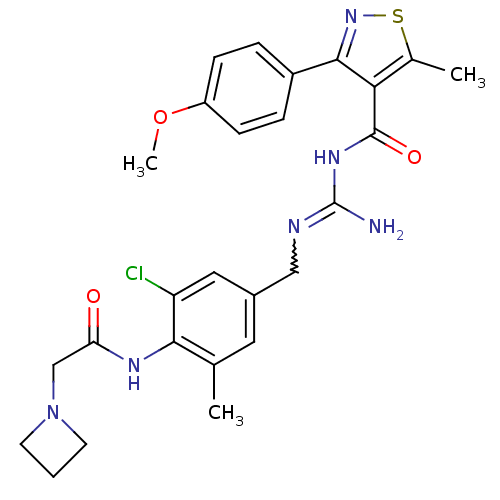
(CHEMBL2178165)Show SMILES COc1ccc(cc1)-c1nsc(C)c1C(=O)NC(N)=NCc1cc(C)c(NC(=O)CN2CCC2)c(Cl)c1 |w:19.21| Show InChI InChI=1S/C26H29ClN6O3S/c1-15-11-17(12-20(27)23(15)30-21(34)14-33-9-4-10-33)13-29-26(28)31-25(35)22-16(2)37-32-24(22)18-5-7-19(36-3)8-6-18/h5-8,11-12H,4,9-10,13-14H2,1-3H3,(H,30,34)(H3,28,29,31,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398868
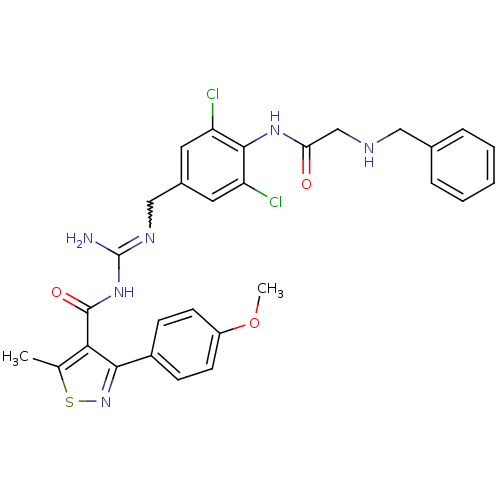
(CHEMBL2178195)Show SMILES COc1ccc(cc1)-c1nsc(C)c1C(=O)NC(N)=NCc1cc(Cl)c(NC(=O)CNCc2ccccc2)c(Cl)c1 |w:19.21| Show InChI InChI=1S/C29H28Cl2N6O3S/c1-17-25(26(37-41-17)20-8-10-21(40-2)11-9-20)28(39)36-29(32)34-15-19-12-22(30)27(23(31)13-19)35-24(38)16-33-14-18-6-4-3-5-7-18/h3-13,33H,14-16H2,1-2H3,(H,35,38)(H3,32,34,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150442

(CHEMBL182070 | N*4*-Phenethyl-N*2*-(1-phenyl-ethyl...)Show InChI InChI=1S/C20H22N4/c1-16(18-10-6-3-7-11-18)23-20-22-15-13-19(24-20)21-14-12-17-8-4-2-5-9-17/h2-11,13,15-16H,12,14H2,1H3,(H2,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50429537
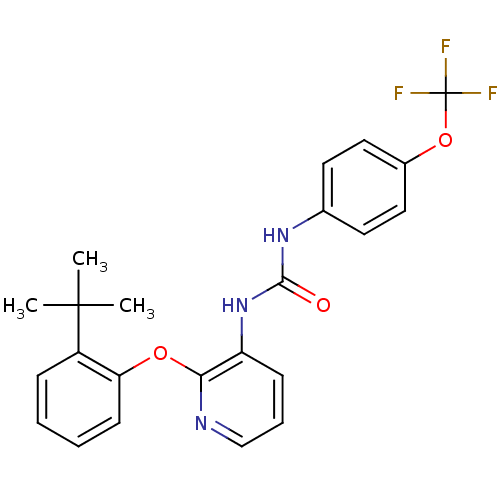
(CHEMBL2333770)Show SMILES CC(C)(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C23H22F3N3O3/c1-22(2,3)17-7-4-5-9-19(17)31-20-18(8-6-14-27-20)29-21(30)28-15-10-12-16(13-11-15)32-23(24,25)26/h4-14H,1-3H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150444
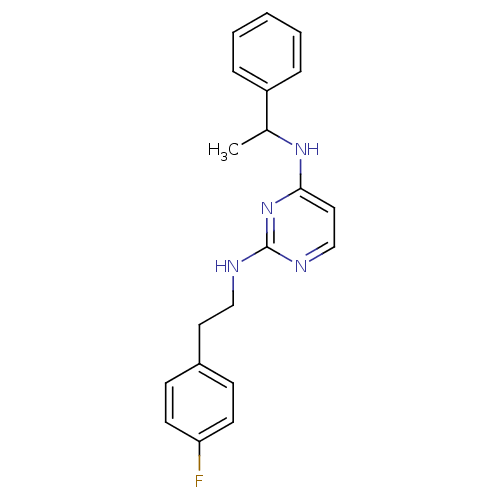
(CHEMBL185437 | N*2*-[2-(4-Fluoro-phenyl)-ethyl]-N*...)Show InChI InChI=1S/C20H21FN4/c1-15(17-5-3-2-4-6-17)24-19-12-14-23-20(25-19)22-13-11-16-7-9-18(21)10-8-16/h2-10,12,14-15H,11,13H2,1H3,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449919
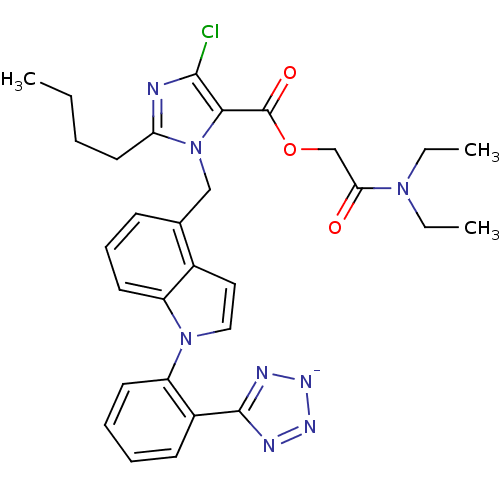
(CHEMBL2021415)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OCC(=O)N(CC)CC)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C30H32ClN8O3.K/c1-4-7-15-25-32-28(31)27(30(41)42-19-26(40)37(5-2)6-3)39(25)18-20-11-10-14-23-21(20)16-17-38(23)24-13-9-8-12-22(24)29-33-35-36-34-29;/h8-14,16-17H,4-7,15,18-19H2,1-3H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50366331

(CHEMBL1790055)Show SMILES CCCCc1nc(Cl)c(C[O-])n1Cc1cccc2n(ccc12)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H23ClN7O/c1-2-3-11-22-26-23(25)21(15-33)32(22)14-16-7-6-10-19-17(16)12-13-31(19)20-9-5-4-8-18(20)24-27-29-30-28-24/h4-10,12-13H,2-3,11,14-15H2,1H3,(H,27,28,29,30)/q-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50429541
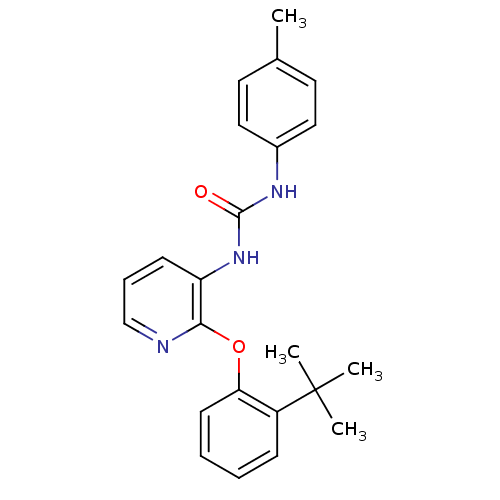
(CHEMBL2333766)Show SMILES Cc1ccc(NC(=O)Nc2cccnc2Oc2ccccc2C(C)(C)C)cc1 Show InChI InChI=1S/C23H25N3O2/c1-16-11-13-17(14-12-16)25-22(27)26-19-9-7-15-24-21(19)28-20-10-6-5-8-18(20)23(2,3)4/h5-15H,1-4H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50429535
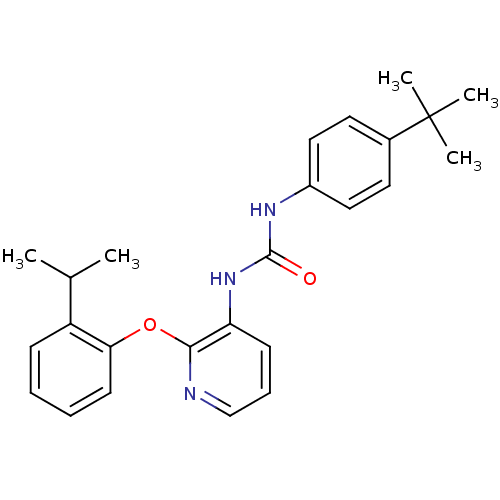
(CHEMBL2333772)Show SMILES CC(C)c1ccccc1Oc1ncccc1NC(=O)Nc1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C25H29N3O2/c1-17(2)20-9-6-7-11-22(20)30-23-21(10-8-16-26-23)28-24(29)27-19-14-12-18(13-15-19)25(3,4)5/h6-17H,1-5H3,(H2,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449910

(CHEMBL2079782)Show SMILES [K+].CCCCOC(=O)c1c(Cl)nc(CCCC)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C28H29ClN7O2.K/c1-3-5-14-24-30-26(29)25(28(37)38-17-6-4-2)36(24)18-19-10-9-13-22-20(19)15-16-35(22)23-12-8-7-11-21(23)27-31-33-34-32-27;/h7-13,15-16H,3-6,14,17-18H2,1-2H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 1
(Homo sapiens (Human)) | BDBM50429536
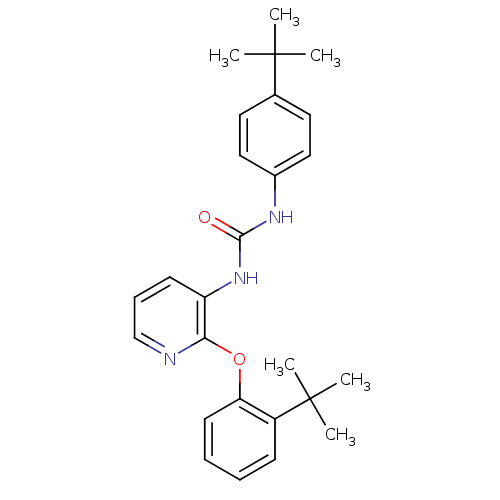
(CHEMBL2333771)Show SMILES CC(C)(C)c1ccc(NC(=O)Nc2cccnc2Oc2ccccc2C(C)(C)C)cc1 Show InChI InChI=1S/C26H31N3O2/c1-25(2,3)18-13-15-19(16-14-18)28-24(30)29-21-11-9-17-27-23(21)31-22-12-8-7-10-20(22)26(4,5)6/h7-17H,1-6H3,(H2,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [beta-33P]-2MeS-ADP from human P2Y1 receptor transfected in HEK293 cells assessed as residual [beta-33P] bound to plate after 1 hr by... |
J Med Chem 56: 1704-14 (2013)
Article DOI: 10.1021/jm301708u
BindingDB Entry DOI: 10.7270/Q2ST7R6G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150405

(CHEMBL182797 | N-(2-Phenoxy-ethyl)-N''-(1-phenyl-e...)Show InChI InChI=1S/C19H22N6O/c1-14(15-8-4-2-5-9-15)22-19-24-17(20)23-18(25-19)21-12-13-26-16-10-6-3-7-11-16/h2-11,14H,12-13H2,1H3,(H4,20,21,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150421
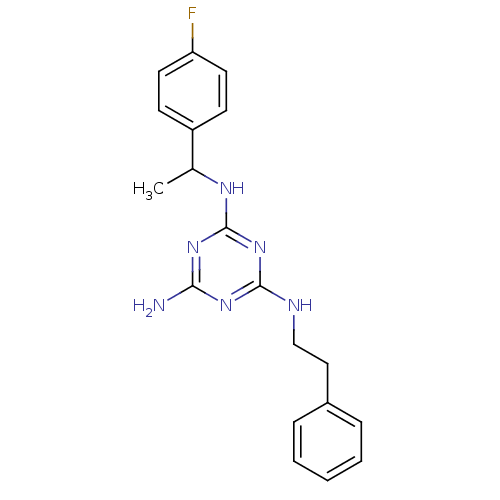
(CHEMBL183462 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-p...)Show InChI InChI=1S/C19H21FN6/c1-13(15-7-9-16(20)10-8-15)23-19-25-17(21)24-18(26-19)22-12-11-14-5-3-2-4-6-14/h2-10,13H,11-12H2,1H3,(H4,21,22,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449912
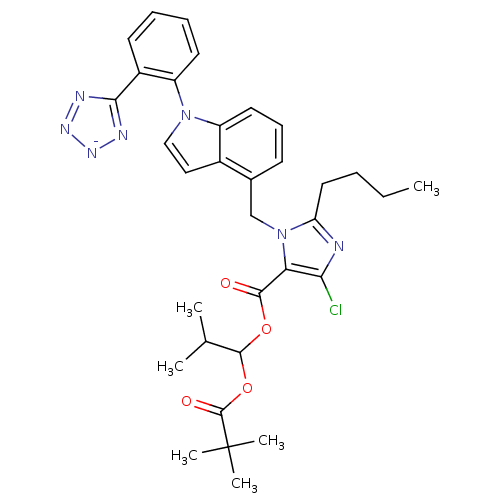
(CHEMBL2079781)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(OC(=O)C(C)(C)C)C(C)C)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C33H37ClN7O4.K/c1-7-8-16-26-35-28(34)27(30(42)44-31(20(2)3)45-32(43)33(4,5)6)41(26)19-21-12-11-15-24-22(21)17-18-40(24)25-14-10-9-13-23(25)29-36-38-39-37-29;/h9-15,17-18,20,31H,7-8,16,19H2,1-6H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150406
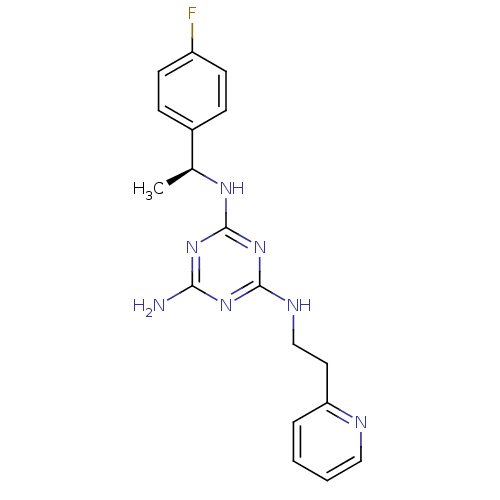
(CHEMBL182418 | N-[(S)-1-(4-Fluoro-phenyl)-ethyl]-N...)Show SMILES C[C@H](Nc1nc(N)nc(NCCc2ccccn2)n1)c1ccc(F)cc1 Show InChI InChI=1S/C18H20FN7/c1-12(13-5-7-14(19)8-6-13)23-18-25-16(20)24-17(26-18)22-11-9-15-4-2-3-10-21-15/h2-8,10,12H,9,11H2,1H3,(H4,20,22,23,24,25,26)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398883
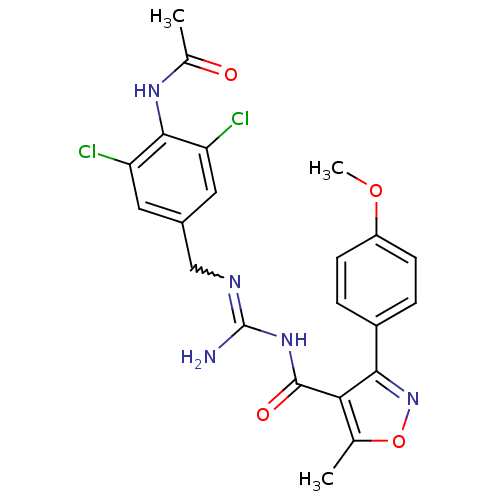
(CHEMBL2178179)Show SMILES COc1ccc(cc1)-c1noc(C)c1C(=O)NC(N)=NCc1cc(Cl)c(NC(C)=O)c(Cl)c1 |w:19.21| Show InChI InChI=1S/C22H21Cl2N5O4/c1-11-18(19(29-33-11)14-4-6-15(32-3)7-5-14)21(31)28-22(25)26-10-13-8-16(23)20(17(24)9-13)27-12(2)30/h4-9H,10H2,1-3H3,(H,27,30)(H3,25,26,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50150407

(CHEMBL180086 | N-[1-(4-Fluoro-phenyl)-ethyl]-N''-(...)Show InChI InChI=1S/C17H19FN6S/c1-11(12-4-6-13(18)7-5-12)21-17-23-15(19)22-16(24-17)20-9-8-14-3-2-10-25-14/h2-7,10-11H,8-9H2,1H3,(H4,19,20,21,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 2C receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150420
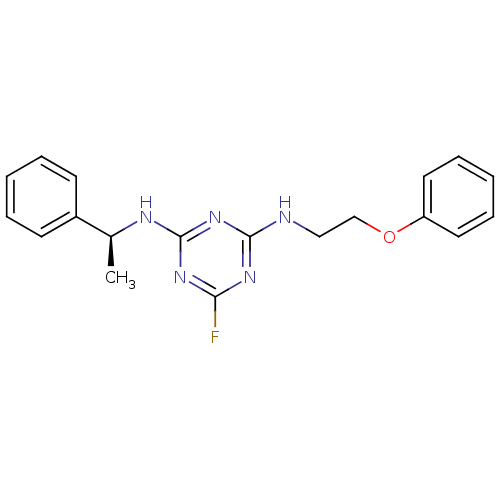
(6-Fluoro-N-(2-phenoxy-ethyl)-N''-((S)-1-phenyl-eth...)Show InChI InChI=1S/C19H20FN5O/c1-14(15-8-4-2-5-9-15)22-19-24-17(20)23-18(25-19)21-12-13-26-16-10-6-3-7-11-16/h2-11,14H,12-13H2,1H3,(H2,21,22,23,24,25)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.008
BindingDB Entry DOI: 10.7270/Q2GM86RJ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398867
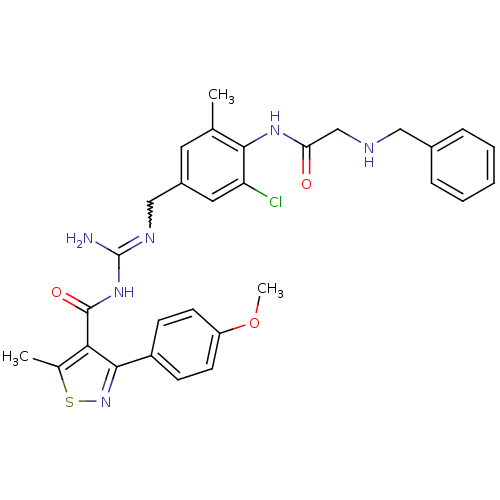
(CHEMBL2178161)Show SMILES COc1ccc(cc1)-c1nsc(C)c1C(=O)NC(N)=NCc1cc(C)c(NC(=O)CNCc2ccccc2)c(Cl)c1 |w:19.21| Show InChI InChI=1S/C30H31ClN6O3S/c1-18-13-21(14-24(31)27(18)35-25(38)17-33-15-20-7-5-4-6-8-20)16-34-30(32)36-29(39)26-19(2)41-37-28(26)22-9-11-23(40-3)12-10-22/h4-14,33H,15-17H2,1-3H3,(H,35,38)(H3,32,34,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50398866
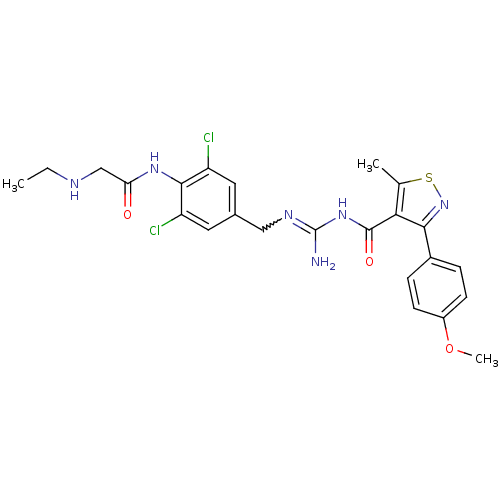
(CHEMBL2178162)Show SMILES CCNCC(=O)Nc1c(Cl)cc(CN=C(N)NC(=O)c2c(C)snc2-c2ccc(OC)cc2)cc1Cl |w:13.12| Show InChI InChI=1S/C24H26Cl2N6O3S/c1-4-28-12-19(33)30-22-17(25)9-14(10-18(22)26)11-29-24(27)31-23(34)20-13(2)36-32-21(20)15-5-7-16(35-3)8-6-15/h5-10,28H,4,11-12H2,1-3H3,(H,30,33)(H3,27,29,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]BMS-599240 from BACE1 expressed in HEK293 cells |
J Med Chem 55: 9208-23 (2012)
Article DOI: 10.1021/jm300931y
BindingDB Entry DOI: 10.7270/Q20G3M8M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50150427
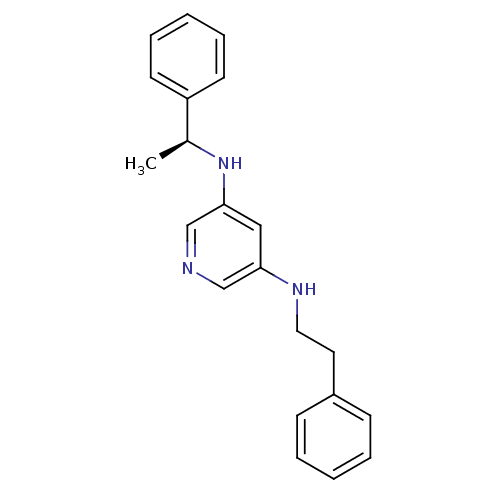
(CHEMBL182356 | N-Phenethyl-N''-((S)-1-phenyl-ethyl...)Show InChI InChI=1S/C21H23N3/c1-17(19-10-6-3-7-11-19)24-21-14-20(15-22-16-21)23-13-12-18-8-4-2-5-9-18/h2-11,14-17,23-24H,12-13H2,1H3/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 7 receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50150428

(CHEMBL183423 | N*4*-[2-(4-Fluoro-phenoxy)-ethyl]-N...)Show InChI InChI=1S/C21H22FN3O/c1-16(17-5-3-2-4-6-17)25-21-15-19(11-12-24-21)23-13-14-26-20-9-7-18(22)8-10-20/h2-12,15-16H,13-14H2,1H3,(H2,23,24,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity against 5-Hydroxy tryptamine 2C receptor |
Bioorg Med Chem Lett 14: 4249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.007
BindingDB Entry DOI: 10.7270/Q2BV7G34 |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor B
(RAT) | BDBM50449909
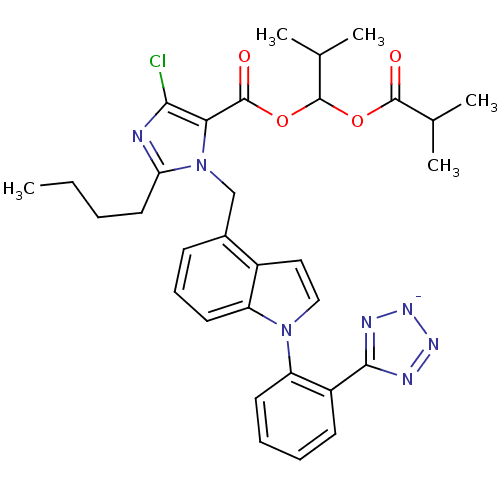
(CHEMBL2079769)Show SMILES [K+].CCCCc1nc(Cl)c(C(=O)OC(OC(=O)C(C)C)C(C)C)n1Cc1cccc2n(ccc12)-c1ccccc1-c1nn[n-]n1 Show InChI InChI=1S/C32H35ClN7O4.K/c1-6-7-15-26-34-28(33)27(31(42)44-32(20(4)5)43-30(41)19(2)3)40(26)18-21-11-10-14-24-22(21)16-17-39(24)25-13-9-8-12-23(25)29-35-37-38-36-29;/h8-14,16-17,19-20,32H,6-7,15,18H2,1-5H3;/q-1;+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes |
Bioorg Med Chem Lett 4: 145-150 (1994)
Article DOI: 10.1016/S0960-894X(01)81137-9
BindingDB Entry DOI: 10.7270/Q2V988KF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data