 Found 640 hits with Last Name = 'pour' and Initial = 'm'
Found 640 hits with Last Name = 'pour' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496270

(CHEMBL3127679)Show SMILES CC(C)N1CCN(CC1)C(=O)C1CC2(C1)CCN(CC2)C1CCOCC1 Show InChI InChI=1S/C21H37N3O2/c1-17(2)22-9-11-24(12-10-22)20(25)18-15-21(16-18)5-7-23(8-6-21)19-3-13-26-14-4-19/h17-19H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50334346
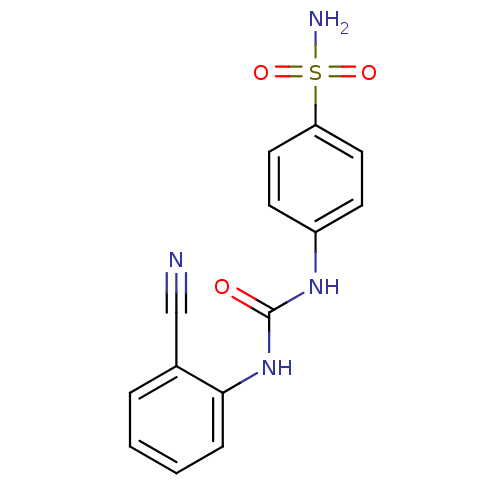
(4-(3-(2-cyanophenyl)ureido)benzenesulfonamide | 4-...)Show InChI InChI=1S/C14H12N4O3S/c15-9-10-3-1-2-4-13(10)18-14(19)17-11-5-7-12(8-6-11)22(16,20)21/h1-8H,(H2,16,20,21)(H2,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
| Assay Description
The inhibition constants (Ki) of FEC to four human CA isoenzymes I, II, IX and XII were determined by CA catalyzed CO2 hydration assays following pre... |
J Enzyme Inhib Med Chem 29: 249-55 (2014)
Article DOI: 10.3109/14756366.2013.773994
BindingDB Entry DOI: 10.7270/Q2VM4B6X |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(Homo sapiens (Human)) | BDBM50066009
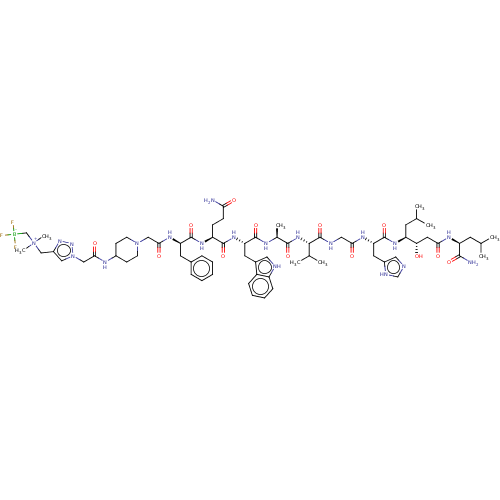
(CHEMBL3401466)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](Cc1ccccc1)NC(=O)CN1CCC(CC1)NC(=O)Cn1cc(C[N+](C)(C)C[B-](F)(F)F)nn1)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(N)=O |r| Show InChI InChI=1S/C70H104BF3N20O13/c1-40(2)25-52(57(95)30-59(97)83-53(64(76)101)26-41(3)4)87-69(106)56(29-47-32-77-39-80-47)84-60(98)33-79-70(107)63(42(5)6)89-65(102)43(7)81-67(104)55(28-45-31-78-50-18-14-13-17-49(45)50)88-66(103)51(19-20-58(75)96)86-68(105)54(27-44-15-11-10-12-16-44)85-61(99)35-92-23-21-46(22-24-92)82-62(100)36-93-34-48(90-91-93)37-94(8,9)38-71(72,73)74/h10-18,31-32,34,39-43,46,51-57,63,78,95H,19-30,33,35-38H2,1-9H3,(H2,75,96)(H2,76,101)(H,77,80)(H,79,107)(H,81,104)(H,82,100)(H,83,97)(H,84,98)(H,85,99)(H,86,105)(H,87,106)(H,88,103)(H,89,102)/t43-,51-,52-,53-,54+,55-,56-,57-,63-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr4]bombesin from GRPR (unknown origin) expressed in human PC3 cells after 45 mins by gamma counting analysis |
Bioorg Med Chem 23: 1500-6 (2015)
Article DOI: 10.1016/j.bmc.2015.02.009
BindingDB Entry DOI: 10.7270/Q27H1M8C |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364921
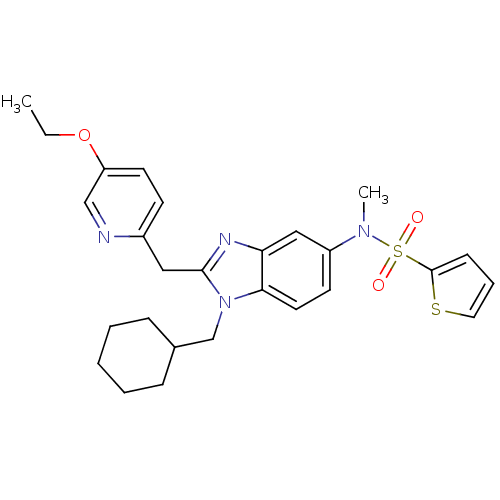
(CHEMBL1950329)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCCC2)N(C)S(=O)(=O)c2cccs2)nc1 Show InChI InChI=1S/C27H32N4O3S2/c1-3-34-23-13-11-21(28-18-23)16-26-29-24-17-22(30(2)36(32,33)27-10-7-15-35-27)12-14-25(24)31(26)19-20-8-5-4-6-9-20/h7,10-15,17-18,20H,3-6,8-9,16,19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496289

(CHEMBL3124968)Show SMILES CC(C)N1CCN(CC1)C(=O)[C@H]1CC11CCN(CC1)C1CCOCC1 |r| Show InChI InChI=1S/C20H35N3O2/c1-16(2)21-9-11-23(12-10-21)19(24)18-15-20(18)5-7-22(8-6-20)17-3-13-25-14-4-17/h16-18H,3-15H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50334349
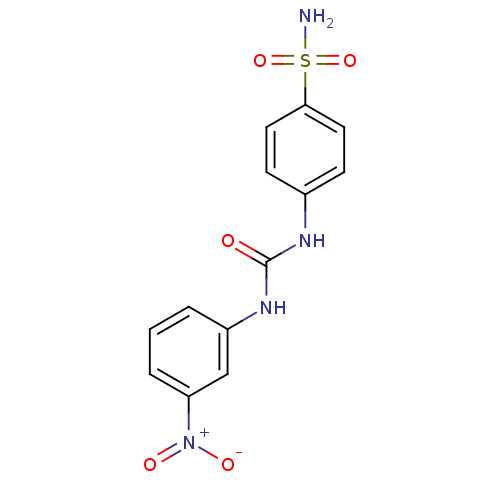
(4-(3-(3-nitrophenyl)ureido)benzenesulfonamide | 4-...)Show SMILES NS(=O)(=O)c1ccc(NC(=O)Nc2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C13H12N4O5S/c14-23(21,22)12-6-4-9(5-7-12)15-13(18)16-10-2-1-3-11(8-10)17(19)20/h1-8H,(H2,14,21,22)(H2,15,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
| Assay Description
The inhibition constants (Ki) of FEC to four human CA isoenzymes I, II, IX and XII were determined by CA catalyzed CO2 hydration assays following pre... |
J Enzyme Inhib Med Chem 29: 249-55 (2014)
Article DOI: 10.3109/14756366.2013.773994
BindingDB Entry DOI: 10.7270/Q2VM4B6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496273
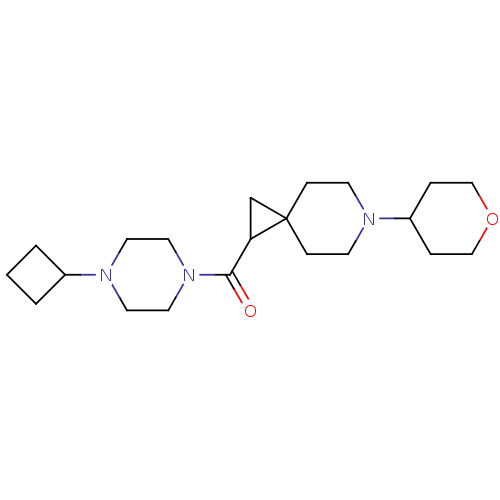
(CHEMBL3127700)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)C1CCC1 Show InChI InChI=1S/C21H35N3O2/c25-20(24-12-10-23(11-13-24)17-2-1-3-17)19-16-21(19)6-8-22(9-7-21)18-4-14-26-15-5-18/h17-19H,1-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496272
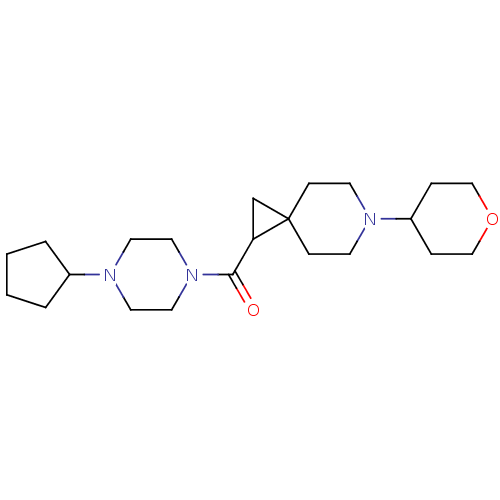
(CHEMBL3127701)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)C1CCCC1 Show InChI InChI=1S/C22H37N3O2/c26-21(25-13-11-24(12-14-25)18-3-1-2-4-18)20-17-22(20)7-9-23(10-8-22)19-5-15-27-16-6-19/h18-20H,1-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496281

(CHEMBL3127698)Show InChI InChI=1S/C20H35N3O2/c1-16(2)21-9-11-23(12-10-21)19(24)18-15-20(18)5-7-22(8-6-20)17-3-13-25-14-4-17/h16-18H,3-15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364921

(CHEMBL1950329)Show SMILES CCOc1ccc(Cc2nc3cc(ccc3n2CC2CCCCC2)N(C)S(=O)(=O)c2cccs2)nc1 Show InChI InChI=1S/C27H32N4O3S2/c1-3-34-23-13-11-21(28-18-23)16-26-29-24-17-22(30(2)36(32,33)27-10-7-15-35-27)12-14-25(24)31(26)19-20-8-5-4-6-9-20/h7,10-15,17-18,20H,3-6,8-9,16,19H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496296
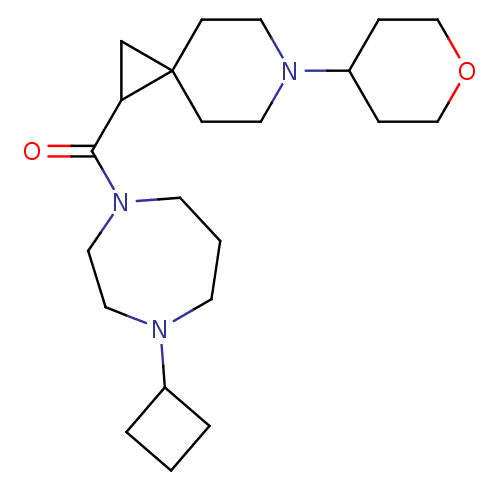
(CHEMBL3127704)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCCN(CC1)C1CCC1 Show InChI InChI=1S/C22H37N3O2/c26-21(25-10-2-9-23(13-14-25)18-3-1-4-18)20-17-22(20)7-11-24(12-8-22)19-5-15-27-16-6-19/h18-20H,1-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50334360
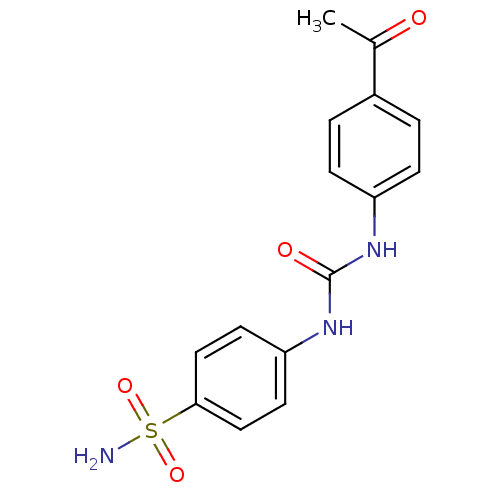
(4-(3-(4-acetylphenyl)ureido)benzenesulfonamide | 4...)Show SMILES CC(=O)c1ccc(NC(=O)Nc2ccc(cc2)S(N)(=O)=O)cc1 Show InChI InChI=1S/C15H15N3O4S/c1-10(19)11-2-4-12(5-3-11)17-15(20)18-13-6-8-14(9-7-13)23(16,21)22/h2-9H,1H3,(H2,16,21,22)(H2,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
| Assay Description
The inhibition constants (Ki) of FEC to four human CA isoenzymes I, II, IX and XII were determined by CA catalyzed CO2 hydration assays following pre... |
J Enzyme Inhib Med Chem 29: 249-55 (2014)
Article DOI: 10.3109/14756366.2013.773994
BindingDB Entry DOI: 10.7270/Q2VM4B6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496290

(CHEMBL3127705)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)c1ccncc1 Show InChI InChI=1S/C22H32N4O2/c27-21(26-13-11-25(12-14-26)18-1-7-23-8-2-18)20-17-22(20)5-9-24(10-6-22)19-3-15-28-16-4-19/h1-2,7-8,19-20H,3-6,9-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364920
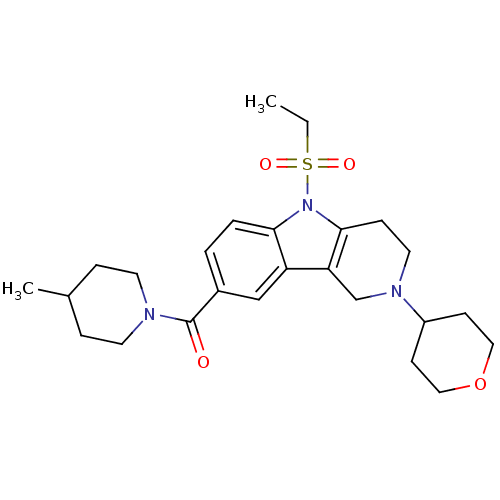
(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364923
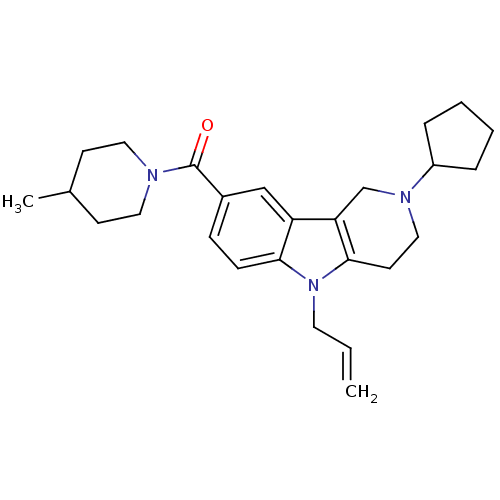
(CHEMBL1950333)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C26H35N3O/c1-3-13-29-24-9-8-20(26(30)27-14-10-19(2)11-15-27)17-22(24)23-18-28(16-12-25(23)29)21-6-4-5-7-21/h3,8-9,17,19,21H,1,4-7,10-16,18H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496297

(CHEMBL3127699)Show InChI InChI=1S/C20H33N3O2/c24-19(23-11-9-22(10-12-23)16-1-2-16)18-15-20(18)5-7-21(8-6-20)17-3-13-25-14-4-17/h16-18H,1-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50364922
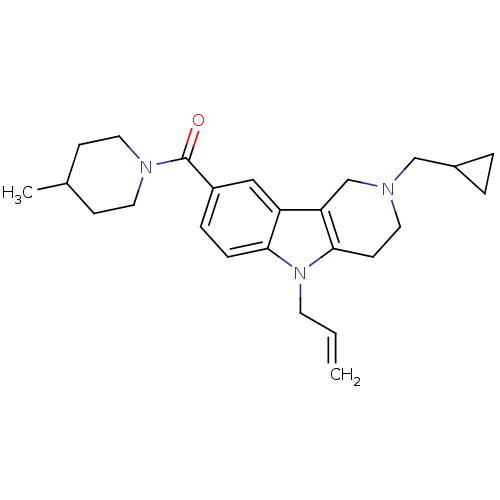
(CHEMBL1950330)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(CC4CC4)Cc3c2c1 Show InChI InChI=1S/C25H33N3O/c1-3-11-28-23-7-6-20(25(29)27-13-8-18(2)9-14-27)15-21(23)22-17-26(12-10-24(22)28)16-19-4-5-19/h3,6-7,15,18-19H,1,4-5,8-14,16-17H2,2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364925

(CHEMBL1950340)Show SMILES CCCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C26H37N3O/c1-3-13-29-24-9-8-20(26(30)27-14-10-19(2)11-15-27)17-22(24)23-18-28(16-12-25(23)29)21-6-4-5-7-21/h8-9,17,19,21H,3-7,10-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496259
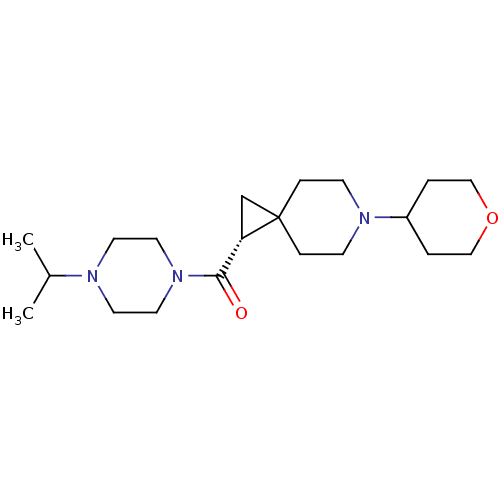
(CHEMBL3127672)Show SMILES CC(C)N1CCN(CC1)C(=O)[C@@H]1CC11CCN(CC1)C1CCOCC1 |r| Show InChI InChI=1S/C20H35N3O2/c1-16(2)21-9-11-23(12-10-21)19(24)18-15-20(18)5-7-22(8-6-20)17-3-13-25-14-4-17/h16-18H,3-15H2,1-2H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364935

(CHEMBL1950350)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C25H35N3O3S/c1-3-32(30,31)28-23-9-8-19(25(29)26-13-10-18(2)11-14-26)16-21(23)22-17-27(15-12-24(22)28)20-6-4-5-7-20/h8-9,16,18,20H,3-7,10-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496295

(CHEMBL3127708)Show SMILES O=C(C1CC11CCN(CC1)C1CCCCC1)N1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C24H41N3O/c28-23(27-17-15-26(16-18-27)21-9-5-2-6-10-21)22-19-24(22)11-13-25(14-12-24)20-7-3-1-4-8-20/h20-22H,1-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50218116
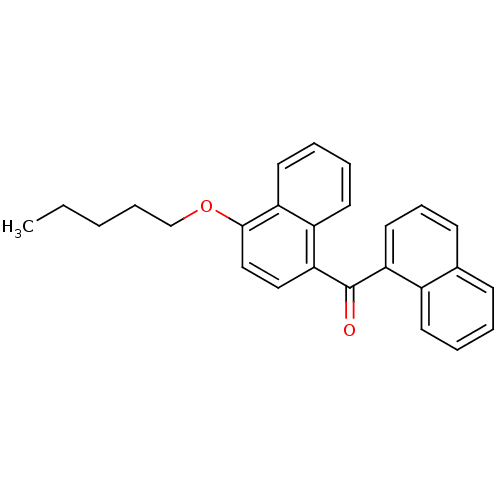
(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496276

(CHEMBL3127670)Show SMILES O=C(C1CC11CCN(CCc2ccccc2)CC1)N1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C26H39N3O/c30-25(29-19-17-28(18-20-29)23-9-5-2-6-10-23)24-21-26(24)12-15-27(16-13-26)14-11-22-7-3-1-4-8-22/h1,3-4,7-8,23-24H,2,5-6,9-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50440441
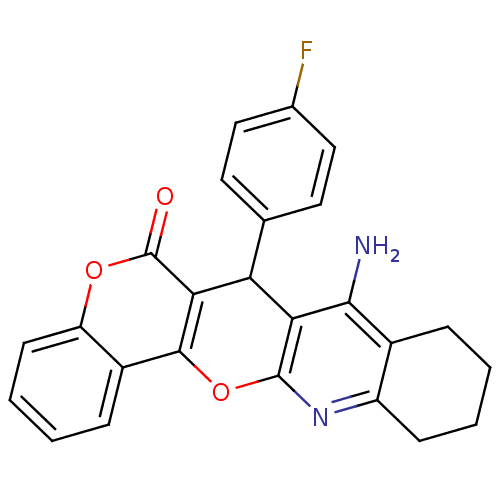
(CHEMBL2425846)Show SMILES Nc1c2CCCCc2nc2Oc3c(C(c4ccc(F)cc4)c12)c(=O)oc1ccccc31 Show InChI InChI=1S/C25H19FN2O3/c26-14-11-9-13(10-12-14)19-20-22(27)15-5-1-3-7-17(15)28-24(20)31-23-16-6-2-4-8-18(16)30-25(29)21(19)23/h2,4,6,8-12,19H,1,3,5,7H2,(H2,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tehran University of Medical Sciences
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis |
Eur J Med Chem 68: 291-300 (2013)
Article DOI: 10.1016/j.ejmech.2013.07.045
BindingDB Entry DOI: 10.7270/Q2794637 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364938
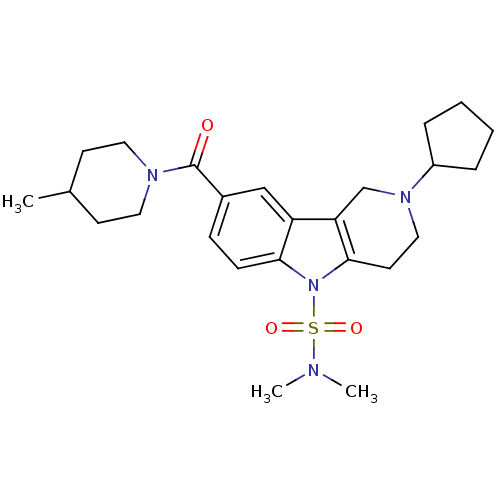
(CHEMBL1950354)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCCC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H36N4O3S/c1-18-10-13-27(14-11-18)25(30)19-8-9-23-21(16-19)22-17-28(20-6-4-5-7-20)15-12-24(22)29(23)33(31,32)26(2)3/h8-9,16,18,20H,4-7,10-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364932

(CHEMBL1950347)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C26H37N3O3S/c1-3-16-33(31,32)29-24-9-8-20(26(30)27-13-10-19(2)11-14-27)17-22(24)23-18-28(15-12-25(23)29)21-6-4-5-7-21/h8-9,17,19,21H,3-7,10-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496280
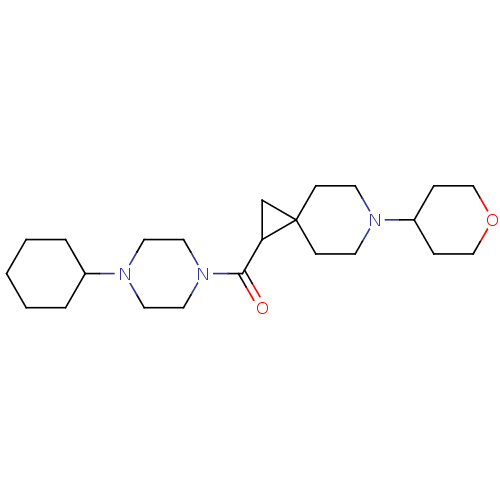
(CHEMBL3127702)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C23H39N3O2/c27-22(26-14-12-25(13-15-26)19-4-2-1-3-5-19)21-18-23(21)8-10-24(11-9-23)20-6-16-28-17-7-20/h19-21H,1-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50003896
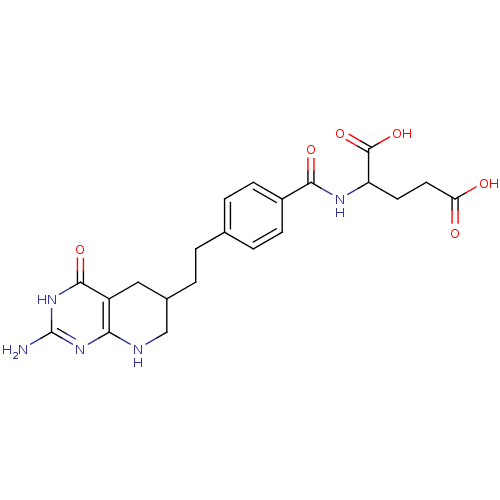
((DDATHF) 5,10-Dideazatetrahydrofolic acid2-{4-[2-(...)Show SMILES Nc1nc2NCC(CCc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)Cc2c(=O)[nH]1 Show InChI InChI=1S/C21H25N5O6/c22-21-25-17-14(19(30)26-21)9-12(10-23-17)2-1-11-3-5-13(6-4-11)18(29)24-15(20(31)32)7-8-16(27)28/h3-6,12,15H,1-2,7-10H2,(H,24,29)(H,27,28)(H,31,32)(H4,22,23,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Princeton University
Curated by ChEMBL
| Assay Description
Compound was evaluated for competitive inhibition of recombinant mouse thymidylate synthase |
J Med Chem 35: 4450-4 (1992)
BindingDB Entry DOI: 10.7270/Q2RR1ZV6 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364937

(CHEMBL1950353)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H35N3O4S/c1-21-9-14-30(15-10-21)29(33)22-7-8-27-25(19-22)26-20-31(23-12-17-36-18-13-23)16-11-28(26)32(27)37(34,35)24-5-3-2-4-6-24/h2-8,19,21,23H,9-18,20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364928
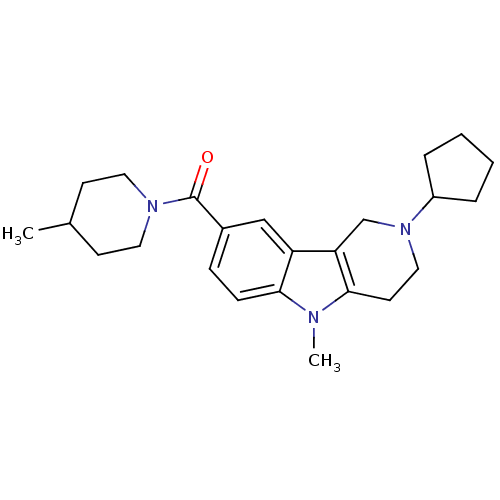
(CHEMBL1950343)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C)c3CCN(Cc3c2c1)C1CCCC1 Show InChI InChI=1S/C24H33N3O/c1-17-9-12-26(13-10-17)24(28)18-7-8-22-20(15-18)21-16-27(19-5-3-4-6-19)14-11-23(21)25(22)2/h7-8,15,17,19H,3-6,9-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364929

(CHEMBL1950344)Show SMILES CCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCCC1 Show InChI InChI=1S/C25H35N3O/c1-3-28-23-9-8-19(25(29)26-13-10-18(2)11-14-26)16-21(23)22-17-27(15-12-24(22)28)20-6-4-5-7-20/h8-9,16,18,20H,3-7,10-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50218116

(CHEMBL244403 | naphthalen-1-yl-(4-pentyloxynaphtha...)Show InChI InChI=1S/C26H24O2/c1-2-3-8-18-28-25-17-16-24(21-13-6-7-14-22(21)25)26(27)23-15-9-11-19-10-4-5-12-20(19)23/h4-7,9-17H,2-3,8,18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364933

(CHEMBL1950348)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCCC1)S(C)(=O)=O Show InChI InChI=1S/C24H33N3O3S/c1-17-9-12-25(13-10-17)24(28)18-7-8-22-20(15-18)21-16-26(19-5-3-4-6-19)14-11-23(21)27(22)31(2,29)30/h7-8,15,17,19H,3-6,9-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496263

(CHEMBL3127709)Show InChI InChI=1S/C21H37N3O/c1-17(2)22-10-8-21(9-11-22)16-19(21)20(25)24-14-12-23(13-15-24)18-6-4-3-5-7-18/h17-19H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364941
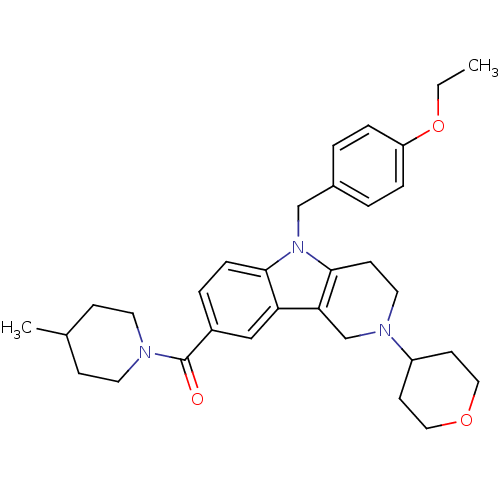
(CHEMBL1950357)Show SMILES CCOc1ccc(Cn2c3CCN(Cc3c3cc(ccc23)C(=O)N2CCC(C)CC2)C2CCOCC2)cc1 Show InChI InChI=1S/C32H41N3O3/c1-3-38-27-7-4-24(5-8-27)21-35-30-9-6-25(32(36)33-15-10-23(2)11-16-33)20-28(30)29-22-34(17-12-31(29)35)26-13-18-37-19-14-26/h4-9,20,23,26H,3,10-19,21-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496275
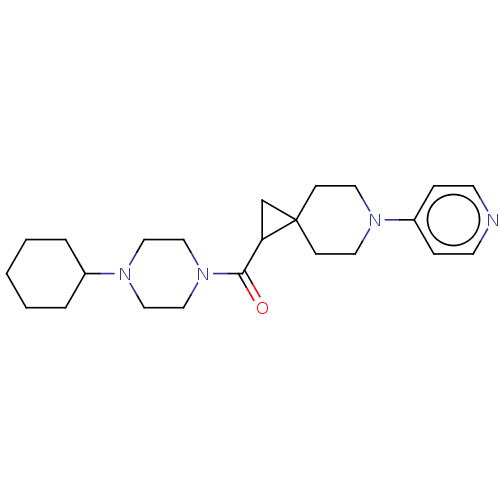
(CHEMBL3127671)Show SMILES O=C(C1CC11CCN(CC1)c1ccncc1)N1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C23H34N4O/c28-22(27-16-14-26(15-17-27)19-4-2-1-3-5-19)21-18-23(21)8-12-25(13-9-23)20-6-10-24-11-7-20/h6-7,10-11,19,21H,1-5,8-9,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364924

(CHEMBL1950335)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(CC=C)c3CCN(Cc3c2c1)C1CCCCC1 Show InChI InChI=1S/C27H37N3O/c1-3-14-30-25-10-9-21(27(31)28-15-11-20(2)12-16-28)18-23(25)24-19-29(17-13-26(24)30)22-7-5-4-6-8-22/h3,9-10,18,20,22H,1,4-8,11-17,19H2,2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364926

(CHEMBL1950341)Show SMILES CCCn1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O2/c1-3-11-29-24-5-4-20(26(30)27-12-6-19(2)7-13-27)17-22(24)23-18-28(14-8-25(23)29)21-9-15-31-16-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM246610
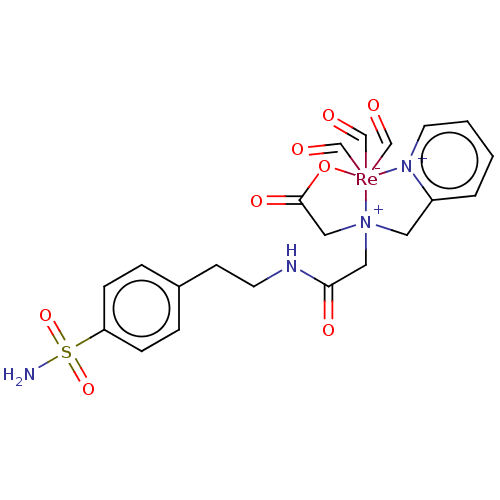
(CA IX inhibitor, (B)2)Show SMILES NS(=O)(=O)c1ccc(CCNC(=O)C[N+]23CC(=O)O[Re-]2(C=O)(C=O)(C=O)[n+]2ccccc2C3)cc1 Show InChI InChI=1S/C18H22N4O5S.3CHO.Re/c19-28(26,27)16-6-4-14(5-7-16)8-10-21-17(23)12-22(13-18(24)25)11-15-3-1-2-9-20-15;3*1-2;/h1-7,9H,8,10-13H2,(H,21,23)(H,24,25)(H2,19,26,27);3*1H;/q;;;;+2/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BC Cancer Agency
| Assay Description
The inhibition constants (Ki) of FEC to four human CA isoenzymes I, II, IX and XII were determined by CA catalyzed CO2 hydration assays following pre... |
J Enzyme Inhib Med Chem 29: 249-55 (2014)
Article DOI: 10.3109/14756366.2013.773994
BindingDB Entry DOI: 10.7270/Q2VM4B6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496279
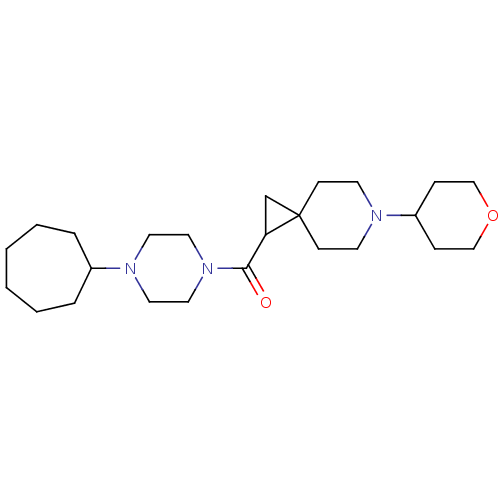
(CHEMBL3127703)Show SMILES O=C(C1CC11CCN(CC1)C1CCOCC1)N1CCN(CC1)C1CCCCCC1 Show InChI InChI=1S/C24H41N3O2/c28-23(27-15-13-26(14-16-27)20-5-3-1-2-4-6-20)22-19-24(22)9-11-25(12-10-24)21-7-17-29-18-8-21/h20-22H,1-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496278
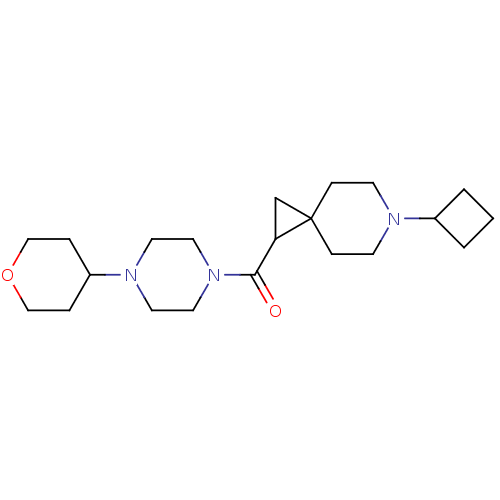
(CHEMBL3127706)Show SMILES O=C(C1CC11CCN(CC1)C1CCC1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C21H35N3O2/c25-20(24-12-10-23(11-13-24)18-4-14-26-15-5-18)19-16-21(19)6-8-22(9-7-21)17-2-1-3-17/h17-19H,1-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364946

(CHEMBL1950489)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(C)c3CCN(Cc3c2c1)C1CCOCC1 Show InChI InChI=1S/C24H33N3O2/c1-17-5-10-26(11-6-17)24(28)18-3-4-22-20(15-18)21-16-27(12-7-23(21)25(22)2)19-8-13-29-14-9-19/h3-4,15,17,19H,5-14,16H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496261
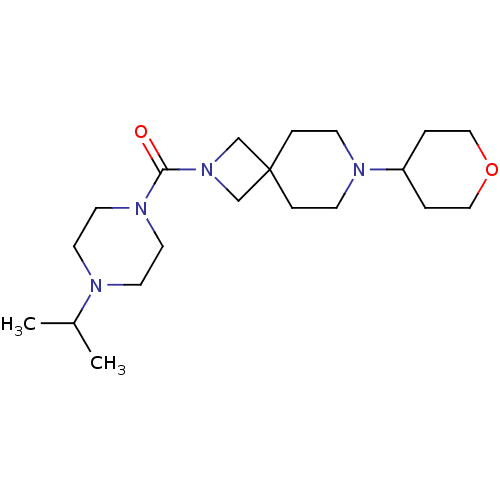
(CHEMBL3127680)Show SMILES CC(C)N1CCN(CC1)C(=O)N1CC2(C1)CCN(CC2)C1CCOCC1 Show InChI InChI=1S/C20H36N4O2/c1-17(2)21-9-11-23(12-10-21)19(25)24-15-20(16-24)5-7-22(8-6-20)18-3-13-26-14-4-18/h17-18H,3-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364930

(CHEMBL1950345)Show SMILES CCCC(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C27H37N3O3/c1-3-4-26(31)30-24-6-5-20(27(32)28-12-7-19(2)8-13-28)17-22(24)23-18-29(14-9-25(23)30)21-10-15-33-16-11-21/h5-6,17,19,21H,3-4,7-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50496287

(CHEMBL3127669)Show SMILES O=C(C1CC11CCN(Cc2ccccc2)CC1)N1CCN(CC1)C1CCCCC1 Show InChI InChI=1S/C25H37N3O/c29-24(28-17-15-27(16-18-28)22-9-5-2-6-10-22)23-19-25(23)11-13-26(14-12-25)20-21-7-3-1-4-8-21/h1,3-4,7-8,22-23H,2,5-6,9-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay |
J Med Chem 57: 733-58 (2014)
Article DOI: 10.1021/jm4014828
BindingDB Entry DOI: 10.7270/Q2FT8Q13 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364939

(CHEMBL1950355)Show SMILES CC1CCN(CC1)C(=O)c1ccc2n(c3CCN(Cc3c2c1)C1CCOCC1)S(=O)(=O)N(C)C Show InChI InChI=1S/C25H36N4O4S/c1-18-6-11-27(12-7-18)25(30)19-4-5-23-21(16-19)22-17-28(20-9-14-33-15-10-20)13-8-24(22)29(23)34(31,32)26(2)3/h4-5,16,18,20H,6-15,17H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364931

(CHEMBL1950346)Show SMILES CCCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C26H37N3O4S/c1-3-16-34(31,32)29-24-5-4-20(26(30)27-11-6-19(2)7-12-27)17-22(24)23-18-28(13-8-25(23)29)21-9-14-33-15-10-21/h4-5,17,19,21H,3,6-16,18H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50364920

(CHEMBL1950351)Show SMILES CCS(=O)(=O)n1c2CCN(Cc2c2cc(ccc12)C(=O)N1CCC(C)CC1)C1CCOCC1 Show InChI InChI=1S/C25H35N3O4S/c1-3-33(30,31)28-23-5-4-19(25(29)26-11-6-18(2)7-12-26)16-21(23)22-17-27(13-8-24(22)28)20-9-14-32-15-10-20/h4-5,16,18,20H,3,6-15,17H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Montreal
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 1619-24 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.124
BindingDB Entry DOI: 10.7270/Q24J0FJX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data