 Found 619 hits with Last Name = 'pryor' and Initial = 'k'
Found 619 hits with Last Name = 'pryor' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Trypsin
(Sus scrofa) | BDBM50080139
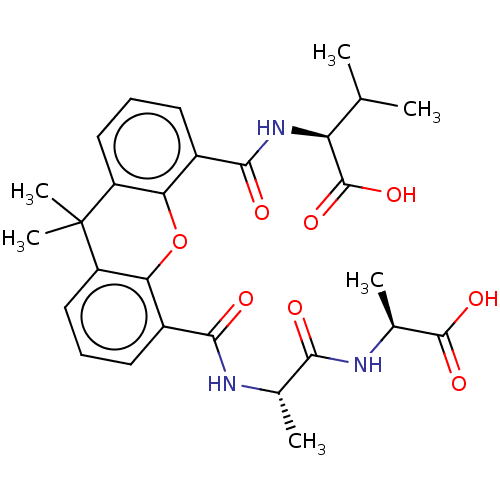
((R)-2-({5-[(S)-1-((R)-1-Carboxy-ethylcarbamoyl)-et...)Show SMILES CC(C)[C@H](NC(=O)c1cccc2c1Oc1c(cccc1C2(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H33N3O8/c1-13(2)20(27(37)38)31-25(34)17-10-8-12-19-22(17)39-21-16(9-7-11-18(21)28(19,5)6)24(33)29-14(3)23(32)30-15(4)26(35)36/h7-15,20H,1-6H3,(H,29,33)(H,30,32)(H,31,34)(H,35,36)(H,37,38)/t14-,15-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant (Ki) of the compound when mixed with p-nitroanilide against trypsin enzyme for the conversion of water-soluble compound to fluore... |
Bioorg Med Chem Lett 9: 2291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2QR4W91 |
More data for this
Ligand-Target Pair | |
Trypsin
(Sus scrofa) | BDBM50080139

((R)-2-({5-[(S)-1-((R)-1-Carboxy-ethylcarbamoyl)-et...)Show SMILES CC(C)[C@H](NC(=O)c1cccc2c1Oc1c(cccc1C2(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C28H33N3O8/c1-13(2)20(27(37)38)31-25(34)17-10-8-12-19-22(17)39-21-16(9-7-11-18(21)28(19,5)6)24(33)29-14(3)23(32)30-15(4)26(35)36/h7-15,20H,1-6H3,(H,29,33)(H,30,32)(H,31,34)(H,35,36)(H,37,38)/t14-,15-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant (Ki) of the compound when mixed with p-nitroanilide against trypsin enzyme for the conversion of water-soluble compound to fluore... |
Bioorg Med Chem Lett 9: 2291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2QR4W91 |
More data for this
Ligand-Target Pair | |
Trypsin
(Sus scrofa) | BDBM50080138
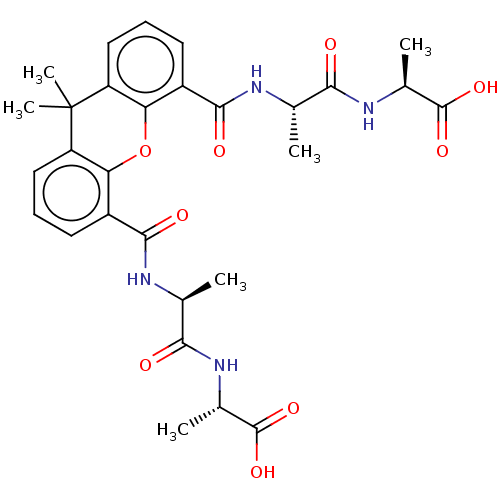
((S)-2-[(R)-2-({5-[(S)-1-((R)-1-Carboxy-ethylcarbam...)Show SMILES C[C@H](NC(=O)[C@H](C)NC(=O)c1cccc2c1Oc1c(cccc1C2(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C29H34N4O9/c1-13(23(34)32-15(3)27(38)39)30-25(36)17-9-7-11-19-21(17)42-22-18(10-8-12-20(22)29(19,5)6)26(37)31-14(2)24(35)33-16(4)28(40)41/h7-16H,1-6H3,(H,30,36)(H,31,37)(H,32,34)(H,33,35)(H,38,39)(H,40,41)/t13-,14-,15-,16-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant (Ki) against trypsin enzyme. |
Bioorg Med Chem Lett 9: 2291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2QR4W91 |
More data for this
Ligand-Target Pair | |
Trypsin
(Sus scrofa) | BDBM50080140
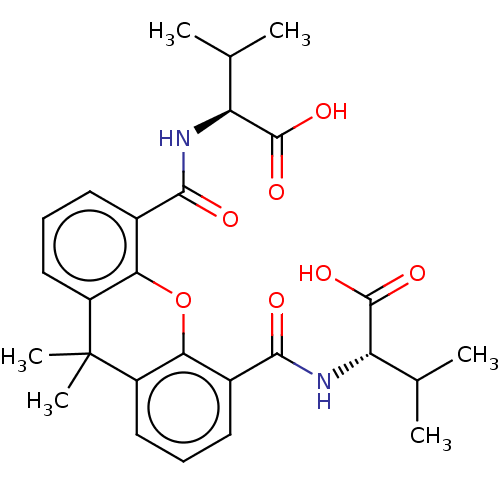
((R)-2-{[5-((S)-1-Carboxy-2-methyl-propylcarbamoyl)...)Show SMILES CC(C)[C@H](NC(=O)c1cccc2c1Oc1c(cccc1C2(C)C)C(=O)N[C@@H](C(C)C)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C27H32N2O7/c1-13(2)19(25(32)33)28-23(30)15-9-7-11-17-21(15)36-22-16(10-8-12-18(22)27(17,5)6)24(31)29-20(14(3)4)26(34)35/h7-14,19-20H,1-6H3,(H,28,30)(H,29,31)(H,32,33)(H,34,35)/t19-,20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition constant (Ki) against trypsin enzyme. |
Bioorg Med Chem Lett 9: 2291-6 (1999)
BindingDB Entry DOI: 10.7270/Q2QR4W91 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50371255

(CHEMBL1203953)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)[C@H]1Cc1ccc(F)cc1)Cc1cc(F)c(F)cc1F |r| Show InChI InChI=1S/C23H20F7N5O/c24-14-3-1-12(2-4-14)7-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-15(31)8-13-9-17(26)18(27)11-16(13)25/h1-4,9,11,15,19H,5-8,10,31H2/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232520

((2R)-4-oxo-4-[3-(trifluoromethyl)-8-[2-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1C(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C24H20F9N5O/c25-16-11-18(27)17(26)8-13(16)7-14(34)10-20(39)37-5-6-38-21(35-36-22(38)24(31,32)33)19(37)9-12-3-1-2-4-15(12)23(28,29)30/h1-4,8,11,14,19H,5-7,9-10,34H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232520

((2R)-4-oxo-4-[3-(trifluoromethyl)-8-[2-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1C(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C24H20F9N5O/c25-16-11-18(27)17(26)8-13(16)7-14(34)10-20(39)37-5-6-38-21(35-36-22(38)24(31,32)33)19(37)9-12-3-1-2-4-15(12)23(28,29)30/h1-4,8,11,14,19H,5-7,9-10,34H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232506
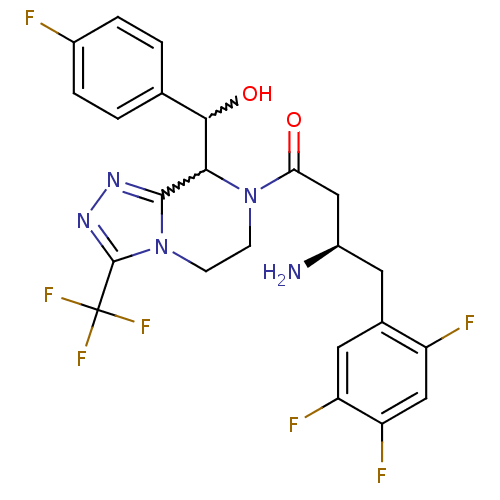
(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1C(O)c1ccc(F)cc1)Cc1cc(F)c(F)cc1F |w:17.17,18.20| Show InChI InChI=1S/C23H20F7N5O2/c24-13-3-1-11(2-4-13)20(37)19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)18(36)9-14(31)7-12-8-16(26)17(27)10-15(12)25/h1-4,8,10,14,19-20,37H,5-7,9,31H2/t14-,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232518
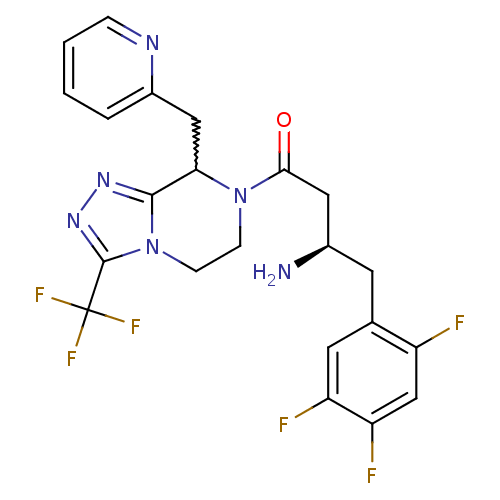
((2R)-4-oxo-4-[8-(pyridin-2-ylmethyl)-3-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccn1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C22H20F6N6O/c23-15-11-17(25)16(24)8-12(15)7-13(29)9-19(35)33-5-6-34-20(31-32-21(34)22(26,27)28)18(33)10-14-3-1-2-4-30-14/h1-4,8,11,13,18H,5-7,9-10,29H2/t13-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232518

((2R)-4-oxo-4-[8-(pyridin-2-ylmethyl)-3-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccn1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C22H20F6N6O/c23-15-11-17(25)16(24)8-12(15)7-13(29)9-19(35)33-5-6-34-20(31-32-21(34)22(26,27)28)18(33)10-14-3-1-2-4-30-14/h1-4,8,11,13,18H,5-7,9-10,29H2/t13-,18?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232503
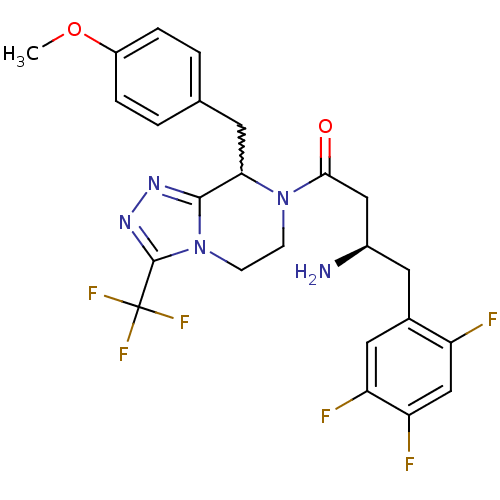
((2R)-4-[8-(4-methoxybenzyl)-3-(trifluoromethyl)-5,...)Show SMILES COc1ccc(CC2N(CCn3c2nnc3C(F)(F)F)C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1 |w:7.6| Show InChI InChI=1S/C24H23F6N5O2/c1-37-16-4-2-13(3-5-16)8-20-22-32-33-23(24(28,29)30)35(22)7-6-34(20)21(36)11-15(31)9-14-10-18(26)19(27)12-17(14)25/h2-5,10,12,15,20H,6-9,11,31H2,1H3/t15-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232503

((2R)-4-[8-(4-methoxybenzyl)-3-(trifluoromethyl)-5,...)Show SMILES COc1ccc(CC2N(CCn3c2nnc3C(F)(F)F)C(=O)C[C@H](N)Cc2cc(F)c(F)cc2F)cc1 |w:7.6| Show InChI InChI=1S/C24H23F6N5O2/c1-37-16-4-2-13(3-5-16)8-20-22-32-33-23(24(28,29)30)35(22)7-6-34(20)21(36)11-15(31)9-14-10-18(26)19(27)12-17(14)25/h2-5,10,12,15,20H,6-9,11,31H2,1H3/t15-,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232501

((2R)-4-[8-(2-fluorobenzyl)-3-(trifluoromethyl)-5,6...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H20F7N5O/c24-15-4-2-1-3-12(15)9-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-14(31)7-13-8-17(26)18(27)11-16(13)25/h1-4,8,11,14,19H,5-7,9-10,31H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232501

((2R)-4-[8-(2-fluorobenzyl)-3-(trifluoromethyl)-5,6...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H20F7N5O/c24-15-4-2-1-3-12(15)9-19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)20(36)10-14(31)7-13-8-17(26)18(27)11-16(13)25/h1-4,8,11,14,19H,5-7,9-10,31H2/t14-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232506

(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1C(O)c1ccc(F)cc1)Cc1cc(F)c(F)cc1F |w:17.17,18.20| Show InChI InChI=1S/C23H20F7N5O2/c24-13-3-1-11(2-4-13)20(37)19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)18(36)9-14(31)7-12-8-16(26)17(27)10-15(12)25/h1-4,8,10,14,19-20,37H,5-7,9,31H2/t14-,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232506

(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1C(O)c1ccc(F)cc1)Cc1cc(F)c(F)cc1F |w:17.17,18.20| Show InChI InChI=1S/C23H20F7N5O2/c24-13-3-1-11(2-4-13)20(37)19-21-32-33-22(23(28,29)30)35(21)6-5-34(19)18(36)9-14(31)7-12-8-16(26)17(27)10-15(12)25/h1-4,8,10,14,19-20,37H,5-7,9,31H2/t14-,19?,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232510

((2R)-4-[8-benzyl-3-(trifluoromethyl)-5,6-dihydro[1...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H21F6N5O/c24-16-12-18(26)17(25)10-14(16)9-15(30)11-20(35)33-6-7-34-21(31-32-22(34)23(27,28)29)19(33)8-13-4-2-1-3-5-13/h1-5,10,12,15,19H,6-9,11,30H2/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232510

((2R)-4-[8-benzyl-3-(trifluoromethyl)-5,6-dihydro[1...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1ccccc1)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C23H21F6N5O/c24-16-12-18(26)17(25)10-14(16)9-15(30)11-20(35)33-6-7-34-21(31-32-22(34)23(27,28)29)19(33)8-13-4-2-1-3-5-13/h1-5,10,12,15,19H,6-9,11,30H2/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523
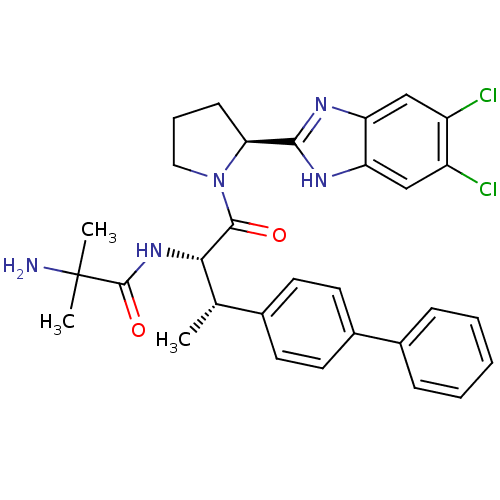
(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328527
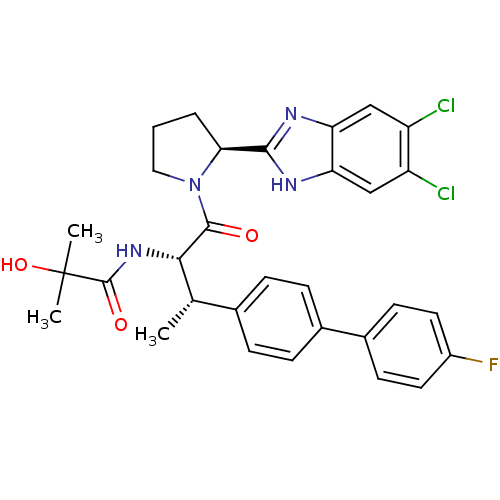
(CHEMBL1259208 | N-[(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H31Cl2FN4O3/c1-17(18-6-8-19(9-7-18)20-10-12-21(34)13-11-20)27(37-30(40)31(2,3)41)29(39)38-14-4-5-26(38)28-35-24-15-22(32)23(33)16-25(24)36-28/h6-13,15-17,26-27,41H,4-5,14H2,1-3H3,(H,35,36)(H,37,40)/t17-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328527

(CHEMBL1259208 | N-[(2S,3S)-1-[(2S)-2-(5,6-Dichloro...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)O)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C31H31Cl2FN4O3/c1-17(18-6-8-19(9-7-18)20-10-12-21(34)13-11-20)27(37-30(40)31(2,3)41)29(39)38-14-4-5-26(38)28-35-24-15-22(32)23(33)16-25(24)36-28/h6-13,15-17,26-27,41H,4-5,14H2,1-3H3,(H,35,36)(H,37,40)/t17-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50328523

(2-Amino-N-{(2S,3S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-18(19-11-13-21(14-12-19)20-8-5-4-6-9-20)27(37-30(40)31(2,3)34)29(39)38-15-7-10-26(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h4-6,8-9,11-14,16-18,26-27H,7,10,15,34H2,1-3H3,(H,35,36)(H,37,40)/t18-,26-,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221449

((3R)-1-(8-((1H-imidazol-1-yl)methyl)-2-(trifluorom...)Show SMILES N[C@@H](CC(=O)N1CCn2nc(nc2C1Cn1ccnc1)C(F)(F)F)Cc1cc(F)c(F)cc1F |w:13.15| Show InChI InChI=1S/C20H19F6N7O/c21-13-8-15(23)14(22)6-11(13)5-12(27)7-17(34)32-3-4-33-18(29-19(30-33)20(24,25)26)16(32)9-31-2-1-28-10-31/h1-2,6,8,10,12,16H,3-5,7,9,27H2/t12-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 5934-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.100
BindingDB Entry DOI: 10.7270/Q20C4VH0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221444
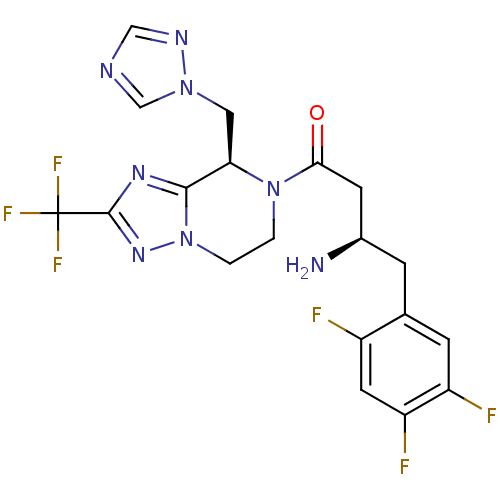
((R)-1-((R)-8-((1H-1,2,4-triazol-1-yl)methyl)-2-(tr...)Show SMILES N[C@@H](CC(=O)N1CCn2nc(nc2[C@H]1Cn1cncn1)C(F)(F)F)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C19H18F6N8O/c20-12-6-14(22)13(21)4-10(12)3-11(26)5-16(34)32-1-2-33-17(29-18(30-33)19(23,24)25)15(32)7-31-9-27-8-28-31/h4,6,8-9,11,15H,1-3,5,7,26H2/t11-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 5934-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.100
BindingDB Entry DOI: 10.7270/Q20C4VH0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232507
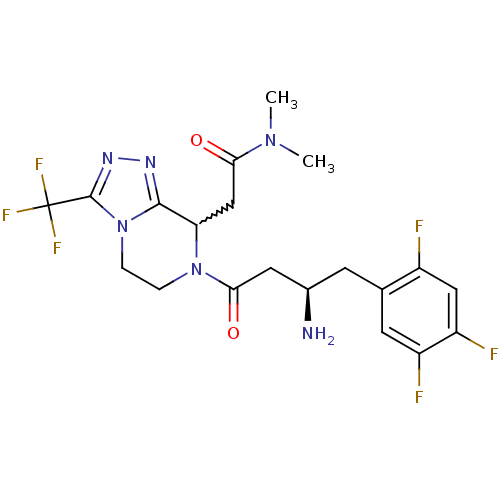
(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CN(C)C(=O)CC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:6.5| Show InChI InChI=1S/C20H22F6N6O2/c1-30(2)16(33)9-15-18-28-29-19(20(24,25)26)32(18)4-3-31(15)17(34)7-11(27)5-10-6-13(22)14(23)8-12(10)21/h6,8,11,15H,3-5,7,9,27H2,1-2H3/t11-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232507

(2-[7-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butano...)Show SMILES CN(C)C(=O)CC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:6.5| Show InChI InChI=1S/C20H22F6N6O2/c1-30(2)16(33)9-15-18-28-29-19(20(24,25)26)32(18)4-3-31(15)17(34)7-11(27)5-10-6-13(22)14(23)8-12(10)21/h6,8,11,15H,3-5,7,9,27H2,1-2H3/t11-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328537

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(CN)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,18-34)30(40)37-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)29(39)38-14-6-9-27(38)28-35-24-16-22(32)23(33)17-25(24)36-28/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,36)(H,37,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50377234
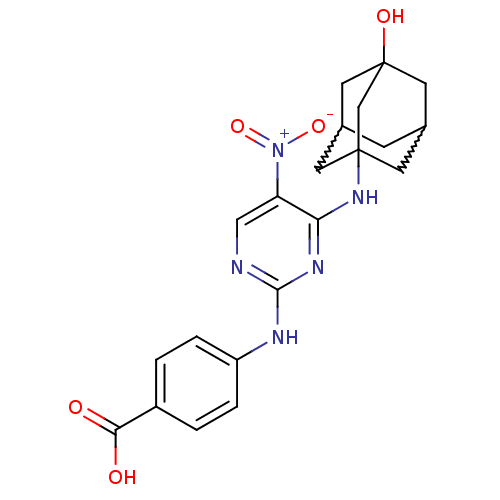
(CHEMBL402902)Show SMILES OC(=O)c1ccc(Nc2ncc(c(NC34CC5CC(CC(O)(C5)C3)C4)n2)[N+]([O-])=O)cc1 |w:18.25,16.15,TLB:13:14:17:22.19.20,21:20:17.18.24:15,THB:19:18:15:22.20.23,19:20:17.18.24:15,23:20:17:24.14.15,23:14:17:22.19.20,21:20:17:24.14.15,TEB:24:18:22:14.15.23| Show InChI InChI=1S/C21H23N5O5/c27-18(28)14-1-3-15(4-2-14)23-19-22-10-16(26(30)31)17(24-19)25-20-6-12-5-13(7-20)9-21(29,8-12)11-20/h1-4,10,12-13,29H,5-9,11H2,(H,27,28)(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kemia, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3-beta |
Bioorg Med Chem Lett 18: 3578-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.001
BindingDB Entry DOI: 10.7270/Q2571CXM |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221442

((3R)-1-(8-((1H-pyrazol-1-yl)methyl)-2-(trifluorome...)Show SMILES N[C@@H](CC(=O)N1CCn2nc(nc2C1Cn1cccn1)C(F)(F)F)Cc1cc(F)c(F)cc1F |w:13.15| Show InChI InChI=1S/C20H19F6N7O/c21-13-9-15(23)14(22)7-11(13)6-12(27)8-17(34)32-4-5-33-18(29-19(30-33)20(24,25)26)16(32)10-31-3-1-2-28-31/h1-3,7,9,12,16H,4-6,8,10,27H2/t12-,16?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 5934-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.100
BindingDB Entry DOI: 10.7270/Q20C4VH0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221452

((R)-1-((R)-8-(4-fluorobenzyl)-2-(trifluoromethyl)-...)Show SMILES N[C@@H](CC(=O)N1CCn2nc(nc2[C@H]1Cc1ccc(F)cc1)C(F)(F)F)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C23H20F7N5O/c24-14-3-1-12(2-4-14)7-19-21-32-22(23(28,29)30)33-35(21)6-5-34(19)20(36)10-15(31)8-13-9-17(26)18(27)11-16(13)25/h1-4,9,11,15,19H,5-8,10,31H2/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 5934-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.100
BindingDB Entry DOI: 10.7270/Q20C4VH0 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221454

((R)-1-((R)-8-(4-(trifluoromethyl)benzyl)-2-(triflu...)Show SMILES N[C@@H](CC(=O)N1CCn2nc(nc2[C@H]1Cc1ccc(cc1)C(F)(F)F)C(F)(F)F)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C24H20F9N5O/c25-16-11-18(27)17(26)9-13(16)8-15(34)10-20(39)37-5-6-38-21(35-22(36-38)24(31,32)33)19(37)7-12-1-3-14(4-2-12)23(28,29)30/h1-4,9,11,15,19H,5-8,10,34H2/t15-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 5934-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.100
BindingDB Entry DOI: 10.7270/Q20C4VH0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328522

(CHEMBL1259179 | N-{(2S)-3-(Biphenyl-4-yl)-1-[(2S)-...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)O |r| Show InChI InChI=1S/C30H38N4O3/c1-29(2,3)25-19-31-26(33-25)24-12-9-17-34(24)27(35)23(32-28(36)30(4,5)37)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24,37H,9,12,17-18H2,1-5H3,(H,31,33)(H,32,36)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328534
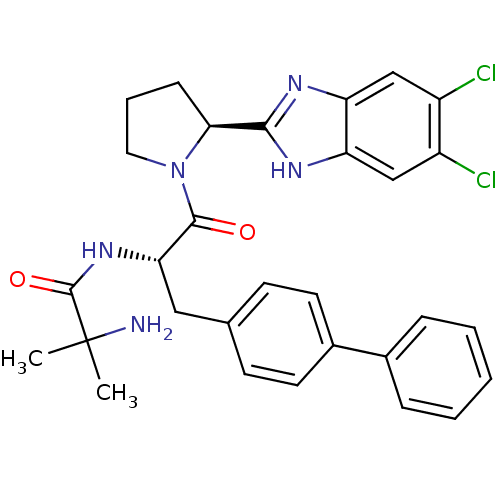
(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C30H31Cl2N5O2/c1-30(2,33)29(39)36-25(15-18-10-12-20(13-11-18)19-7-4-3-5-8-19)28(38)37-14-6-9-26(37)27-34-23-16-21(31)22(32)17-24(23)35-27/h3-5,7-8,10-13,16-17,25-26H,6,9,14-15,33H2,1-2H3,(H,34,35)(H,36,39)/t25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232502
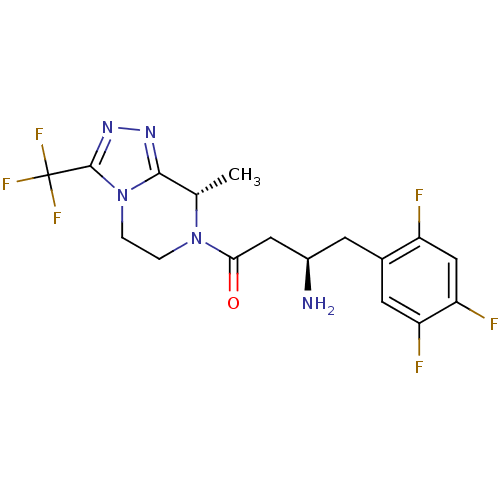
((2R)-4-[(8S)-8-methyl-3-(trifluoromethyl)-5,6-dihy...)Show SMILES C[C@@H]1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F Show InChI InChI=1S/C17H17F6N5O/c1-8-15-25-26-16(17(21,22)23)28(15)3-2-27(8)14(29)6-10(24)4-9-5-12(19)13(20)7-11(9)18/h5,7-8,10H,2-4,6,24H2,1H3/t8-,10+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50377234

(CHEMBL402902)Show SMILES OC(=O)c1ccc(Nc2ncc(c(NC34CC5CC(CC(O)(C5)C3)C4)n2)[N+]([O-])=O)cc1 |w:18.25,16.15,TLB:13:14:17:22.19.20,21:20:17.18.24:15,THB:19:18:15:22.20.23,19:20:17.18.24:15,23:20:17:24.14.15,23:14:17:22.19.20,21:20:17:24.14.15,TEB:24:18:22:14.15.23| Show InChI InChI=1S/C21H23N5O5/c27-18(28)14-1-3-15(4-2-14)23-19-22-10-16(26(30)31)17(24-19)25-20-6-12-5-13(7-20)9-21(29,8-12)11-20/h1-4,10,12-13,29H,5-9,11H2,(H,27,28)(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kemia, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GSK3alpha |
Bioorg Med Chem Lett 18: 3578-81 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.001
BindingDB Entry DOI: 10.7270/Q2571CXM |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328538

(3-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5,6-d...)Show SMILES CC(C)(N)CC(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C31H33Cl2N5O2/c1-31(2,34)18-28(39)35-26(15-19-10-12-21(13-11-19)20-7-4-3-5-8-20)30(40)38-14-6-9-27(38)29-36-24-16-22(32)23(33)17-25(24)37-29/h3-5,7-8,10-13,16-17,26-27H,6,9,14-15,18,34H2,1-2H3,(H,35,39)(H,36,37)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328528
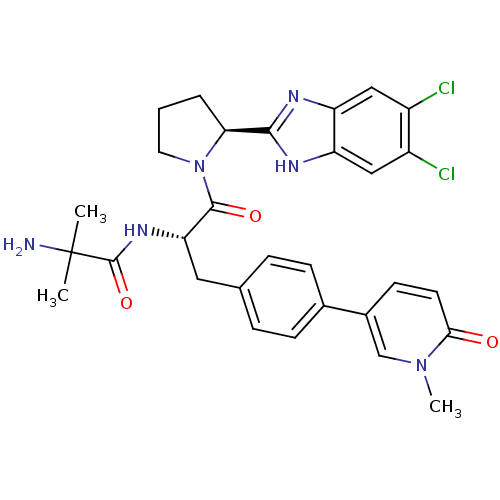
(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-4-(1-methyl...)Show SMILES Cn1cc(ccc1=O)-c1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C30H32Cl2N6O3/c1-30(2,33)29(41)36-24(13-17-6-8-18(9-7-17)19-10-11-26(39)37(3)16-19)28(40)38-12-4-5-25(38)27-34-22-14-20(31)21(32)15-23(22)35-27/h6-11,14-16,24-25H,4-5,12-13,33H2,1-3H3,(H,34,35)(H,36,41)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET in presence of 1% mouse serum albumin |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50221453

((3R)-1-(8-((1H-1,2,4-triazol-1-yl)methyl)-2-(trifl...)Show SMILES N[C@@H](CC(=O)N1CCn2nc(nc2C1Cn1cncn1)C(F)(F)F)Cc1cc(F)c(F)cc1F |w:13.15| Show InChI InChI=1S/C19H18F6N8O/c20-12-6-14(22)13(21)4-10(12)3-11(26)5-16(34)32-1-2-33-17(29-18(30-33)19(23,24)25)15(32)7-31-9-27-8-28-31/h4,6,8-9,11,15H,1-3,5,7,26H2/t11-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 17: 5934-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.100
BindingDB Entry DOI: 10.7270/Q20C4VH0 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328524

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-(betaS)-bet...)Show SMILES C[C@H]([C@H](NC(=O)C(C)(C)N)C(=O)N1CCC[C@H]1c1nc2cc(Cl)c(Cl)cc2[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C25H29Cl2N5O2/c1-14(15-8-5-4-6-9-15)21(31-24(34)25(2,3)28)23(33)32-11-7-10-20(32)22-29-18-12-16(26)17(27)13-19(18)30-22/h4-6,8-9,12-14,20-21H,7,10-11,28H2,1-3H3,(H,29,30)(H,31,34)/t14-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232511

((2R)-4-[8-ethyl-3-(trifluoromethyl)-5,6-dihydro[1,...)Show SMILES CCC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:2.1| Show InChI InChI=1S/C18H19F6N5O/c1-2-14-16-26-27-17(18(22,23)24)29(16)4-3-28(14)15(30)7-10(25)5-9-6-12(20)13(21)8-11(9)19/h6,8,10,14H,2-5,7,25H2,1H3/t10-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232511

((2R)-4-[8-ethyl-3-(trifluoromethyl)-5,6-dihydro[1,...)Show SMILES CCC1N(CCn2c1nnc2C(F)(F)F)C(=O)C[C@H](N)Cc1cc(F)c(F)cc1F |w:2.1| Show InChI InChI=1S/C18H19F6N5O/c1-2-14-16-26-27-17(18(22,23)24)29(16)4-3-28(14)15(30)7-10(25)5-9-6-12(20)13(21)8-11(9)19/h6,8,10,14H,2-5,7,25H2,1H3/t10-,14?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232508
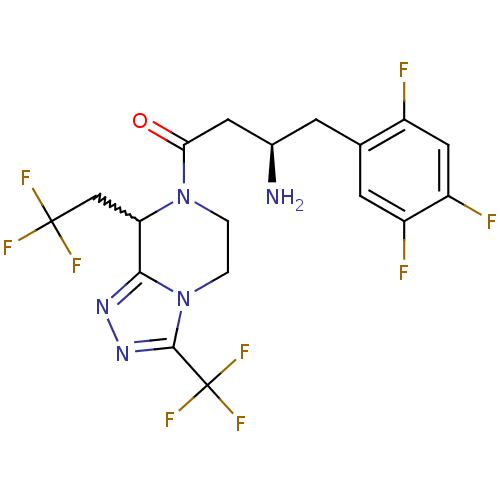
((2R)-4-[8-(2,2,2-trifluoroethyl)-3-(trifluoromethy...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1CC(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C18H16F9N5O/c19-10-6-12(21)11(20)4-8(10)3-9(28)5-14(33)31-1-2-32-15(13(31)7-17(22,23)24)29-30-16(32)18(25,26)27/h4,6,9,13H,1-3,5,7,28H2/t9-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232508

((2R)-4-[8-(2,2,2-trifluoroethyl)-3-(trifluoromethy...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1CC(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C18H16F9N5O/c19-10-6-12(21)11(20)4-8(10)3-9(28)5-14(33)31-1-2-32-15(13(31)7-17(22,23)24)29-30-16(32)18(25,26)27/h4,6,9,13H,1-3,5,7,28H2/t9-,13?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232519

((2R)-4-[8-[3,5-bis(trifluoromethyl)benzyl]-3-(trif...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C25H19F12N5O/c26-16-10-18(28)17(27)7-12(16)6-15(38)9-20(43)41-1-2-42-21(39-40-22(42)25(35,36)37)19(41)5-11-3-13(23(29,30)31)8-14(4-11)24(32,33)34/h3-4,7-8,10,15,19H,1-2,5-6,9,38H2/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50232519

((2R)-4-[8-[3,5-bis(trifluoromethyl)benzyl]-3-(trif...)Show SMILES N[C@@H](CC(=O)N1CCn2c(nnc2C(F)(F)F)C1Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)Cc1cc(F)c(F)cc1F |w:17.19| Show InChI InChI=1S/C25H19F12N5O/c26-16-10-18(28)17(27)7-12(16)6-15(38)9-20(43)41-1-2-42-21(39-40-22(42)25(35,36)37)19(41)5-11-3-13(23(29,30)31)8-14(4-11)24(32,33)34/h3-4,7-8,10,15,19H,1-2,5-6,9,38H2/t15-,19?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
J Med Chem 51: 589-602 (2008)
Article DOI: 10.1021/jm070330v
BindingDB Entry DOI: 10.7270/Q2B27W4M |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328528

(5,6-Dichloro-2-{(2S)-1-[2-methylalanyl-4-(1-methyl...)Show SMILES Cn1cc(ccc1=O)-c1ccc(C[C@H](NC(=O)C(C)(C)N)C(=O)N2CCC[C@H]2c2nc3cc(Cl)c(Cl)cc3[nH]2)cc1 |r| Show InChI InChI=1S/C30H32Cl2N6O3/c1-30(2,33)29(41)36-24(13-17-6-8-18(9-7-17)19-10-11-26(39)37(3)16-19)28(40)38-12-4-5-25(38)27-34-22-14-20(31)21(32)15-23(22)35-27/h6-11,14-16,24-25H,4-5,12-13,33H2,1-3H3,(H,34,35)(H,36,41)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50328539

(2-Amino-N-{(2S)-3-(biphenyl-4-yl)-1-[(2S)-2-(5-ter...)Show SMILES CC(C)(C)c1cnc([nH]1)[C@@H]1CCCN1C(=O)[C@H](Cc1ccc(cc1)-c1ccccc1)NC(=O)C(C)(C)N |r| Show InChI InChI=1S/C30H39N5O2/c1-29(2,3)25-19-32-26(34-25)24-12-9-17-35(24)27(36)23(33-28(37)30(4,5)31)18-20-13-15-22(16-14-20)21-10-7-6-8-11-21/h6-8,10-11,13-16,19,23-24H,9,12,17-18,31H2,1-5H3,(H,32,34)(H,33,37)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP by FRET |
J Med Chem 53: 7251-63 (2010)
Article DOI: 10.1021/jm101013m
BindingDB Entry DOI: 10.7270/Q2QV3MQR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data