 Found 143 hits with Last Name = 'quibell' and Initial = 'm'
Found 143 hits with Last Name = 'quibell' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM100291
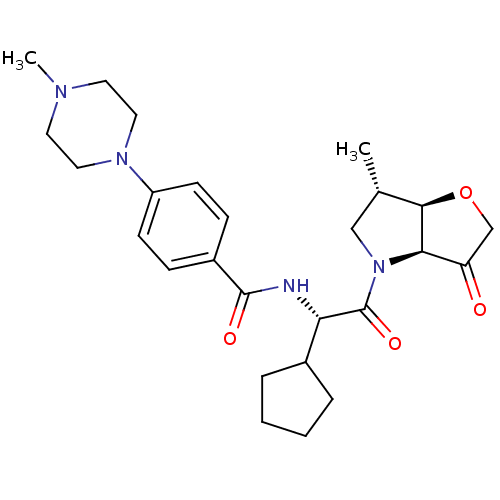
(US8501744, 7)Show SMILES C[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C26H36N4O4/c1-17-15-30(23-21(31)16-34-24(17)23)26(33)22(18-5-3-4-6-18)27-25(32)19-7-9-20(10-8-19)29-13-11-28(2)12-14-29/h7-10,17-18,22-24H,3-6,11-16H2,1-2H3,(H,27,32)/t17-,22-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103367
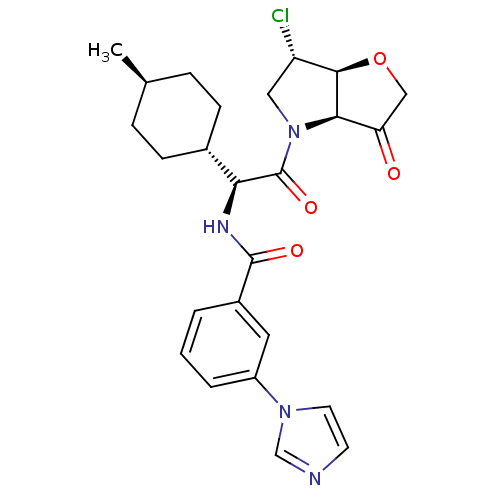
(US8552202, Example 4)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1ccnc1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,wD:26.29,1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C25H29ClN4O4/c1-15-5-7-16(8-6-15)21(25(33)30-12-19(26)23-22(30)20(31)13-34-23)28-24(32)17-3-2-4-18(11-17)29-10-9-27-14-29/h2-4,9-11,14-16,19,21-23H,5-8,12-13H2,1H3,(H,28,32)/t15-,16-,19-,21-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100304
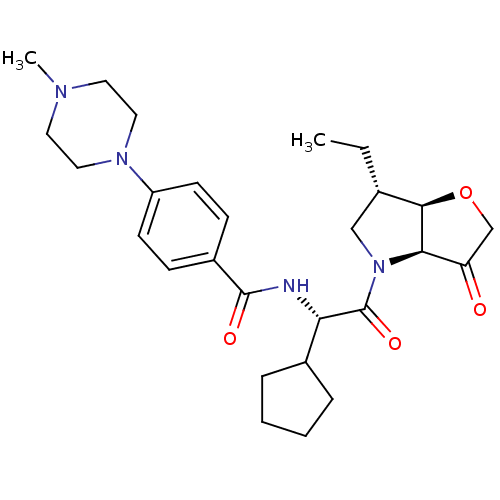
(US8501744, 29)Show SMILES CC[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C27H38N4O4/c1-3-18-16-31(24-22(32)17-35-25(18)24)27(34)23(19-6-4-5-7-19)28-26(33)20-8-10-21(11-9-20)30-14-12-29(2)13-15-30/h8-11,18-19,23-25H,3-7,12-17H2,1-2H3,(H,28,33)/t18-,23-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100305
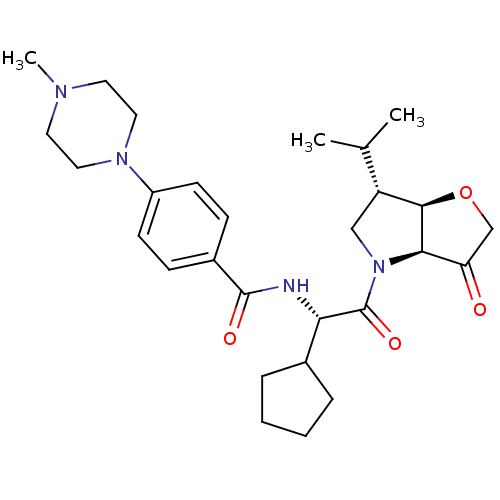
(US8501744, 30)Show SMILES CC(C)[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C28H40N4O4/c1-18(2)22-16-32(25-23(33)17-36-26(22)25)28(35)24(19-6-4-5-7-19)29-27(34)20-8-10-21(11-9-20)31-14-12-30(3)13-15-31/h8-11,18-19,22,24-26H,4-7,12-17H2,1-3H3,(H,29,34)/t22-,24+,25-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100296
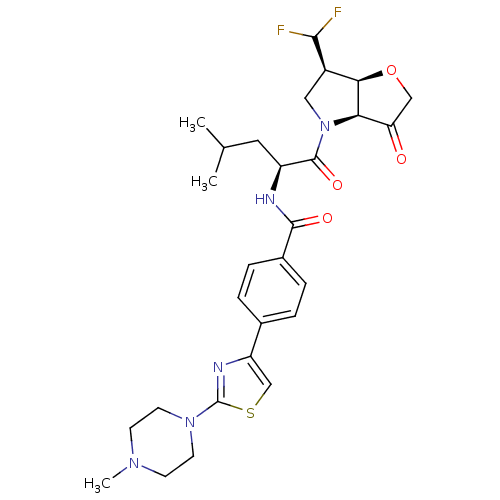
(US8501744, 17)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@@H](C(F)F)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H35F2N5O4S/c1-16(2)12-20(27(38)35-13-19(25(29)30)24-23(35)22(36)14-39-24)31-26(37)18-6-4-17(5-7-18)21-15-40-28(32-21)34-10-8-33(3)9-11-34/h4-7,15-16,19-20,23-25H,8-14H2,1-3H3,(H,31,37)/t19-,20+,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100295

(US8501744, 22)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H37N5O4S/c1-17(2)13-21(27(36)33-14-18(3)25-24(33)23(34)15-37-25)29-26(35)20-7-5-19(6-8-20)22-16-38-28(30-22)32-11-9-31(4)10-12-32/h5-8,16-18,21,24-25H,9-15H2,1-4H3,(H,29,35)/t18-,21-,24+,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100294

(US8501744, 16)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H37N5O4S/c1-17(2)13-21(27(36)33-14-18(3)25-24(33)23(34)15-37-25)29-26(35)20-7-5-19(6-8-20)22-16-38-28(30-22)32-11-9-31(4)10-12-32/h5-8,16-18,21,24-25H,9-15H2,1-4H3,(H,29,35)/t18-,21+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100297
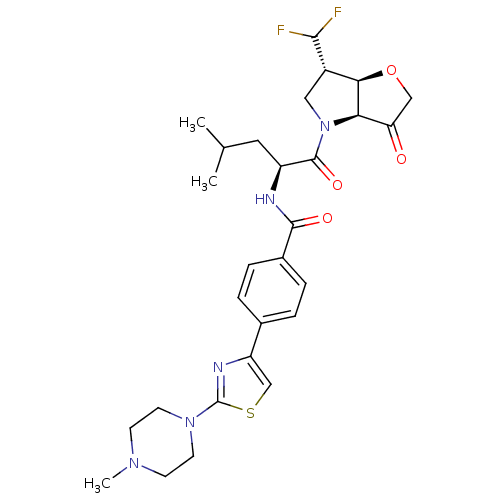
(US8501744, 18)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)-c1csc(n1)N1CCN(C)CC1)C(=O)N1C[C@H](C(F)F)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C28H35F2N5O4S/c1-16(2)12-20(27(38)35-13-19(25(29)30)24-23(35)22(36)14-39-24)31-26(37)18-6-4-17(5-7-18)21-15-40-28(32-21)34-10-8-33(3)9-11-34/h4-7,15-16,19-20,23-25H,8-14H2,1-3H3,(H,31,37)/t19-,20-,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100289
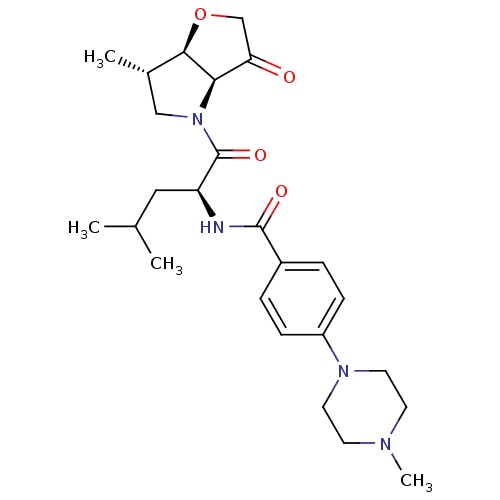
(US8501744, 5)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(=O)N1C[C@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C25H36N4O4/c1-16(2)13-20(25(32)29-14-17(3)23-22(29)21(30)15-33-23)26-24(31)18-5-7-19(8-6-18)28-11-9-27(4)10-12-28/h5-8,16-17,20,22-23H,9-15H2,1-4H3,(H,26,31)/t17-,20-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100301

(US8501744, 26)Show SMILES CC(C)[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H40N4O4/c1-17(2)20-15-31(22-21(32)16-35-23(20)22)26(34)24(27(3,4)5)28-25(33)18-7-9-19(10-8-18)30-13-11-29(6)12-14-30/h7-10,17,20,22-24H,11-16H2,1-6H3,(H,28,33)/t20-,22-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100293
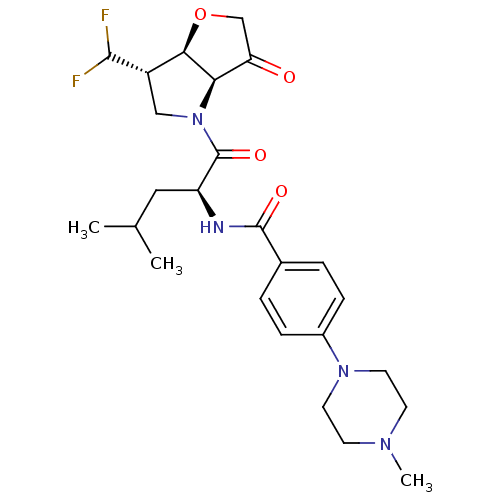
(US8501744, 12)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(=O)N1C[C@H](C(F)F)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C25H34F2N4O4/c1-15(2)12-19(25(34)31-13-18(23(26)27)22-21(31)20(32)14-35-22)28-24(33)16-4-6-17(7-5-16)30-10-8-29(3)9-11-30/h4-7,15,18-19,21-23H,8-14H2,1-3H3,(H,28,33)/t18-,19-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100300
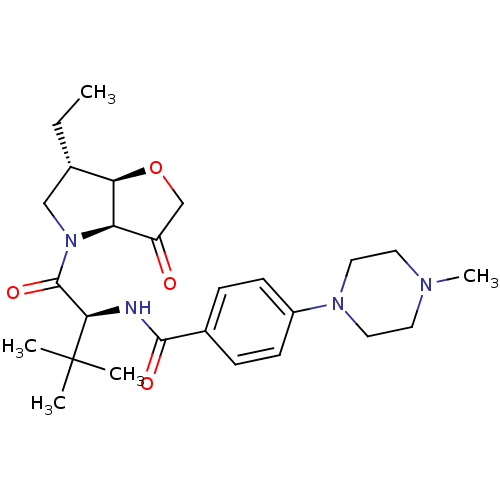
(US8501744, 25)Show SMILES CC[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N4O4/c1-6-17-15-30(21-20(31)16-34-22(17)21)25(33)23(26(2,3)4)27-24(32)18-7-9-19(10-8-18)29-13-11-28(5)12-14-29/h7-10,17,21-23H,6,11-16H2,1-5H3,(H,27,32)/t17-,21+,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100288
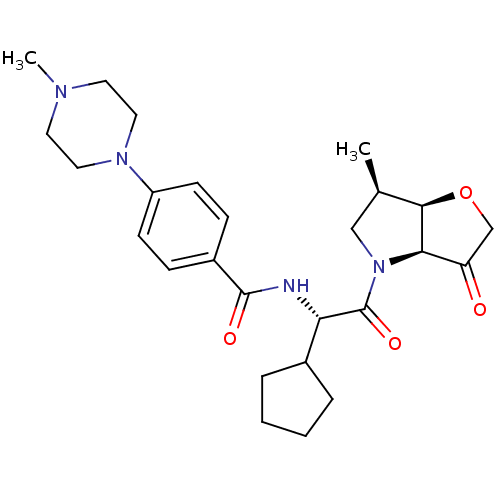
(US8501744, 4)Show SMILES C[C@@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C26H36N4O4/c1-17-15-30(23-21(31)16-34-24(17)23)26(33)22(18-5-3-4-6-18)27-25(32)19-7-9-20(10-8-19)29-13-11-28(2)12-14-29/h7-10,17-18,22-24H,3-6,11-16H2,1-2H3,(H,27,32)/t17-,22+,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100290

(US8501744, 6)Show SMILES C[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(C)(C)C |r| Show InChI InChI=1S/C25H36N4O4/c1-16-14-29(20-19(30)15-33-21(16)20)24(32)22(25(2,3)4)26-23(31)17-6-8-18(9-7-17)28-12-10-27(5)11-13-28/h6-9,16,20-22H,10-15H2,1-5H3,(H,26,31)/t16-,20+,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100302
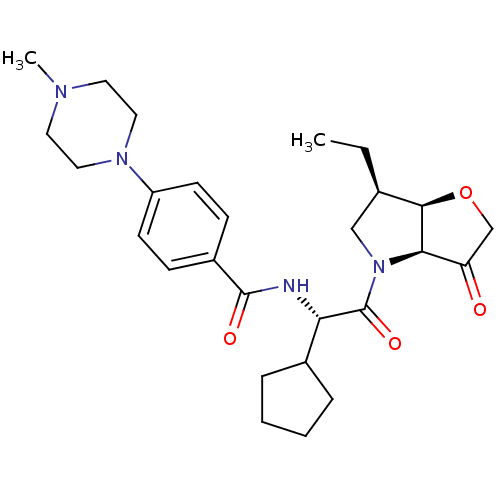
(US8501744, 27)Show SMILES CC[C@@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C27H38N4O4/c1-3-18-16-31(24-22(32)17-35-25(18)24)27(34)23(19-6-4-5-7-19)28-26(33)20-8-10-21(11-9-20)30-14-12-29(2)13-15-30/h8-11,18-19,23-25H,3-7,12-17H2,1-2H3,(H,28,33)/t18-,23+,24-,25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100287
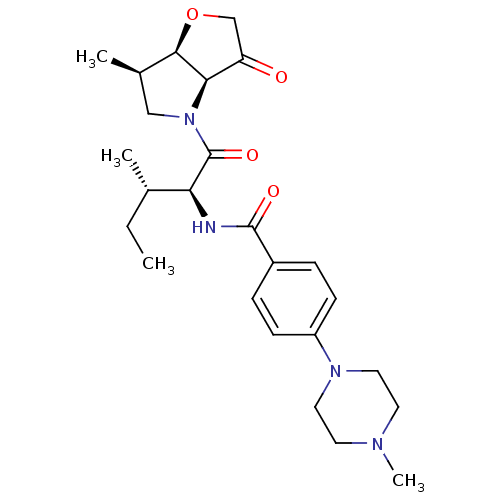
(US8501744, 2)Show SMILES CC[C@H](C)[C@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(=O)N1C[C@@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C25H36N4O4/c1-5-16(2)21(25(32)29-14-17(3)23-22(29)20(30)15-33-23)26-24(31)18-6-8-19(9-7-18)28-12-10-27(4)11-13-28/h6-9,16-17,21-23H,5,10-15H2,1-4H3,(H,26,31)/t16-,17+,21-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100303
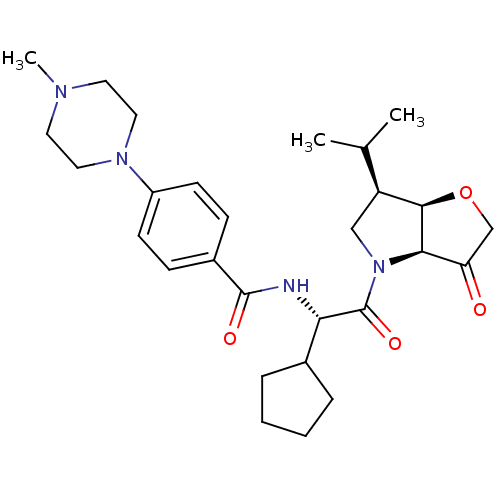
(US8501744, 28)Show SMILES CC(C)[C@@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C1CCCC1 |r| Show InChI InChI=1S/C28H40N4O4/c1-18(2)22-16-32(25-23(33)17-36-26(22)25)28(35)24(19-6-4-5-7-19)29-27(34)20-8-10-21(11-9-20)31-14-12-30(3)13-15-31/h8-11,18-19,22,24-26H,4-7,12-17H2,1-3H3,(H,29,34)/t22-,24-,25+,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100292
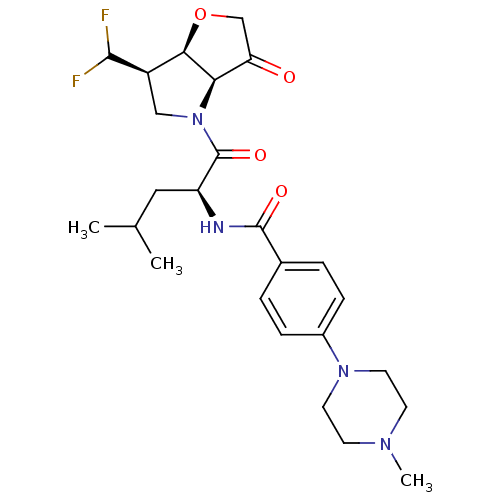
(US8501744, 8)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(=O)N1C[C@@H](C(F)F)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C25H34F2N4O4/c1-15(2)12-19(25(34)31-13-18(23(26)27)22-21(31)20(32)14-35-22)28-24(33)16-4-6-17(7-5-16)30-10-8-29(3)9-11-30/h4-7,15,18-19,21-23H,8-14H2,1-3H3,(H,28,33)/t18-,19+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103364

(US8552202, Compound 13)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1ccnc1)C1CCCCC1 |r| Show InChI InChI=1S/C24H27ClN4O4/c25-18-12-29(21-19(30)13-33-22(18)21)24(32)20(15-5-2-1-3-6-15)27-23(31)16-7-4-8-17(11-16)28-10-9-26-14-28/h4,7-11,14-15,18,20-22H,1-3,5-6,12-13H2,(H,27,31)/t18-,20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103361
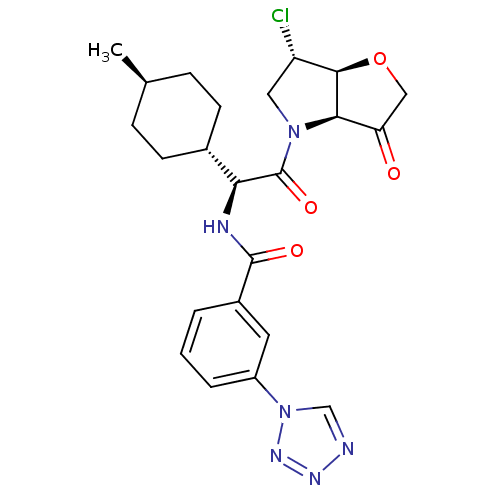
(US8552202, Example 2)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,wD:26.29,1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C23H27ClN6O4/c1-13-5-7-14(8-6-13)19(23(33)29-10-17(24)21-20(29)18(31)11-34-21)26-22(32)15-3-2-4-16(9-15)30-12-25-27-28-30/h2-4,9,12-14,17,19-21H,5-8,10-11H2,1H3,(H,26,32)/t13-,14-,17-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100286
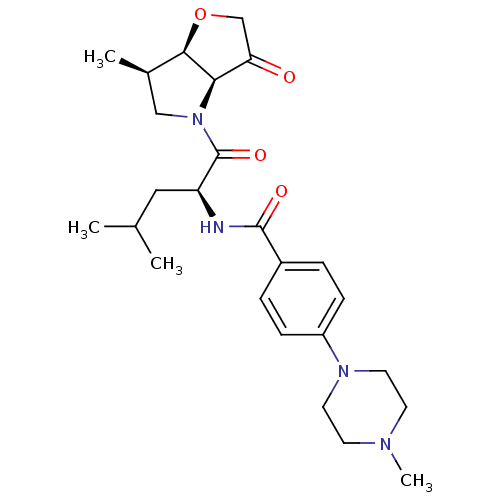
(US8501744, 1)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(=O)N1C[C@@H](C)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C25H36N4O4/c1-16(2)13-20(25(32)29-14-17(3)23-22(29)21(30)15-33-23)26-24(31)18-5-7-19(8-6-18)28-11-9-27(4)10-12-28/h5-8,16-17,20,22-23H,9-15H2,1-4H3,(H,26,31)/t17-,20+,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100299
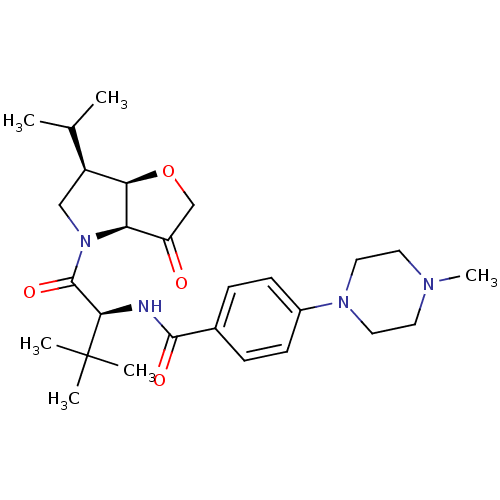
(US8501744, 24)Show SMILES CC(C)[C@@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(C)(C)C |r| Show InChI InChI=1S/C27H40N4O4/c1-17(2)20-15-31(22-21(32)16-35-23(20)22)26(34)24(27(3,4)5)28-25(33)18-7-9-19(10-8-18)30-13-11-29(6)12-14-30/h7-10,17,20,22-24H,11-16H2,1-6H3,(H,28,33)/t20-,22+,23+,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50134127

(CHEMBL607122)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1NC[C@@H]2[C@H]1C(=O)CN2C(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)15-22(31-26(35)19-11-13-21(14-12-19)29(3,4)5)28(37)33-25-23(16-30-33)32(17-24(25)34)27(36)20-9-7-6-8-10-20/h6-14,18,22-23,25,30H,15-17H2,1-5H3,(H,31,35)/t22-,23+,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin K |
Bioorg Med Chem Lett 15: 1327-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.022
BindingDB Entry DOI: 10.7270/Q2SB46HX |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103358
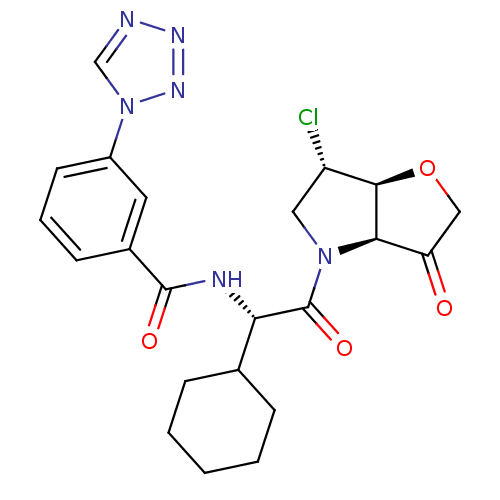
(US8552202, Compound 9)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1cnnn1)C1CCCCC1 |r| Show InChI InChI=1S/C22H25ClN6O4/c23-16-10-28(19-17(30)11-33-20(16)19)22(32)18(13-5-2-1-3-6-13)25-21(31)14-7-4-8-15(9-14)29-12-24-26-27-29/h4,7-9,12-13,16,18-20H,1-3,5-6,10-11H2,(H,25,31)/t16-,18-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100298

(US8501744, 23)Show SMILES CC[C@@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(C)(C)C |r| Show InChI InChI=1S/C26H38N4O4/c1-6-17-15-30(21-20(31)16-34-22(17)21)25(33)23(26(2,3)4)27-24(32)18-7-9-19(10-8-18)29-13-11-28(5)12-14-29/h7-10,17,21-23H,6,11-16H2,1-5H3,(H,27,32)/t17-,21-,22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103354

(US8552202, Example 1)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1ccccc1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,28.30,23.25,4.4,wD:21.23,1.0,(.08,4.62,;.08,3.08,;-1.25,2.31,;-1.25,.77,;.08,,;1.42,.77,;1.42,2.31,;.08,-1.54,;-1.25,-2.31,;-2.58,-1.54,;-2.58,,;-3.92,-2.31,;-5.25,-1.54,;-6.59,-2.31,;-6.59,-3.85,;-5.25,-4.62,;-3.92,-3.85,;1.42,-2.31,;1.42,-3.85,;2.75,-1.54,;2.75,,;4.22,.48,;4.99,1.81,;5.12,-.77,;6.59,-1.25,;6.59,-2.79,;5.12,-3.26,;4.35,-4.6,;4.22,-2.02,)| Show InChI InChI=1S/C22H27ClN2O4/c1-13-7-9-14(10-8-13)18(24-21(27)15-5-3-2-4-6-15)22(28)25-11-16(23)20-19(25)17(26)12-29-20/h2-6,13-14,16,18-20H,7-12H2,1H3,(H,24,27)/t13-,14-,16-,18-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50161337

(N-[1-((S)-(S)-4-Benzoyl-6-(S)-oxo-hexahydro-pyrrol...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N(C)C)C(=O)N1CCC2[C@H]1C(=O)CN2C(=O)c1ccccc1 Show InChI InChI=1S/C28H34N4O4/c1-18(2)16-22(29-26(34)19-10-12-21(13-11-19)30(3)4)28(36)31-15-14-23-25(31)24(33)17-32(23)27(35)20-8-6-5-7-9-20/h5-13,18,22-23,25H,14-17H2,1-4H3,(H,29,34)/t22-,23?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin K |
Bioorg Med Chem Lett 15: 1327-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.022
BindingDB Entry DOI: 10.7270/Q2SB46HX |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103351
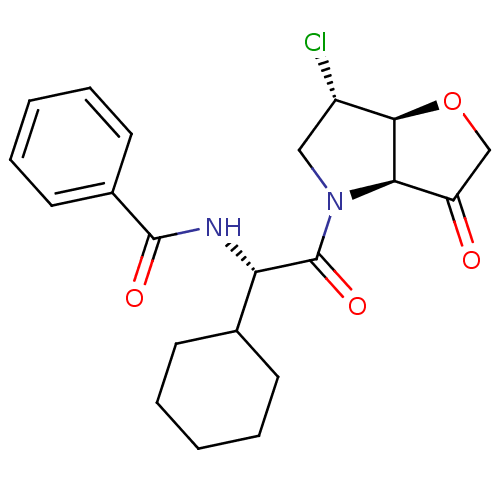
(US8552202, Compound 3)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccccc1)C1CCCCC1 |r| Show InChI InChI=1S/C21H25ClN2O4/c22-15-11-24(18-16(25)12-28-19(15)18)21(27)17(13-7-3-1-4-8-13)23-20(26)14-9-5-2-6-10-14/h2,5-6,9-10,13,15,17-19H,1,3-4,7-8,11-12H2,(H,23,26)/t15-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100285

(CAC1 proteinases 5,5 bicyclic inhibitor 10)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N1CCN(C)CC1)C(=O)N1CC[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C24H34N4O4/c1-16(2)14-19(24(31)28-9-8-21-22(28)20(29)15-32-21)25-23(30)17-4-6-18(7-5-17)27-12-10-26(3)11-13-27/h4-7,16,19,21-22H,8-15H2,1-3H3,(H,25,30)/t19-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50318879
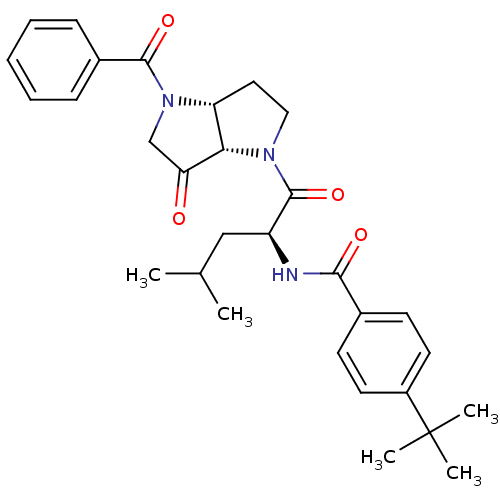
(CHEMBL607169 | N-((S)-1-((3aR,6aS)-4-benzoyl-6-oxo...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1CC[C@@H]2[C@H]1C(=O)CN2C(=O)c1ccccc1 Show InChI InChI=1S/C30H37N3O4/c1-19(2)17-23(31-27(35)20-11-13-22(14-12-20)30(3,4)5)29(37)32-16-15-24-26(32)25(34)18-33(24)28(36)21-9-7-6-8-10-21/h6-14,19,23-24,26H,15-18H2,1-5H3,(H,31,35)/t23-,24+,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin K |
Bioorg Med Chem Lett 15: 1327-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.022
BindingDB Entry DOI: 10.7270/Q2SB46HX |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103363

(US8552202, Example 3)Show SMILES CC1(C)CCC(CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1C[C@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C24H29ClN6O4/c1-24(2)8-6-14(7-9-24)19(23(34)30-11-17(25)21-20(30)18(32)12-35-21)27-22(33)15-4-3-5-16(10-15)31-13-26-28-29-31/h3-5,10,13-14,17,19-21H,6-9,11-12H2,1-2H3,(H,27,33)/t17-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103366

(US8552202, Compound 15)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1ccnc1)C(=O)N1C[C@H](F)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,wD:26.29,1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C25H29FN4O4/c1-15-5-7-16(8-6-15)21(25(33)30-12-19(26)23-22(30)20(31)13-34-23)28-24(32)17-3-2-4-18(11-17)29-10-9-27-14-29/h2-4,9-11,14-16,19,21-23H,5-8,12-13H2,1H3,(H,28,32)/t15-,16-,19-,21-,22+,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM103364

(US8552202, Compound 13)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1ccnc1)C1CCCCC1 |r| Show InChI InChI=1S/C24H27ClN4O4/c25-18-12-29(21-19(30)13-33-22(18)21)24(32)20(15-5-2-1-3-6-15)27-23(31)16-7-4-8-17(11-16)28-10-9-26-14-28/h4,7-11,14-15,18,20-22H,1-3,5-6,12-13H2,(H,27,31)/t18-,20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103360
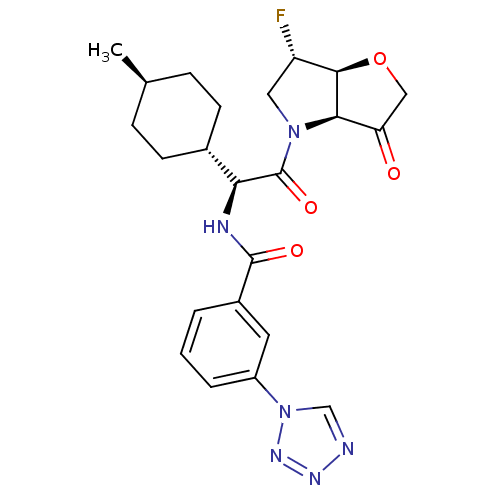
(US8552202, Compound 11)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1C[C@H](F)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,wD:26.29,1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C23H27FN6O4/c1-13-5-7-14(8-6-13)19(23(33)29-10-17(24)21-20(29)18(31)11-34-21)26-22(32)15-3-2-4-16(9-15)30-12-25-27-28-30/h2-4,9,12-14,17,19-21H,5-8,10-11H2,1H3,(H,26,32)/t13-,14-,17-,19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM103358

(US8552202, Compound 9)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1cnnn1)C1CCCCC1 |r| Show InChI InChI=1S/C22H25ClN6O4/c23-16-10-28(19-17(30)11-33-20(16)19)22(32)18(13-5-2-1-3-6-13)25-21(31)14-7-4-8-15(9-14)29-12-24-26-27-29/h4,7-9,12-13,16,18-20H,1-3,5-6,10-11H2,(H,25,31)/t16-,18-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103353
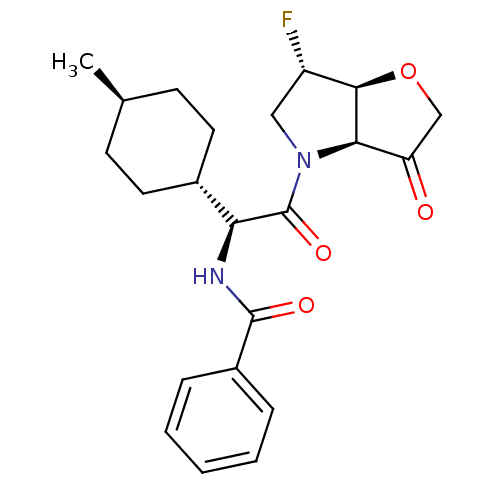
(US8552202, Compound 5)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1ccccc1)C(=O)N1C[C@H](F)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,28.30,23.25,4.4,wD:21.23,1.0,(.08,4.62,;.08,3.08,;-1.25,2.31,;-1.25,.77,;.08,,;1.42,.77,;1.42,2.31,;.08,-1.54,;-1.25,-2.31,;-2.58,-1.54,;-2.58,,;-3.92,-2.31,;-5.25,-1.54,;-6.59,-2.31,;-6.59,-3.85,;-5.25,-4.62,;-3.92,-3.85,;1.42,-2.31,;1.42,-3.85,;2.75,-1.54,;2.75,,;4.22,.48,;4.99,1.81,;5.12,-.77,;6.59,-1.25,;6.59,-2.79,;5.12,-3.26,;4.35,-4.6,;4.22,-2.02,)| Show InChI InChI=1S/C22H27FN2O4/c1-13-7-9-14(10-8-13)18(24-21(27)15-5-3-2-4-6-15)22(28)25-11-16(23)20-19(25)17(26)12-29-20/h2-6,13-14,16,18-20H,7-12H2,1H3,(H,24,27)/t13-,14-,16-,18-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103365
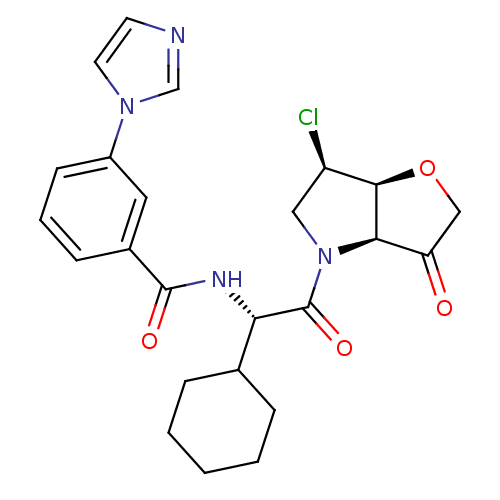
(US8552202, Compound 14)Show SMILES Cl[C@@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1ccnc1)C1CCCCC1 |r| Show InChI InChI=1S/C24H27ClN4O4/c25-18-12-29(21-19(30)13-33-22(18)21)24(32)20(15-5-2-1-3-6-15)27-23(31)16-7-4-8-17(11-16)28-10-9-26-14-28/h4,7-11,14-15,18,20-22H,1-3,5-6,12-13H2,(H,27,31)/t18-,20+,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM103351

(US8552202, Compound 3)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccccc1)C1CCCCC1 |r| Show InChI InChI=1S/C21H25ClN2O4/c22-15-11-24(18-16(25)12-28-19(15)18)21(27)17(13-7-3-1-4-8-13)23-20(26)14-9-5-2-6-10-14/h2,5-6,9-10,13,15,17-19H,1,3-4,7-8,11-12H2,(H,23,26)/t15-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50161334

(N-[1-((S)-(S)-4-Benzoyl-6-(S)-oxo-hexahydro-2-oxa-...)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)N(C)C)C(=O)N1OCC2[C@H]1C(=O)CN2C(=O)c1ccccc1 Show InChI InChI=1S/C27H32N4O5/c1-17(2)14-21(28-25(33)18-10-12-20(13-11-18)29(3)4)27(35)31-24-22(16-36-31)30(15-23(24)32)26(34)19-8-6-5-7-9-19/h5-13,17,21-22,24H,14-16H2,1-4H3,(H,28,33)/t21-,22?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against human cathepsin K |
Bioorg Med Chem Lett 15: 1327-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.022
BindingDB Entry DOI: 10.7270/Q2SB46HX |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103357

(US8552202, Compound 8)Show SMILES F[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1cccc(c1)-n1cnnn1)C1CCCCC1 |r| Show InChI InChI=1S/C22H25FN6O4/c23-16-10-28(19-17(30)11-33-20(16)19)22(32)18(13-5-2-1-3-6-13)25-21(31)14-7-4-8-15(9-14)29-12-24-26-27-29/h4,7-9,12-13,16,18-20H,1-3,5-6,10-11H2,(H,25,31)/t16-,18-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cysteine proteinase B
(Leishmania mexicana) | BDBM50134127

(CHEMBL607122)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1NC[C@@H]2[C@H]1C(=O)CN2C(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)15-22(31-26(35)19-11-13-21(14-12-19)29(3,4)5)28(37)33-25-23(16-30-33)32(17-24(25)34)27(36)20-9-7-6-8-10-20/h6-14,18,22-23,25,30H,15-17H2,1-5H3,(H,31,35)/t22-,23+,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against Leishmania mexicana cysteine peptidase B (CPB) |
Bioorg Med Chem Lett 15: 1327-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.022
BindingDB Entry DOI: 10.7270/Q2SB46HX |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103362
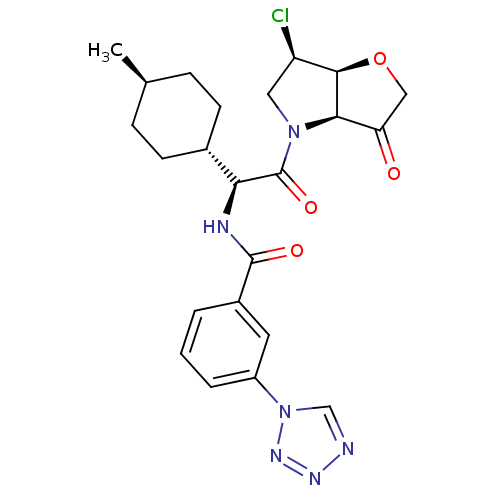
(US8552202, Compound 12)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1C[C@@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,33.36,28.31,4.4,26.29,wD:1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.84,1.81,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C23H27ClN6O4/c1-13-5-7-14(8-6-13)19(23(33)29-10-17(24)21-20(29)18(31)11-34-21)26-22(32)15-3-2-4-16(9-15)30-12-25-27-28-30/h2-4,9,12-14,17,19-21H,5-8,10-11H2,1H3,(H,26,32)/t13-,14-,17-,19+,20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103350
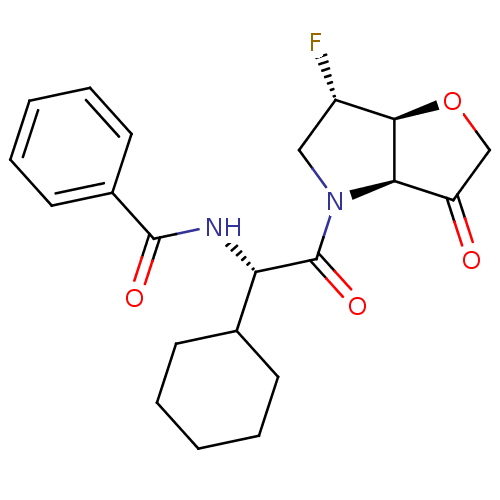
(US8552202, Compound 2)Show SMILES F[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccccc1)C1CCCCC1 |r| Show InChI InChI=1S/C21H25FN2O4/c22-15-11-24(18-16(25)12-28-19(15)18)21(27)17(13-7-3-1-4-8-13)23-20(26)14-9-5-2-6-10-14/h2,5-6,9-10,13,15,17-19H,1,3-4,7-8,11-12H2,(H,23,26)/t15-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103352
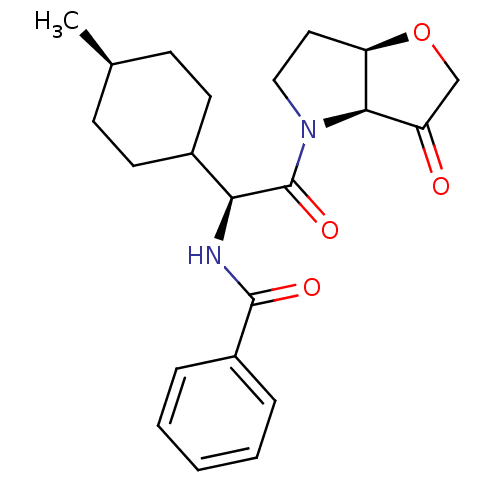
(US8552202, Compound 4)Show SMILES C[C@H]1CCC(CC1)[C@H](NC(=O)c1ccccc1)C(=O)N1CC[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,27.29,22.24,wD:1.0,(.08,4.62,;.08,3.08,;-1.25,2.31,;-1.25,.77,;.08,,;1.42,.77,;1.42,2.31,;.08,-1.54,;-1.25,-2.31,;-2.58,-1.54,;-2.58,,;-3.92,-2.31,;-5.25,-1.54,;-6.59,-2.31,;-6.59,-3.85,;-5.25,-4.62,;-3.92,-3.85,;1.42,-2.31,;1.42,-3.85,;2.75,-1.54,;2.75,,;4.22,.48,;5.12,-.77,;6.59,-1.25,;6.59,-2.79,;5.12,-3.26,;4.35,-4.6,;4.22,-2.02,)| Show InChI InChI=1S/C22H28N2O4/c1-14-7-9-15(10-8-14)19(23-21(26)16-5-3-2-4-6-16)22(27)24-12-11-18-20(24)17(25)13-28-18/h2-6,14-15,18-20H,7-13H2,1H3,(H,23,26)/t14-,15?,18-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103355
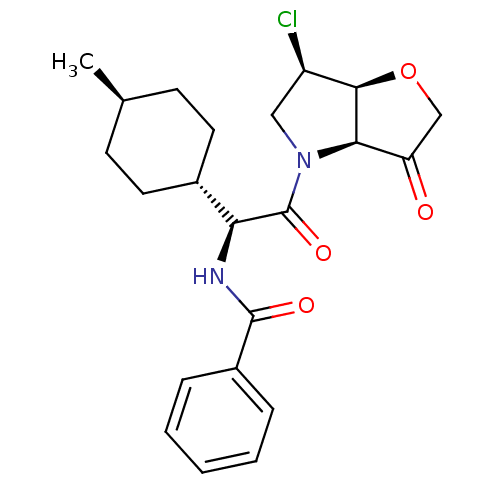
(US8552202, Compound 6)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1ccccc1)C(=O)N1C[C@@H](Cl)[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,28.30,23.25,4.4,21.23,wD:1.0,(.08,4.62,;.08,3.08,;-1.25,2.31,;-1.25,.77,;.08,,;1.42,.77,;1.42,2.31,;.08,-1.54,;-1.25,-2.31,;-2.58,-1.54,;-2.58,,;-3.92,-2.31,;-5.25,-1.54,;-6.59,-2.31,;-6.59,-3.85,;-5.25,-4.62,;-3.92,-3.85,;1.42,-2.31,;1.42,-3.85,;2.75,-1.54,;2.75,,;4.22,.48,;4.99,1.81,;5.12,-.77,;6.59,-1.25,;6.59,-2.79,;5.12,-3.26,;4.35,-4.6,;4.22,-2.02,)| Show InChI InChI=1S/C22H27ClN2O4/c1-13-7-9-14(10-8-13)18(24-21(27)15-5-3-2-4-6-15)22(28)25-11-16(23)20-19(25)17(26)12-29-20/h2-6,13-14,16,18-20H,7-12H2,1H3,(H,24,27)/t13-,14-,16-,18+,19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM103359
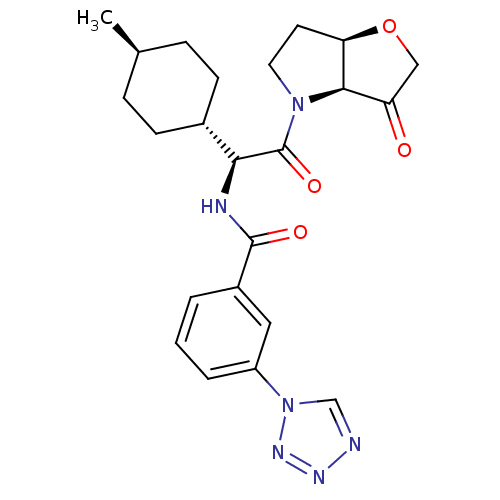
(US8552202, Compound 10)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cccc(c1)-n1cnnn1)C(=O)N1CC[C@H]2OCC(=O)[C@@H]12 |r,wU:7.8,32.35,27.30,4.4,wD:1.0,(1.93,4.62,;1.93,3.08,;.6,2.31,;.6,.77,;1.93,,;3.27,.77,;3.27,2.31,;1.93,-1.54,;.6,-2.31,;-.73,-1.54,;-.73,,;-2.07,-2.31,;-2.07,-3.85,;-3.4,-4.62,;-4.73,-3.85,;-4.73,-2.31,;-3.4,-1.54,;-6.07,-1.54,;-7.53,-2.02,;-8.44,-.77,;-7.53,.48,;-6.07,,;3.27,-2.31,;3.27,-3.85,;4.6,-1.54,;4.6,,;6.07,.48,;6.97,-.77,;8.44,-1.25,;8.44,-2.79,;6.97,-3.26,;6.2,-4.6,;6.07,-2.02,)| Show InChI InChI=1S/C23H28N6O4/c1-14-5-7-15(8-6-14)20(23(32)28-10-9-19-21(28)18(30)12-33-19)25-22(31)16-3-2-4-17(11-16)29-13-24-26-27-29/h2-4,11,13-15,19-21H,5-10,12H2,1H3,(H,25,31)/t14-,15-,19-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM100284

(CAC1 proteinases 5,5 bicyclic inhibitor 45a)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1CC[C@H]2OCC(=O)[C@@H]12 |r| Show InChI InChI=1S/C23H32N2O4/c1-14(2)12-17(22(28)25-11-10-19-20(25)18(26)13-29-19)24-21(27)15-6-8-16(9-7-15)23(3,4)5/h6-9,14,17,19-20H,10-13H2,1-5H3,(H,24,27)/t17-,19+,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 87.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics, Limited
US Patent
| Assay Description
In vitro cathepsin inhibition assay. |
US Patent US8501744 (2013)
BindingDB Entry DOI: 10.7270/Q2D7992S |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM50134127

(CHEMBL607122)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(C)(C)C)C(=O)N1NC[C@@H]2[C@H]1C(=O)CN2C(=O)c1ccccc1 |r| Show InChI InChI=1S/C29H36N4O4/c1-18(2)15-22(31-26(35)19-11-13-21(14-12-19)29(3,4)5)28(37)33-25-23(16-30-33)32(17-24(25)34)27(36)20-9-7-6-8-10-20/h6-14,18,22-23,25,30H,15-17H2,1-5H3,(H,31,35)/t22-,23+,25-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited, Inc
Curated by ChEMBL
| Assay Description
Binding affinity against cruzaine |
Bioorg Med Chem Lett 15: 1327-31 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.022
BindingDB Entry DOI: 10.7270/Q2SB46HX |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM103350

(US8552202, Compound 2)Show SMILES F[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccccc1)C1CCCCC1 |r| Show InChI InChI=1S/C21H25FN2O4/c22-15-11-24(18-16(25)12-28-19(15)18)21(27)17(13-7-3-1-4-8-13)23-20(26)14-9-5-2-6-10-14/h2,5-6,9-10,13,15,17-19H,1,3-4,7-8,11-12H2,(H,23,26)/t15-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM103351

(US8552202, Compound 3)Show SMILES Cl[C@H]1CN([C@H]2[C@@H]1OCC2=O)C(=O)[C@@H](NC(=O)c1ccccc1)C1CCCCC1 |r| Show InChI InChI=1S/C21H25ClN2O4/c22-15-11-24(18-16(25)12-28-19(15)18)21(27)17(13-7-3-1-4-8-13)23-20(26)14-9-5-2-6-10-14/h2,5-6,9-10,13,15,17-19H,1,3-4,7-8,11-12H2,(H,23,26)/t15-,17-,18+,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amura Therapeutics Limited
US Patent
| Assay Description
In vitro inhibition assay using cathepsin. |
US Patent US8552202 (2013)
BindingDB Entry DOI: 10.7270/Q2S46QJ8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data