

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484529 (US10934294, Example 50 | US10934294, Example 51 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484570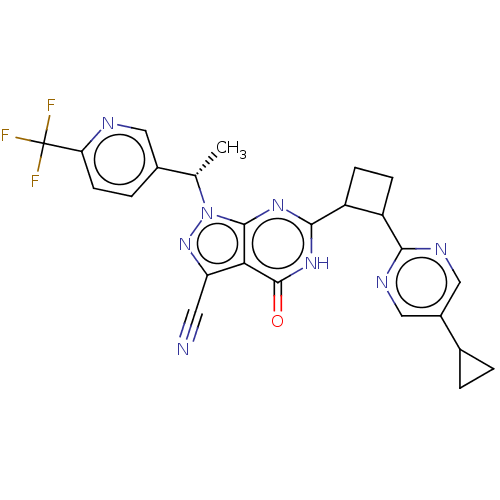 (US10934294, Example 91 | US10934294, Example 92 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484541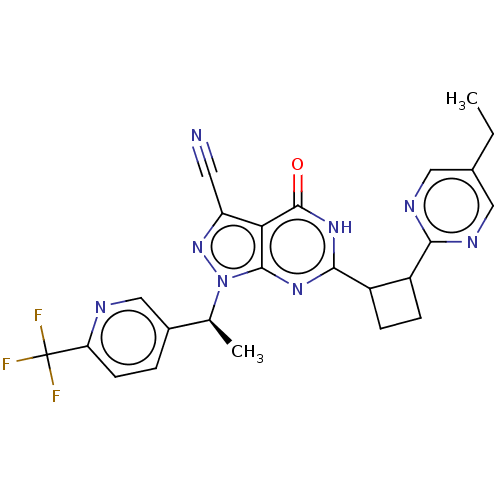 (US10934294, Example 62 | US11028092, Example 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484497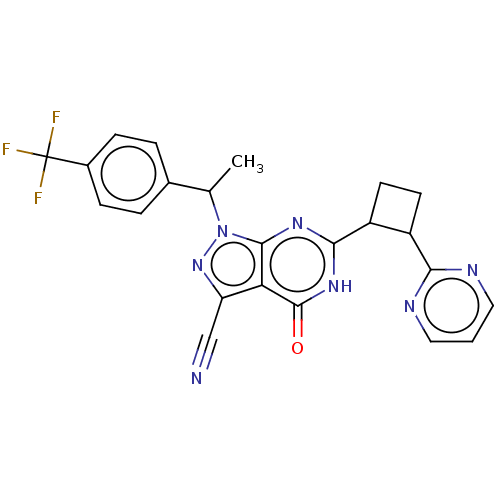 (US10934294, Example 19 | US10934294, Example 20 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325767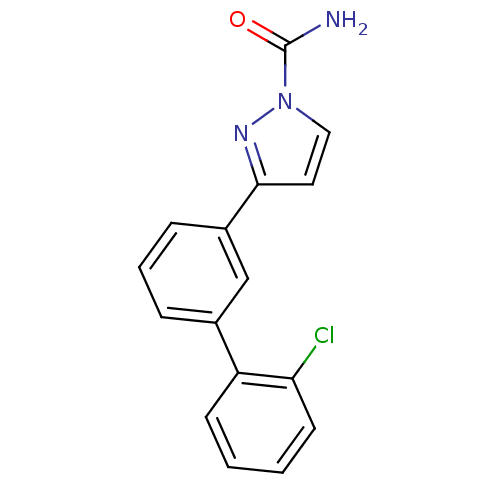 (3-(2'-chlorobiphenyl-3-yl)-1H-pyrazole-1-carboxami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484551 (US10934294, Example 72 | US10934294, Example 73 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484572 (US10934294, Example 93) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484509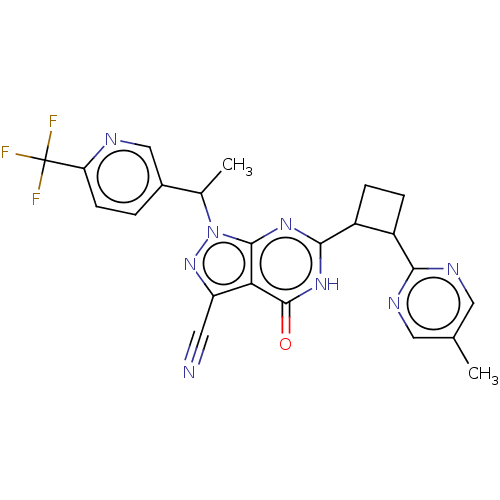 (US10934294, Example 31 | US10934294, Example 32 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484596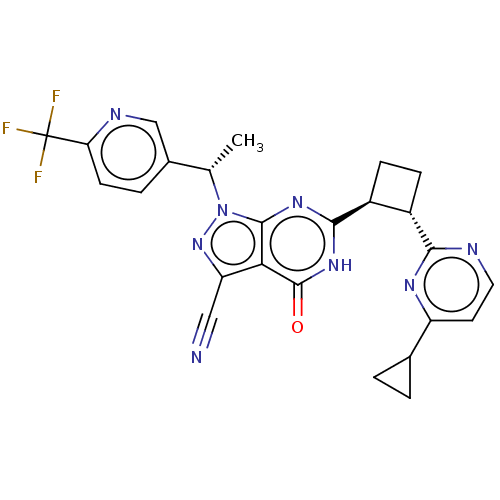 (US10934294, Example 114 | US11028092, Example 114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484593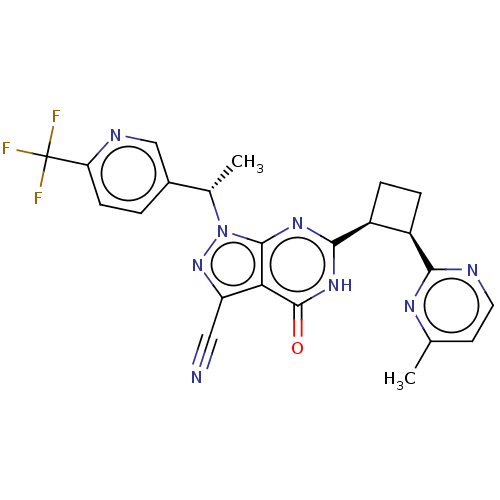 (US10934294, Example 111) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484591 (US10934294, Example 110 | US11028092, Example 110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082827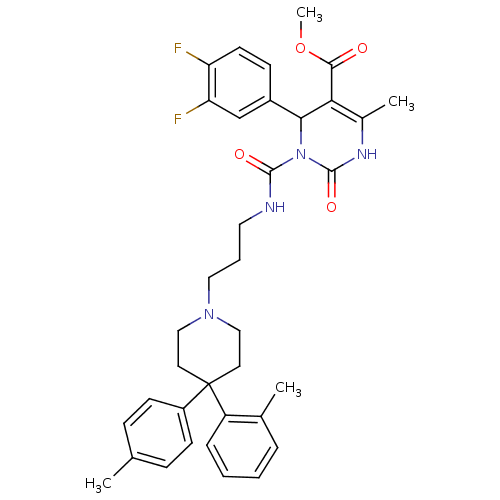 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-o-t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082811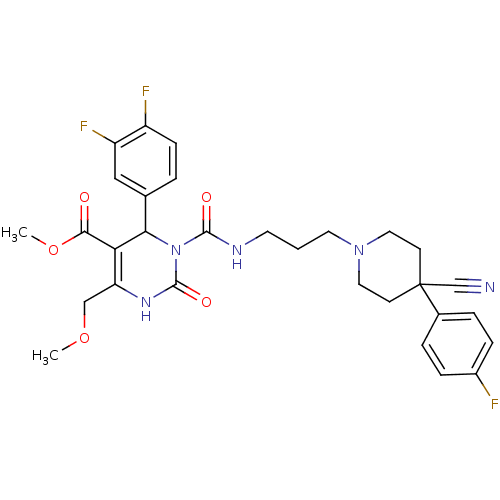 (3-{3-[4-Cyano-4-(4-fluoro-phenyl)-piperidin-1-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484539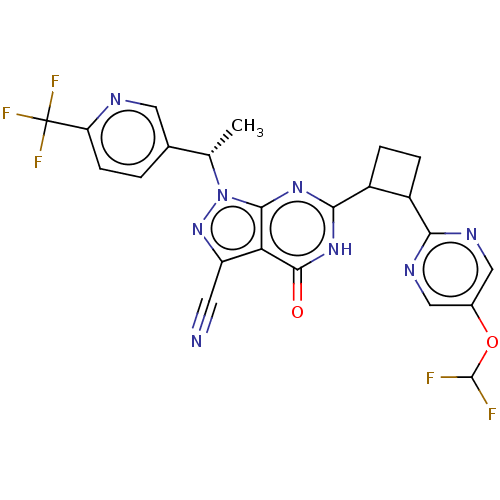 (US10934294, Example 60 | US10934294, Example 61 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484559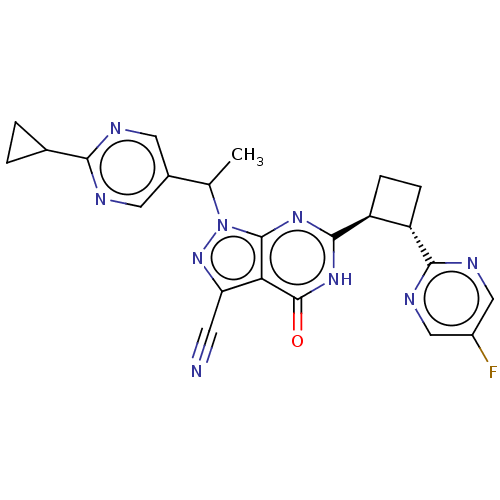 (US10934294, Example 80 | US10934294, Example 81 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082792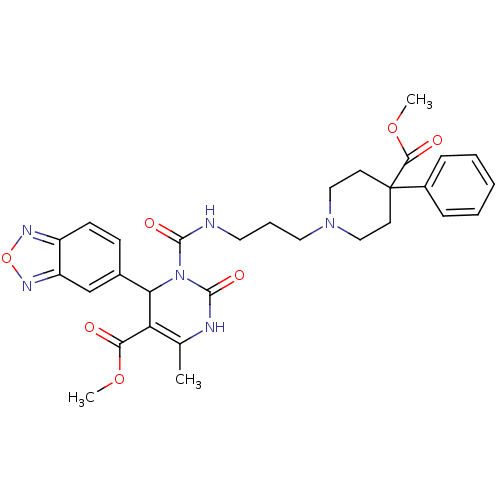 (4-Benzo[1,2,5]oxadiazol-5-yl-3-[3-(4-methoxycarbon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082825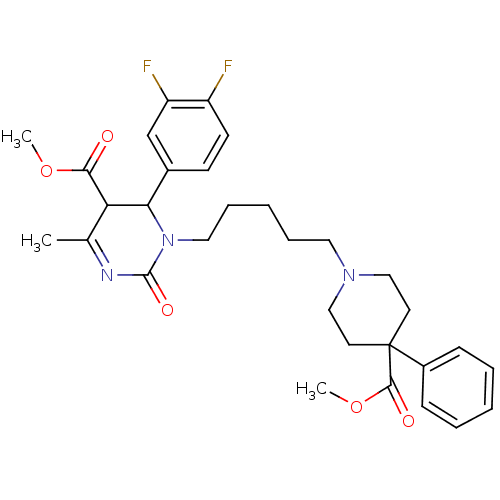 (4-(3,4-Difluoro-phenyl)-3-[5-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082810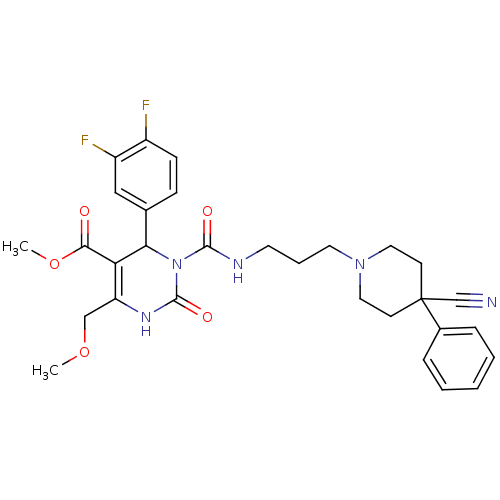 (3-[3-(4-Cyano-4-phenyl-piperidin-1-yl)-propylcarba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082797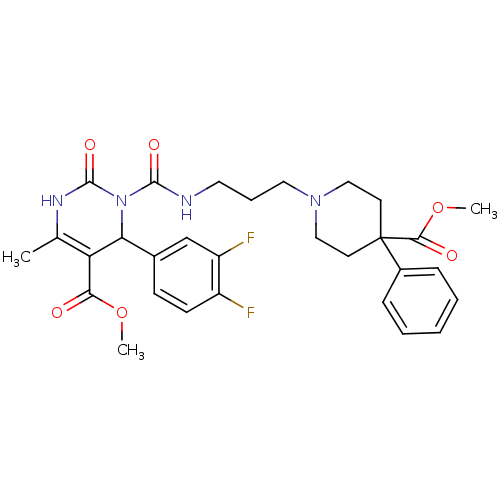 (4-(3,4-Difluoro-phenyl)-3-[3-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484484 (US10934294, Example 6 | US11028092, Example 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484489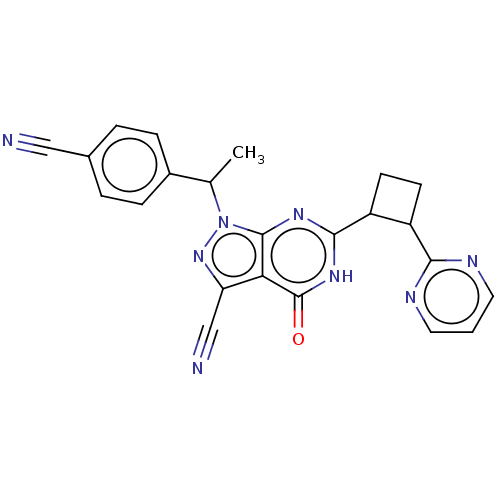 (US10934294, Example 11 | US10934294, Example 12 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484501 (US10934294, Example 23 | US10934294, Example 24 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082797 (4-(3,4-Difluoro-phenyl)-3-[3-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50325766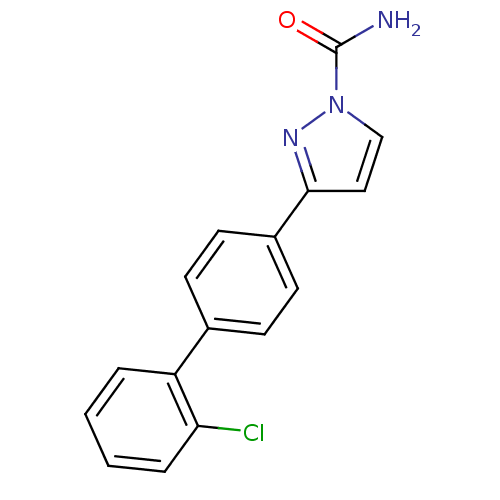 (3-(2'-chlorobiphenyl-4-yl)-1H-pyrazole-1-carboxami...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav 1.7 channel by electrophysiology | Bioorg Med Chem Lett 20: 5480-3 (2010) Article DOI: 10.1016/j.bmcl.2010.07.080 BindingDB Entry DOI: 10.7270/Q2PV6KJX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484537 (US10934294, Example 58 | US10934294, Example 59 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484517 (US10934294, Example 38 | US10934294, Example 39 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484594 (BDBM502802 | US10934294, Example 112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484542 (US10934294, Example 63 | US10934294, Example 97 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484583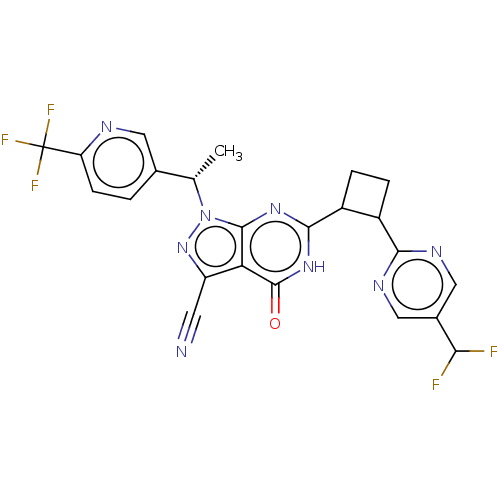 (US10934294, Example 102 | US10934294, Example 103 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082790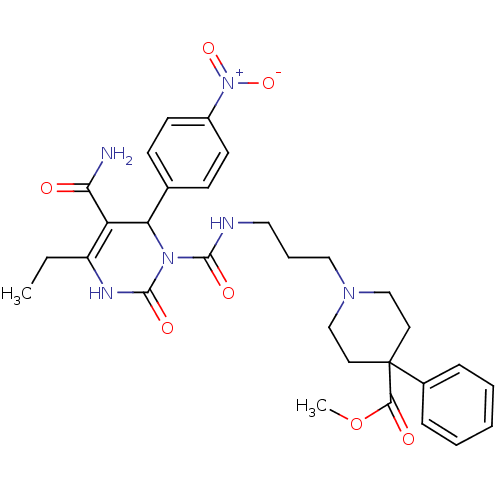 (1-(3-{[5-Carbamoyl-4-ethyl-6-(4-nitro-phenyl)-2-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor in isolated prostate tissue of human | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082794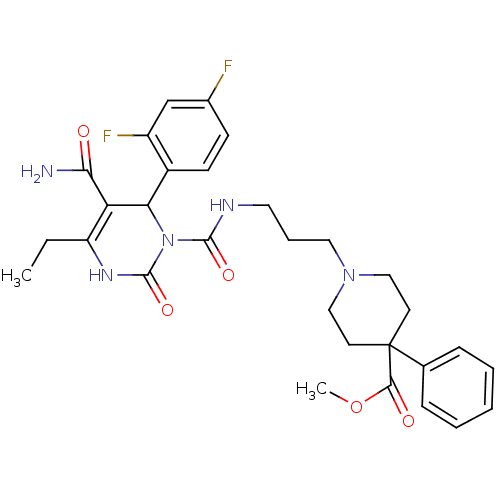 (1-(3-{[5-Carbamoyl-6-(2,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082813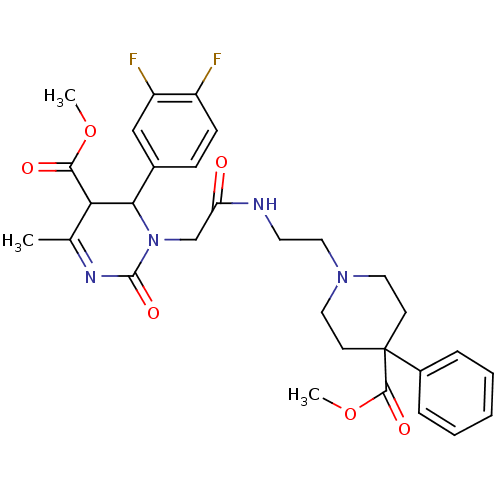 (4-(3,4-Difluoro-phenyl)-3-{[2-(4-methoxycarbonyl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082821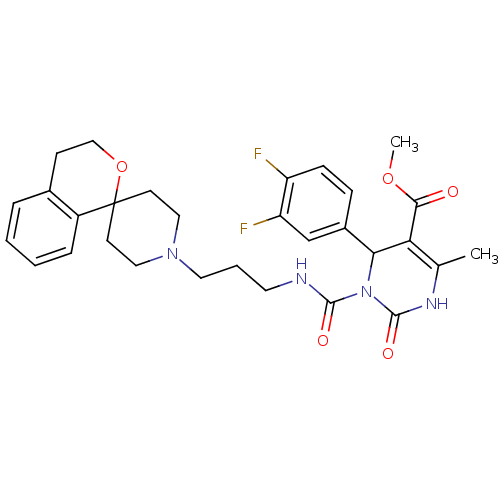 (CHEMBL359012 | methyl 4-(3,4-difluorophenyl)-6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082814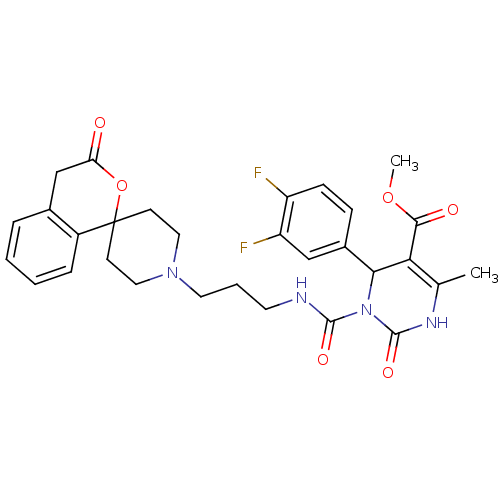 (CHEMBL358785 | methyl 4-(3,4-difluorophenyl)-6-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082819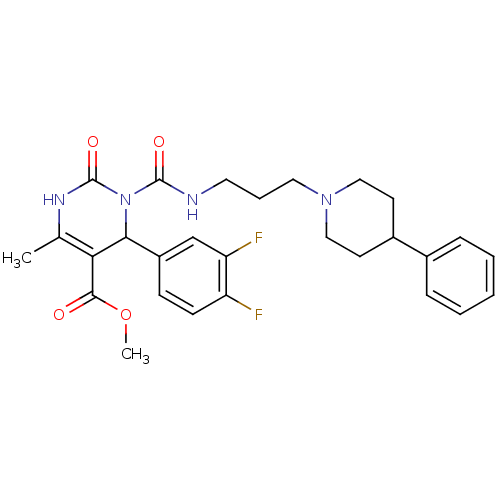 (4-(3,4-Difluoro-phenyl)-6-methyl-2-oxo-3-[3-(4-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484587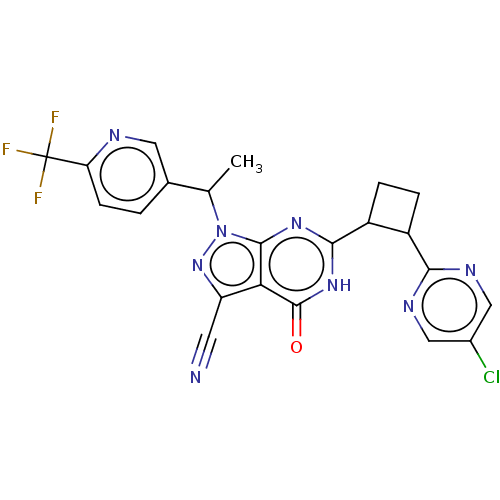 (US10934294, Example 106 | US10934294, Example 107 ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484480 (US10934294, Example 2 | US11028092, Example 2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082798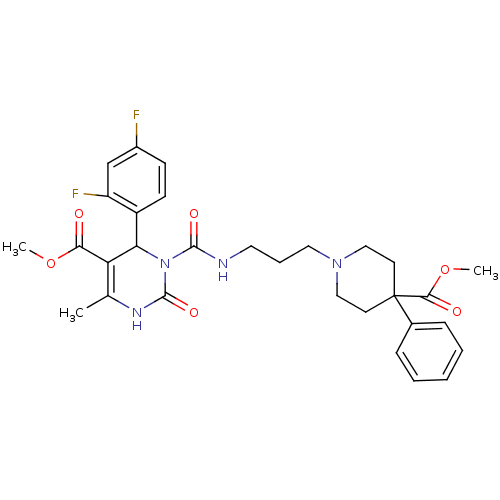 (4-(2,4-Difluoro-phenyl)-3-[3-(4-methoxycarbonyl-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082784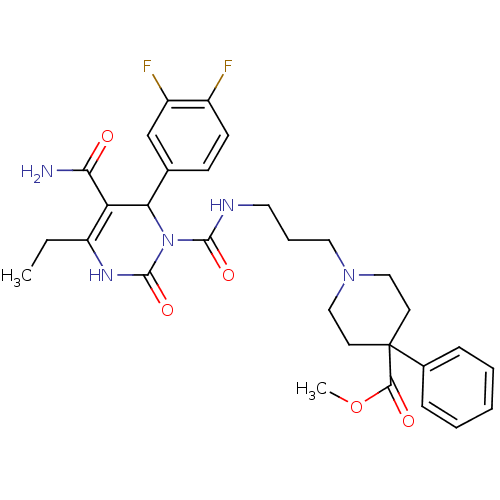 (1-(3-{[5-Carbamoyl-6-(3,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity radioligand | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082790 (1-(3-{[5-Carbamoyl-4-ethyl-6-(4-nitro-phenyl)-2-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082790 (1-(3-{[5-Carbamoyl-4-ethyl-6-(4-nitro-phenyl)-2-ox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082794 (1-(3-{[5-Carbamoyl-6-(2,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082784 (1-(3-{[5-Carbamoyl-6-(3,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor of human liver microsomes. | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082784 (1-(3-{[5-Carbamoyl-6-(3,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082835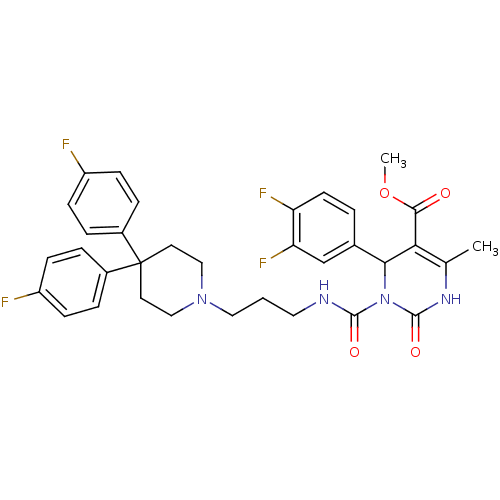 (3-{3-[4,4-Bis-(4-fluoro-phenyl)-piperidin-1-yl]-pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082812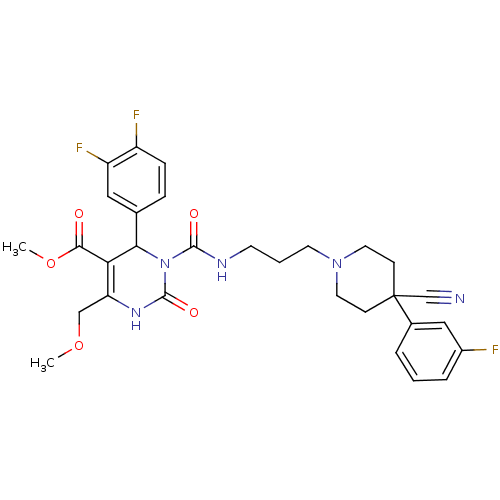 (3-{3-[4-Cyano-4-(3-fluoro-phenyl)-piperidin-1-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against alpha-1A adrenergic receptor of human liver microsomes | J Med Chem 42: 4778-93 (1999) BindingDB Entry DOI: 10.7270/Q2930SCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50082784 (1-(3-{[5-Carbamoyl-6-(3,4-difluoro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Synaptic Pharmaceutical Corporation Curated by ChEMBL | Assay Description In vitro binding affinity against Alpha-1A adrenergic receptor in isolated prostate tissue of human | J Med Chem 42: 4764-77 (1999) BindingDB Entry DOI: 10.7270/Q2W959W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484525 (US10934294, Example 46 | US10934294, Example 47 | ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484561 (US10934294, Example 82 | US11028092, Example 82) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Isoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2) (Homo sapiens (Human)) | BDBM484563 (US10934294, Example 84 | US11028092, Example 84) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol... | US Patent US10934294 (2021) BindingDB Entry DOI: 10.7270/Q2C250JS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2559 total ) | Next | Last >> |