 Found 1086 hits with Last Name = 'raveglia' and Initial = 'l'
Found 1086 hits with Last Name = 'raveglia' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(MOUSE) | BDBM50290872
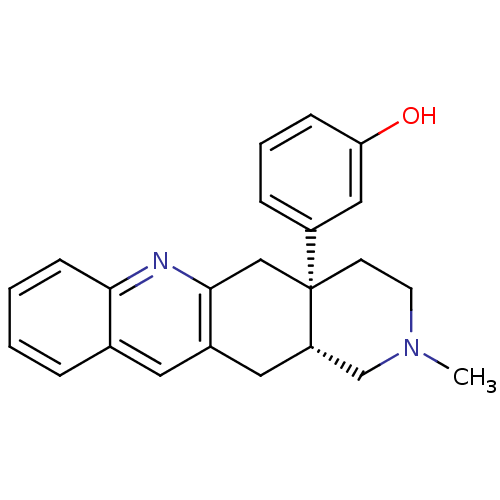
((-)-rel-3-((4aR,12aS)-2-methyl-1,2,3,4,4a,5,12,12a...)Show SMILES CN1CC[C@@]2(Cc3nc4ccccc4cc3C[C@H]2C1)c1cccc(O)c1 |r| Show InChI InChI=1S/C23H24N2O/c1-25-10-9-23(18-6-4-7-20(26)13-18)14-22-17(12-19(23)15-25)11-16-5-2-3-8-21(16)24-22/h2-8,11,13,19,26H,9-10,12,14-15H2,1H3/t19-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604064

(US11660293, Example 171 | US11660293, Example 173)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r,wU:1.0,(.29,4.78,;-1.04,4.01,;-2.37,4.78,;-3.71,4.01,;-3.71,2.47,;-2.37,3.24,;-2.37,1.7,;-1.04,2.47,;.29,1.7,;1.63,2.47,;2.96,1.7,;4.37,2.32,;5.4,1.18,;6.94,1.18,;7.71,-.16,;6.94,-1.49,;7.71,-2.82,;5.4,-1.49,;4.63,-.16,;3.12,.16,;-2.37,.16,;-1.04,-.61,;-3.71,-.61,;-5.04,.16,;-6.37,-.61,;-7.71,.16,;-6.37,-2.15,;-5.04,-2.92,;-3.71,-2.15,;-2.37,-2.92,;-2.21,-4.46,;-.71,-4.78,;.06,-3.44,;-.97,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50450528

(CHEMBL2051964)Show SMILES Cc1ccc(O)c(c1)C(=O)NC[C@]1(C)C[C@H](O)CC(C)(C)C1 |r| Show InChI InChI=1S/C18H27NO3/c1-12-5-6-15(21)14(7-12)16(22)19-11-18(4)9-13(20)8-17(2,3)10-18/h5-7,13,20-21H,8-11H2,1-4H3,(H,19,22)/t13-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50291261
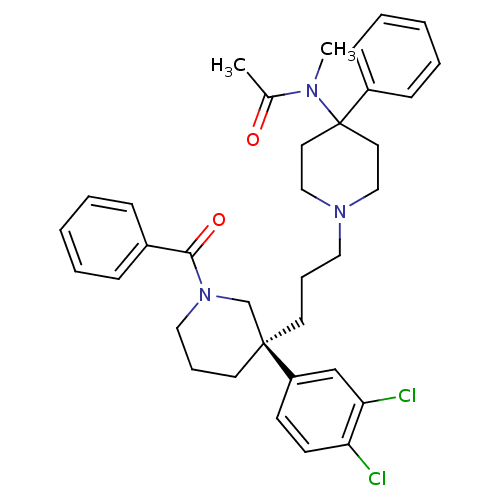
((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]NKA from Tachykinin receptor 2 in rat deodenum membrane |
J Med Chem 39: 2281-4 (1996)
Article DOI: 10.1021/jm9602423
BindingDB Entry DOI: 10.7270/Q22J6CJR |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099628
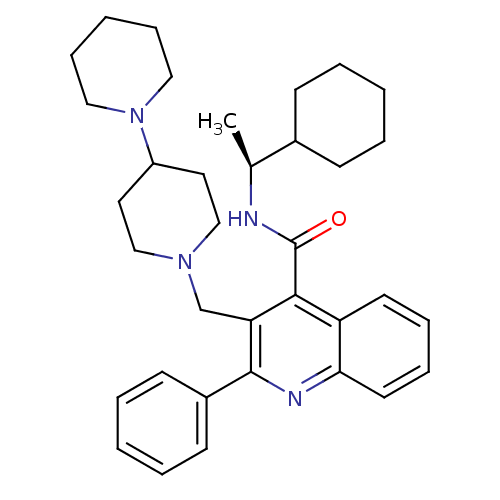
(3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoli...)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C35H46N4O/c1-26(27-13-5-2-6-14-27)36-35(40)33-30-17-9-10-18-32(30)37-34(28-15-7-3-8-16-28)31(33)25-38-23-19-29(20-24-38)39-21-11-4-12-22-39/h3,7-10,15-18,26-27,29H,2,4-6,11-14,19-25H2,1H3,(H,36,40)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208047
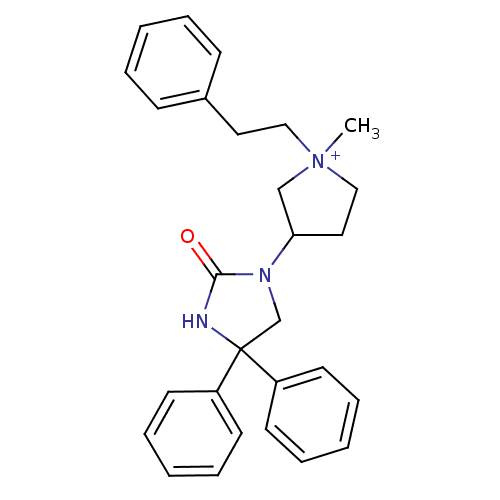
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O/c1-31(19-17-23-11-5-2-6-12-23)20-18-26(21-31)30-22-28(29-27(30)32,24-13-7-3-8-14-24)25-15-9-4-10-16-25/h2-16,26H,17-22H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208057
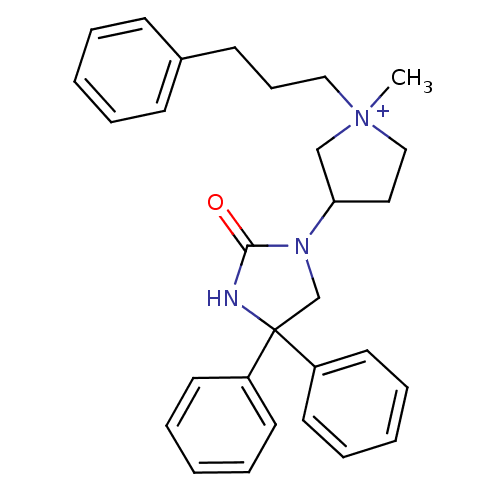
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O/c1-32(20-11-14-24-12-5-2-6-13-24)21-19-27(22-32)31-23-29(30-28(31)33,25-15-7-3-8-16-25)26-17-9-4-10-18-26/h2-10,12-13,15-18,27H,11,14,19-23H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099638
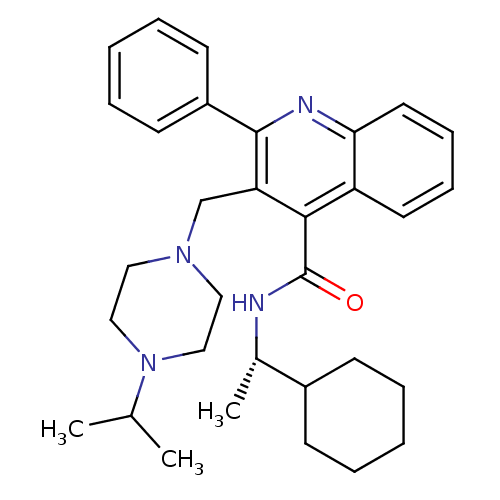
(3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quin...)Show SMILES CC(C)N1CCN(Cc2c(nc3ccccc3c2C(=O)N[C@@H](C)C2CCCCC2)-c2ccccc2)CC1 Show InChI InChI=1S/C32H42N4O/c1-23(2)36-20-18-35(19-21-36)22-28-30(32(37)33-24(3)25-12-6-4-7-13-25)27-16-10-11-17-29(27)34-31(28)26-14-8-5-9-15-26/h5,8-11,14-17,23-25H,4,6-7,12-13,18-22H2,1-3H3,(H,33,37)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208057

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O/c1-32(20-11-14-24-12-5-2-6-13-24)21-19-27(22-32)31-23-29(30-28(31)33,25-15-7-3-8-16-25)26-17-9-4-10-18-26/h2-10,12-13,15-18,27H,11,14,19-23H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208043
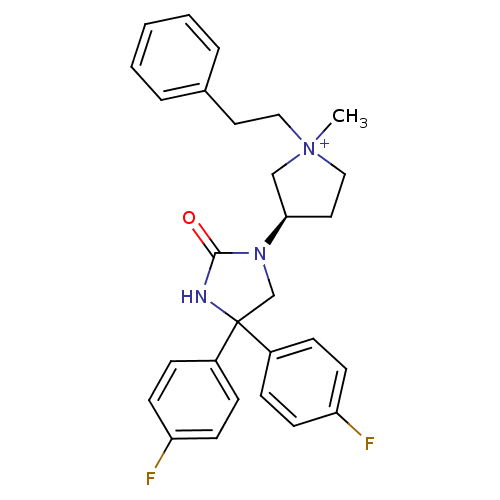
(1-methyl-3-(R)-[4,4-Bis-(4-fluoro-phenyl)-2-oxo-im...)Show SMILES C[N+]1(CCc2ccccc2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c1-33(17-15-21-5-3-2-4-6-21)18-16-26(19-33)32-20-28(31-27(32)34,22-7-11-24(29)12-8-22)23-9-13-25(30)14-10-23/h2-14,26H,15-20H2,1H3/p+1/t26-,33?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM603987

(US11660293, Example 61)Show SMILES Cc1nc(C(=O)N2C3CC(C3)CC2CNc2nc3ccc(F)cc3s2)c(s1)-c1ccccc1 |(-8.47,-.61,;-6.93,-.61,;-5.9,.53,;-4.49,-.1,;-3.16,.67,;-1.82,-.1,;-3.16,2.21,;-4.49,2.98,;-4.49,4.52,;-3.16,5.29,;-3.16,3.75,;-1.82,4.52,;-1.82,2.98,;-.49,2.21,;.85,2.98,;2.18,2.21,;2.34,.68,;3.85,.36,;4.62,-.97,;6.16,-.97,;6.93,.36,;8.47,.36,;6.16,1.7,;4.62,1.7,;3.59,2.84,;-4.65,-1.63,;-6.16,-1.95,;-3.56,-2.72,;-3.96,-4.2,;-2.87,-5.29,;-1.38,-4.89,;-.98,-3.41,;-2.07,-2.32,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099631
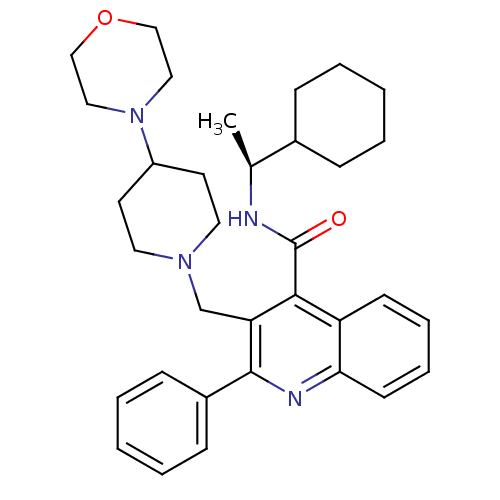
(3-(4-Morpholin-4-yl-piperidin-1-ylmethyl)-2-phenyl...)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCOCC2)c(nc2ccccc12)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C34H44N4O2/c1-25(26-10-4-2-5-11-26)35-34(39)32-29-14-8-9-15-31(29)36-33(27-12-6-3-7-13-27)30(32)24-37-18-16-28(17-19-37)38-20-22-40-23-21-38/h3,6-9,12-15,25-26,28H,2,4-5,10-11,16-24H2,1H3,(H,35,39)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50099631

(3-(4-Morpholin-4-yl-piperidin-1-ylmethyl)-2-phenyl...)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCOCC2)c(nc2ccccc12)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C34H44N4O2/c1-25(26-10-4-2-5-11-26)35-34(39)32-29-14-8-9-15-31(29)36-33(27-12-6-3-7-13-27)30(32)24-37-18-16-28(17-19-37)38-20-22-40-23-21-38/h3,6-9,12-15,25-26,28H,2,4-5,10-11,16-24H2,1H3,(H,35,39)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604048

(US11660293, Example 152 | US11660293, Example 154)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1nc(C)ccc1-n1nccn1 |r,wU:1.0,(-.48,4.78,;-1.81,4.01,;-3.14,4.78,;-4.48,4.01,;-4.48,2.47,;-3.14,3.24,;-3.14,1.7,;-1.81,2.47,;-.48,1.7,;.86,2.47,;2.19,1.7,;2.35,.16,;3.86,-.16,;4.63,-1.49,;6.17,-1.49,;6.94,-.16,;8.48,-.16,;6.17,1.18,;4.63,1.18,;3.6,2.32,;-3.14,.16,;-1.81,-.61,;-4.48,-.61,;-5.81,.16,;-7.14,-.61,;-8.48,.16,;-7.14,-2.15,;-5.81,-2.92,;-4.48,-2.15,;-3.14,-2.92,;-2.98,-4.46,;-1.48,-4.78,;-.71,-3.44,;-1.74,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.871 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50061061
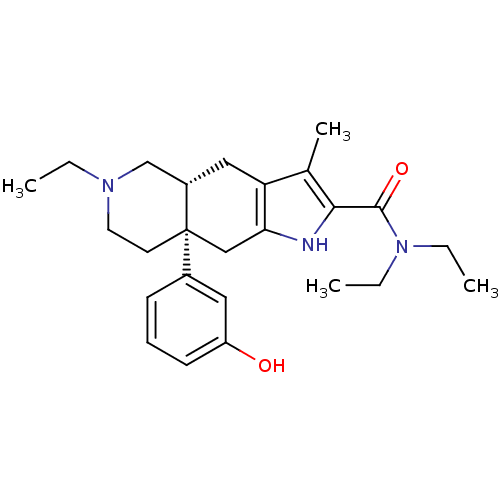
((4aR,8aS)-6-Ethyl-8a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN(CC)C(=O)c1[nH]c2C[C@]3(CCN(CC)C[C@@H]3Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C25H35N3O2/c1-5-27-12-11-25(18-9-8-10-20(29)13-18)15-22-21(14-19(25)16-27)17(4)23(26-22)24(30)28(6-2)7-3/h8-10,13,19,26,29H,5-7,11-12,14-16H2,1-4H3/t19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50207996
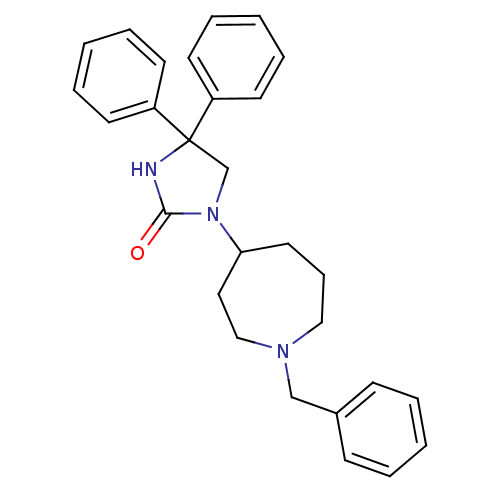
(3-(1-benzyl-azepin-4-yl)-5,5-diphenyl-imidazolidin...)Show SMILES O=C1NC(CN1C1CCCN(Cc2ccccc2)CC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O/c32-27-29-28(24-13-6-2-7-14-24,25-15-8-3-9-16-25)22-31(27)26-17-10-19-30(20-18-26)21-23-11-4-1-5-12-23/h1-9,11-16,26H,10,17-22H2,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHO K1 cells |
J Med Chem 50: 1571-83 (2007)
Article DOI: 10.1021/jm061159a
BindingDB Entry DOI: 10.7270/Q2QV3M61 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051293
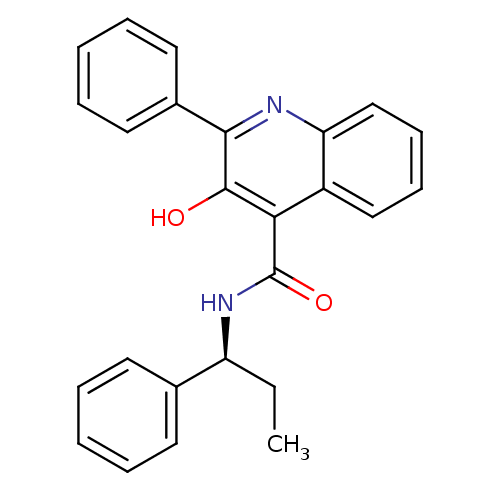
((S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinol...)Show SMILES CC[C@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding Affinity of [125I]-MePhe7-NKB towards Tachykinin receptor 3-CHO cell membranes(n=3-8) |
J Med Chem 39: 2281-4 (1996)
Article DOI: 10.1021/jm9602423
BindingDB Entry DOI: 10.7270/Q22J6CJR |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099640
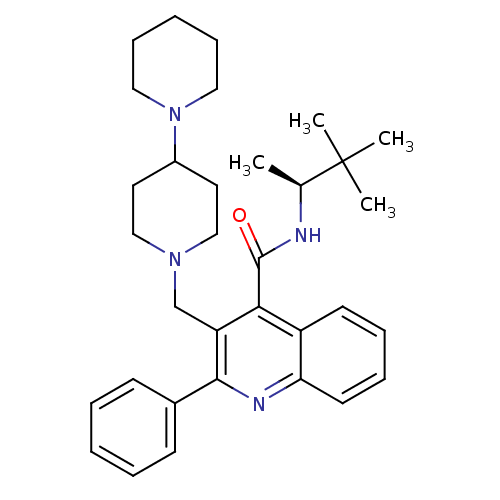
(3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoli...)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1ccccc1)C(C)(C)C Show InChI InChI=1S/C33H44N4O/c1-24(33(2,3)4)34-32(38)30-27-15-9-10-16-29(27)35-31(25-13-7-5-8-14-25)28(30)23-36-21-17-26(18-22-36)37-19-11-6-12-20-37/h5,7-10,13-16,24,26H,6,11-12,17-23H2,1-4H3,(H,34,38)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604115
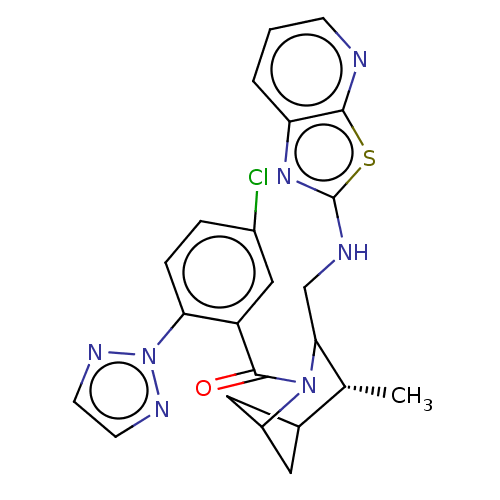
(US11660293, Example 234 | US11660293, Example 236)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2cccnc2s1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r,wU:1.0,(.29,4.78,;-1.04,4.01,;-2.37,4.78,;-3.71,4.01,;-3.71,2.47,;-2.37,3.24,;-2.37,1.7,;-1.04,2.47,;.29,1.7,;1.63,2.47,;2.96,1.7,;4.37,2.32,;5.4,1.18,;6.94,1.18,;7.71,-.16,;6.94,-1.49,;5.4,-1.49,;4.63,-.16,;3.12,.16,;-2.37,.16,;-1.04,-.61,;-3.71,-.61,;-5.04,.16,;-6.37,-.61,;-7.71,.16,;-6.37,-2.15,;-5.04,-2.92,;-3.71,-2.15,;-2.37,-2.92,;-2.21,-4.46,;-.71,-4.78,;.06,-3.44,;-.97,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.02 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208044
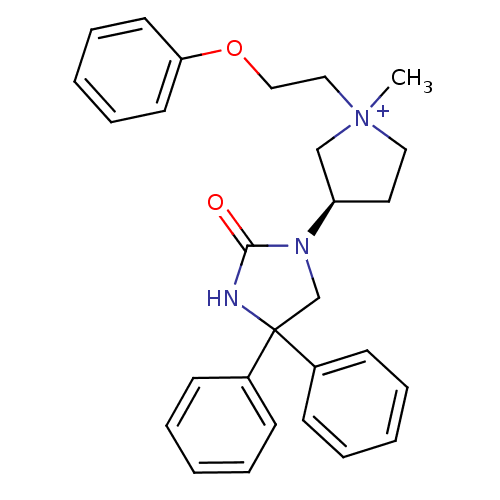
(1-methyl-3-(R)-(2-oxo-4,4-diphenyl-imidazolidin-1-...)Show SMILES C[N+]1(CCOc2ccccc2)CC[C@H](C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1/t25-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604067
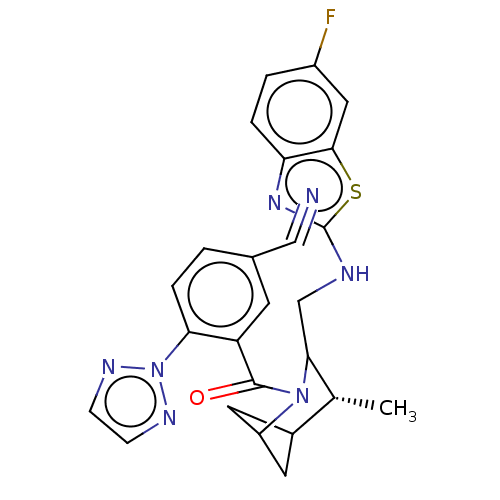
(US11660293, Example 175 | US11660293, Example 177)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1cc(ccc1-n1nccn1)C#N |r,wU:1.0,(.96,4.78,;-.37,4.01,;-1.71,4.78,;-3.04,4.01,;-3.04,2.47,;-1.71,3.24,;-1.71,1.7,;-.37,2.47,;.96,1.7,;2.29,2.47,;3.63,1.7,;5.03,2.32,;6.07,1.18,;7.61,1.18,;8.38,-.16,;7.61,-1.49,;8.38,-2.82,;6.07,-1.49,;5.3,-.16,;3.79,.16,;-1.71,.16,;-.37,-.61,;-3.04,-.61,;-4.37,.16,;-5.71,-.61,;-5.71,-2.15,;-4.37,-2.92,;-3.04,-2.15,;-1.71,-2.92,;-1.55,-4.46,;-.04,-4.78,;.73,-3.44,;-.3,-2.3,;-7.04,.16,;-8.38,.93,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604057
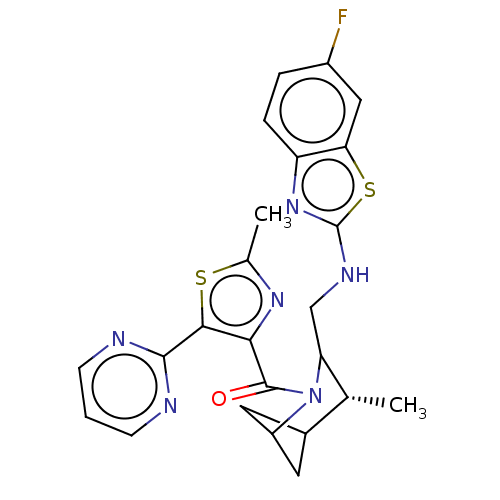
(US11660293, Example 162 | US11660293, Example 164)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1nc(C)sc1-c1ncccn1 |r,wU:1.0,(.26,5.29,;-1.08,4.52,;-2.41,5.29,;-3.75,4.52,;-3.75,2.98,;-2.41,3.75,;-2.41,2.21,;-1.08,2.98,;.26,2.21,;1.59,2.98,;2.92,2.21,;4.33,2.84,;5.36,1.7,;6.9,1.7,;7.67,.36,;6.9,-.97,;7.67,-2.31,;5.36,-.97,;4.59,.36,;3.08,.68,;-2.41,.67,;-1.08,-.1,;-3.75,-.1,;-5.15,.53,;-6.18,-.61,;-7.67,-.22,;-5.41,-1.95,;-3.91,-1.63,;-2.82,-2.72,;-3.22,-4.2,;-2.13,-5.29,;-.64,-4.89,;-.24,-3.41,;-1.33,-2.32,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50099643
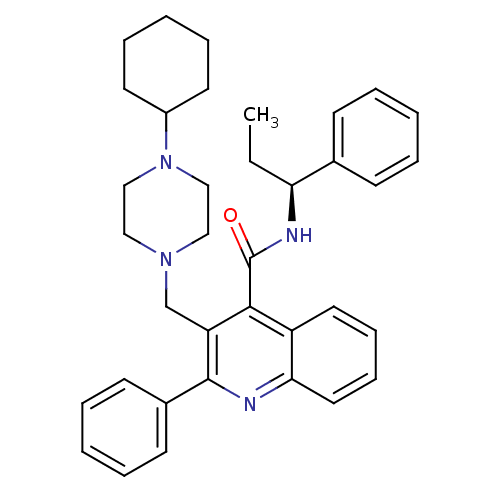
(3-(4-Cyclohexyl-piperazin-1-ylmethyl)-2-phenyl-qui...)Show SMILES CC[C@H](NC(=O)c1c(CN2CCN(CC2)C2CCCCC2)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H42N4O/c1-2-32(27-14-6-3-7-15-27)38-36(41)34-30-20-12-13-21-33(30)37-35(28-16-8-4-9-17-28)31(34)26-39-22-24-40(25-23-39)29-18-10-5-11-19-29/h3-4,6-9,12-17,20-21,29,32H,2,5,10-11,18-19,22-26H2,1H3,(H,38,41)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261

((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding Affinity of [125I]-MePhe7-NKB towards Tachykinin receptor 3-CHO cell membranes(n=3-8) |
J Med Chem 39: 2281-4 (1996)
Article DOI: 10.1021/jm9602423
BindingDB Entry DOI: 10.7270/Q22J6CJR |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099637

(3-(4-Isobutyryl-piperazin-1-ylmethyl)-2-phenyl-qui...)Show SMILES CC(C)C(=O)N1CCN(Cc2c(nc3ccccc3c2C(=O)N[C@@H](C)C2CCCCC2)-c2ccccc2)CC1 Show InChI InChI=1S/C33H42N4O2/c1-23(2)33(39)37-20-18-36(19-21-37)22-28-30(32(38)34-24(3)25-12-6-4-7-13-25)27-16-10-11-17-29(27)35-31(28)26-14-8-5-9-15-26/h5,8-11,14-17,23-25H,4,6-7,12-13,18-22H2,1-3H3,(H,34,38)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261

((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50099632
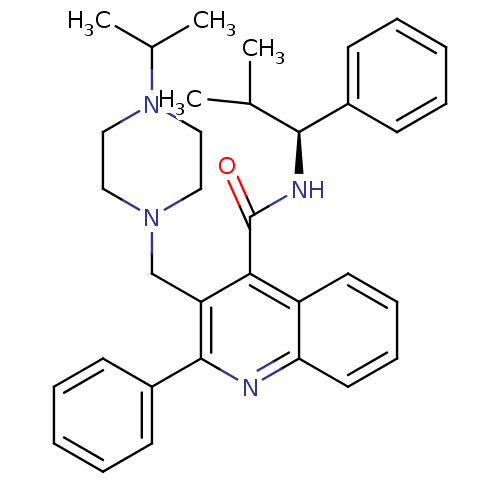
(3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quin...)Show SMILES CC(C)[C@H](NC(=O)c1c(CN2CCN(CC2)C(C)C)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C34H40N4O/c1-24(2)32(26-13-7-5-8-14-26)36-34(39)31-28-17-11-12-18-30(28)35-33(27-15-9-6-10-16-27)29(31)23-37-19-21-38(22-20-37)25(3)4/h5-18,24-25,32H,19-23H2,1-4H3,(H,36,39)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074789
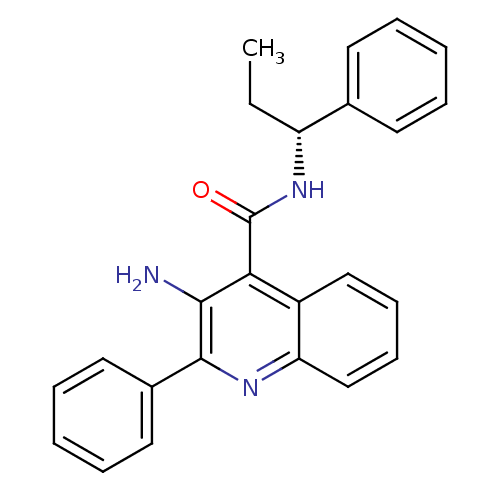
(3-Amino-2-phenyl-quinoline-4-carboxylic acid ((R)-...)Show SMILES CC[C@@H](NC(=O)c1c(N)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H23N3O/c1-2-20(17-11-5-3-6-12-17)28-25(29)22-19-15-9-10-16-21(19)27-24(23(22)26)18-13-7-4-8-14-18/h3-16,20H,2,26H2,1H3,(H,28,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604092

(US11660293, Example 210 | US11660293, Example 211)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1ccc(cn1)C(F)(F)F)C(=O)c1nn(C)cc1-c1ccccc1 |r,wU:1.0,(-.01,5.26,;-1.34,4.49,;-2.68,5.26,;-4.01,4.49,;-4.01,2.95,;-2.68,3.72,;-2.68,2.18,;-1.34,2.95,;-.01,2.18,;1.32,2.95,;2.66,2.18,;2.66,.64,;3.99,-.13,;5.32,.64,;5.32,2.18,;3.99,2.95,;6.66,-.13,;7.99,.64,;6.66,-1.67,;7.99,-.9,;-2.68,.64,;-1.34,-.13,;-4.01,-.13,;-5.26,.78,;-6.5,-.13,;-7.99,.27,;-6.03,-1.59,;-4.49,-1.59,;-3.4,-2.68,;-1.91,-2.28,;-.82,-3.37,;-1.22,-4.86,;-2.71,-5.26,;-3.8,-4.17,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604064

(US11660293, Example 171 | US11660293, Example 173)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1cc(Cl)ccc1-n1nccn1 |r,wU:1.0,(.29,4.78,;-1.04,4.01,;-2.37,4.78,;-3.71,4.01,;-3.71,2.47,;-2.37,3.24,;-2.37,1.7,;-1.04,2.47,;.29,1.7,;1.63,2.47,;2.96,1.7,;4.37,2.32,;5.4,1.18,;6.94,1.18,;7.71,-.16,;6.94,-1.49,;7.71,-2.82,;5.4,-1.49,;4.63,-.16,;3.12,.16,;-2.37,.16,;-1.04,-.61,;-3.71,-.61,;-5.04,.16,;-6.37,-.61,;-7.71,.16,;-6.37,-2.15,;-5.04,-2.92,;-3.71,-2.15,;-2.37,-2.92,;-2.21,-4.46,;-.71,-4.78,;.06,-3.44,;-.97,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM603991

(US11660293, Example 89)Show SMILES C[C@H]1C2CC(C2)N([C@H]1COc1cc2ccccc2cn1)C(=O)c1nc(C)sc1-c1ccccc1 |r,wD:7.9,1.0,(-.04,5.29,;-1.37,4.52,;-2.71,5.29,;-4.04,4.52,;-4.04,2.98,;-2.71,3.75,;-2.71,2.21,;-1.37,2.98,;-.04,2.21,;1.3,2.98,;2.63,2.21,;3.96,2.98,;5.3,2.21,;6.63,2.98,;7.96,2.21,;7.96,.67,;6.63,-.1,;5.3,.67,;3.96,-.1,;2.63,.67,;-2.71,.67,;-1.37,-.1,;-4.04,-.1,;-5.45,.53,;-6.48,-.61,;-7.96,-.22,;-5.71,-1.95,;-4.2,-1.63,;-3.11,-2.72,;-1.62,-2.32,;-.53,-3.41,;-.93,-4.89,;-2.42,-5.29,;-3.51,-4.2,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604062

(US11660293, Example 168 | US11660293, Example 170)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1cc(F)ccc1-n1nccn1 |r,wU:1.0,(.29,4.78,;-1.04,4.01,;-2.37,4.78,;-3.71,4.01,;-3.71,2.47,;-2.37,3.24,;-2.37,1.7,;-1.04,2.47,;.29,1.7,;1.63,2.47,;2.96,1.7,;4.37,2.32,;5.4,1.18,;6.94,1.18,;7.71,-.16,;6.94,-1.49,;7.71,-2.82,;5.4,-1.49,;4.63,-.16,;3.12,.16,;-2.37,.16,;-1.04,-.61,;-3.71,-.61,;-5.04,.16,;-6.37,-.61,;-7.71,.16,;-6.37,-2.15,;-5.04,-2.92,;-3.71,-2.15,;-2.37,-2.92,;-2.21,-4.46,;-.71,-4.78,;.06,-3.44,;-.97,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051293

((S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinol...)Show SMILES CC[C@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074791
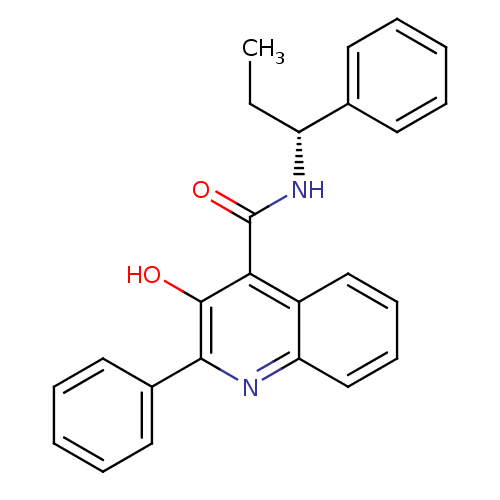
(3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((R...)Show SMILES CC[C@@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50099637

(3-(4-Isobutyryl-piperazin-1-ylmethyl)-2-phenyl-qui...)Show SMILES CC(C)C(=O)N1CCN(Cc2c(nc3ccccc3c2C(=O)N[C@@H](C)C2CCCCC2)-c2ccccc2)CC1 Show InChI InChI=1S/C33H42N4O2/c1-23(2)33(39)37-20-18-36(19-21-37)22-28-30(32(38)34-24(3)25-12-6-4-7-13-25)27-16-10-11-17-29(27)35-31(28)26-14-8-5-9-15-26/h5,8-11,14-17,23-25H,4,6-7,12-13,18-22H2,1-3H3,(H,34,38)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604057

(US11660293, Example 162 | US11660293, Example 164)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccc(F)cc2s1)C(=O)c1nc(C)sc1-c1ncccn1 |r,wU:1.0,(.26,5.29,;-1.08,4.52,;-2.41,5.29,;-3.75,4.52,;-3.75,2.98,;-2.41,3.75,;-2.41,2.21,;-1.08,2.98,;.26,2.21,;1.59,2.98,;2.92,2.21,;4.33,2.84,;5.36,1.7,;6.9,1.7,;7.67,.36,;6.9,-.97,;7.67,-2.31,;5.36,-.97,;4.59,.36,;3.08,.68,;-2.41,.67,;-1.08,-.1,;-3.75,-.1,;-5.15,.53,;-6.18,-.61,;-7.67,-.22,;-5.41,-1.95,;-3.91,-1.63,;-2.82,-2.72,;-3.22,-4.2,;-2.13,-5.29,;-.64,-4.89,;-.24,-3.41,;-1.33,-2.32,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50099626
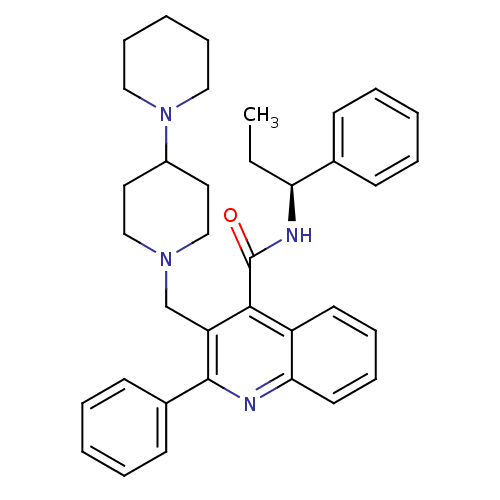
(3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoli...)Show SMILES CC[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H42N4O/c1-2-32(27-14-6-3-7-15-27)38-36(41)34-30-18-10-11-19-33(30)37-35(28-16-8-4-9-17-28)31(34)26-39-24-20-29(21-25-39)40-22-12-5-13-23-40/h3-4,6-11,14-19,29,32H,2,5,12-13,20-26H2,1H3,(H,38,41)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50290869
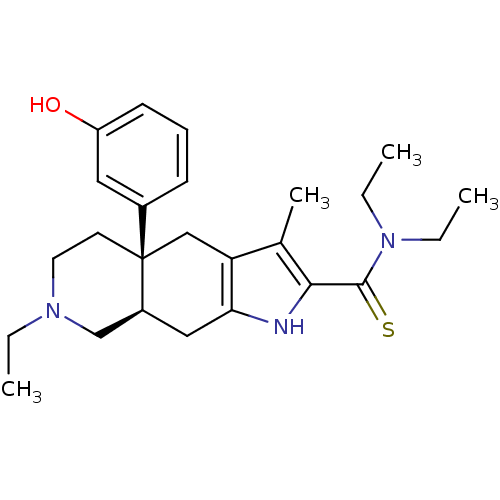
((4aR,8aR)-7-Ethyl-4a-(3-hydroxy-phenyl)-3-methyl-4...)Show SMILES CCN(CC)C(=S)c1[nH]c2C[C@H]3CN(CC)CC[C@@]3(Cc2c1C)c1cccc(O)c1 Show InChI InChI=1S/C25H35N3OS/c1-5-27-12-11-25(18-9-8-10-20(29)13-18)15-21-17(4)23(24(30)28(6-2)7-3)26-22(21)14-19(25)16-27/h8-10,13,19,26,29H,5-7,11-12,14-16H2,1-4H3/t19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
inhibition of [3H]DADLE binding to delta-opioid receptor of mouse brain homogenates |
Bioorg Med Chem Lett 7: 2967-2972 (1997)
Article DOI: 10.1016/S0960-894X(97)10119-6
BindingDB Entry DOI: 10.7270/Q26110VD |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074785
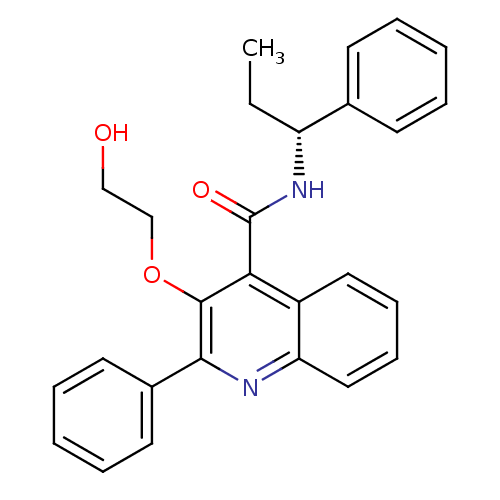
(3-(2-Hydroxy-ethoxy)-2-phenyl-quinoline-4-carboxyl...)Show SMILES CC[C@@H](NC(=O)c1c(OCCO)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H26N2O3/c1-2-22(19-11-5-3-6-12-19)29-27(31)24-21-15-9-10-16-23(21)28-25(26(24)32-18-17-30)20-13-7-4-8-14-20/h3-16,22,30H,2,17-18H2,1H3,(H,29,31)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099632

(3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quin...)Show SMILES CC(C)[C@H](NC(=O)c1c(CN2CCN(CC2)C(C)C)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C34H40N4O/c1-24(2)32(26-13-7-5-8-14-26)36-34(39)31-28-17-11-12-18-30(28)35-33(27-15-9-6-10-16-27)29(31)23-37-19-21-38(22-20-37)25(3)4/h5-18,24-25,32H,19-23H2,1-4H3,(H,36,39)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604066

(US11660293, Example 174)Show SMILES CCOc1ccc(C)nc1C(=O)N1C2CC(C2)[C@@H](C)C1CNc1nc2ccc(F)cc2s1 |r,wU:17.19,(-1.04,-5,;-2.37,-4.23,;-2.37,-2.69,;-3.71,-1.93,;-5.04,-2.69,;-6.37,-1.93,;-6.37,-.38,;-7.71,.38,;-5.04,.38,;-3.71,-.38,;-2.37,.38,;-1.04,-.38,;-2.37,1.93,;-3.71,2.69,;-3.71,4.23,;-2.37,5,;-2.37,3.47,;-1.04,4.23,;.29,5,;-1.04,2.69,;.29,1.93,;1.63,2.69,;2.96,1.93,;4.37,2.55,;5.4,1.41,;6.94,1.41,;7.71,.07,;6.94,-1.26,;7.71,-2.59,;5.4,-1.26,;4.63,.07,;3.12,.39,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM85845
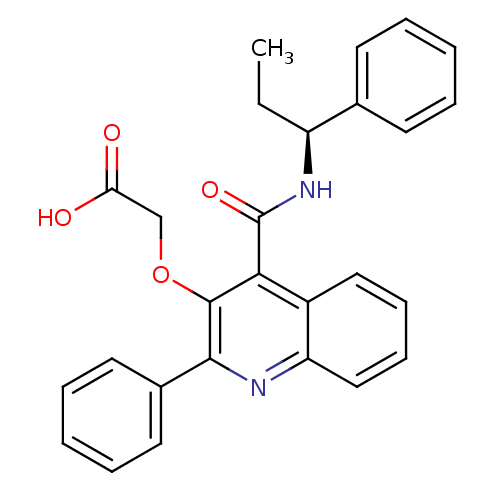
(N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)...)Show SMILES CC[C@H](NC(=O)c1c(OCC(O)=O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H24N2O4/c1-2-21(18-11-5-3-6-12-18)29-27(32)24-20-15-9-10-16-22(20)28-25(19-13-7-4-8-14-19)26(24)33-17-23(30)31/h3-16,21H,2,17H2,1H3,(H,29,32)(H,30,31)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 314-23 (2002)
Article DOI: 10.1124/jpet.300.1.314
BindingDB Entry DOI: 10.7270/Q2SB449D |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50099643

(3-(4-Cyclohexyl-piperazin-1-ylmethyl)-2-phenyl-qui...)Show SMILES CC[C@H](NC(=O)c1c(CN2CCN(CC2)C2CCCCC2)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C36H42N4O/c1-2-32(27-14-6-3-7-15-27)38-36(41)34-30-20-12-13-21-33(30)37-35(28-16-8-4-9-17-28)31(34)26-39-22-24-40(25-23-39)29-18-10-5-11-19-29/h3-4,6-9,12-17,20-21,29,32H,2,5,10-11,18-19,22-26H2,1H3,(H,38,41)/t32-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208035
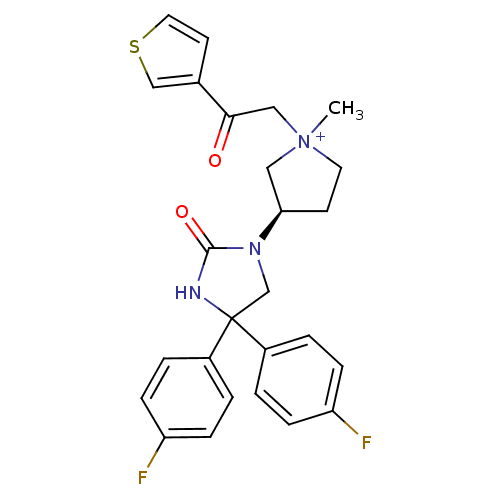
(1-methyl-3-(R)-3-[4,4-bis-(4-fluoro-phenyl)-2-oxo-...)Show SMILES C[N+]1(CC(=O)c2ccsc2)CC[C@H](C1)N1CC(NC1=O)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C26H25F2N3O2S/c1-31(15-24(32)18-11-13-34-16-18)12-10-23(14-31)30-17-26(29-25(30)33,19-2-6-21(27)7-3-19)20-4-8-22(28)9-5-20/h2-9,11,13,16,23H,10,12,14-15,17H2,1H3/p+1/t23-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604069

(US11660293, Example 178 | US11660293, Example 180)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1nc2ccccc2s1)C(=O)c1nc(C)ccc1-n1nccn1 |r,wU:1.0,(.29,4.78,;-1.04,4.01,;-2.37,4.78,;-3.71,4.01,;-3.71,2.47,;-2.37,3.24,;-2.37,1.7,;-1.04,2.47,;.29,1.7,;1.63,2.47,;2.96,1.7,;4.37,2.32,;5.4,1.18,;6.94,1.18,;7.71,-.16,;6.94,-1.49,;5.4,-1.49,;4.63,-.16,;3.12,.16,;-2.37,.16,;-1.04,-.61,;-3.71,-.61,;-5.04,.16,;-6.37,-.61,;-7.71,.16,;-6.37,-2.15,;-5.04,-2.92,;-3.71,-2.15,;-2.37,-2.92,;-2.21,-4.46,;-.71,-4.78,;.06,-3.44,;-.97,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50208038
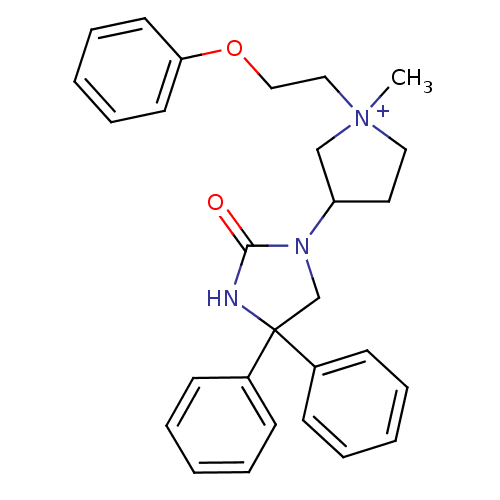
(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCOc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H31N3O2/c1-31(19-20-33-26-15-9-4-10-16-26)18-17-25(21-31)30-22-28(29-27(30)32,23-11-5-2-6-12-23)24-13-7-3-8-14-24/h2-16,25H,17-22H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M2 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50099628

(3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoli...)Show SMILES C[C@H](NC(=O)c1c(CN2CCC(CC2)N2CCCCC2)c(nc2ccccc12)-c1ccccc1)C1CCCCC1 Show InChI InChI=1S/C35H46N4O/c1-26(27-13-5-2-6-14-27)36-35(40)33-30-17-9-10-18-32(30)37-34(28-15-7-3-8-16-28)31(33)25-38-23-19-29(20-24-38)39-21-11-4-12-22-39/h3,7-10,15-18,26-27,29H,2,4-6,11-14,19-25H2,1H3,(H,36,40)/t26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-[MePhe]-NKB binding to hNK-3-CHO (Chinese hamster ovary) membranes |
J Med Chem 44: 1675-89 (2001)
BindingDB Entry DOI: 10.7270/Q29C6WQN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208018
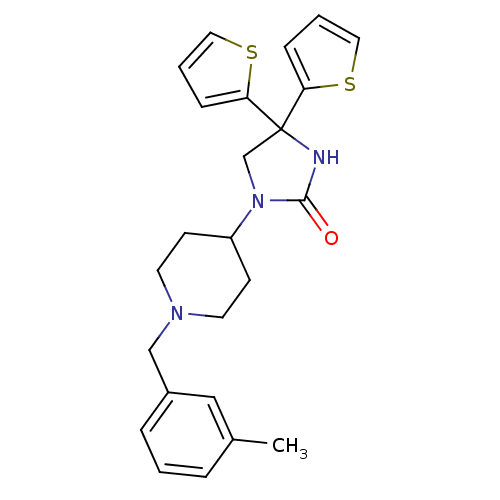
(3-(1-(3-methylbenzyl)-piperidin-4-yl)-5,5-di-(2-th...)Show SMILES Cc1cccc(CN2CCC(CC2)N2CC(NC2=O)(c2cccs2)c2cccs2)c1 Show InChI InChI=1S/C24H27N3OS2/c1-18-5-2-6-19(15-18)16-26-11-9-20(10-12-26)27-17-24(25-23(27)28,21-7-3-13-29-21)22-8-4-14-30-22/h2-8,13-15,20H,9-12,16-17H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHO K1 cells |
J Med Chem 50: 1571-83 (2007)
Article DOI: 10.1021/jm061159a
BindingDB Entry DOI: 10.7270/Q2QV3M61 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50208056

(1-methyl-3-(2-oxo-4,4-diphenyl-imidazolidin-1-yl)-...)Show SMILES C[N+]1(CCCOc2ccccc2)CCC(C1)N1CC(NC1=O)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33N3O2/c1-32(19-11-21-34-27-16-9-4-10-17-27)20-18-26(22-32)31-23-29(30-28(31)33,24-12-5-2-6-13-24)25-14-7-3-8-15-25/h2-10,12-17,26H,11,18-23H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Via Zambeletti 25
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methyl-scopalamine from human muscarinic M3 receptor expressed in CHOK1 cells |
J Med Chem 50: 1693-7 (2007)
Article DOI: 10.1021/jm061160+
BindingDB Entry DOI: 10.7270/Q2M32VF5 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM604132

(US11660293, Example 254 | US11660293, Example 256)Show SMILES C[C@@H]1C2CC(C2)N(C1CNc1cnc(cn1)C(F)(F)F)C(=O)c1cc(C)ccc1-n1nccn1 |r,wU:1.0,(-.1,4.78,;-1.44,4.01,;-2.77,4.78,;-4.1,4.01,;-4.1,2.47,;-2.77,3.24,;-2.77,1.7,;-1.44,2.47,;-.1,1.7,;1.23,2.47,;2.56,1.7,;2.56,.16,;3.9,-.61,;5.28,.13,;5.23,1.7,;3.9,2.47,;6.61,-.64,;7.95,-1.41,;5.84,-1.98,;7.38,.69,;-2.77,.16,;-1.44,-.61,;-4.1,-.61,;-5.44,.16,;-6.77,-.61,;-8.11,.16,;-6.77,-2.15,;-5.44,-2.92,;-4.1,-2.15,;-2.77,-2.92,;-2.61,-4.46,;-1.1,-4.78,;-.33,-3.44,;-1.36,-2.3,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2280CJQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data