 Found 403 hits with Last Name = 'reich' and Initial = 'r'
Found 403 hits with Last Name = 'reich' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant VEGFR2 |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant VEGFR3 |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028965
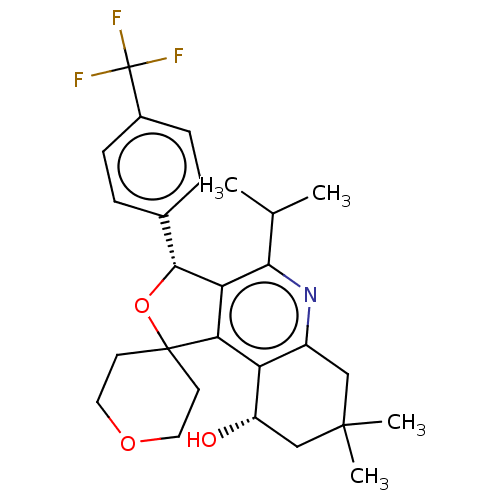
(CHEMBL3360279)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCOCC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H32F3NO3/c1-15(2)23-21-22(20-18(31-23)13-25(3,4)14-19(20)32)26(9-11-33-12-10-26)34-24(21)16-5-7-17(8-6-16)27(28,29)30/h5-8,15,19,24,32H,9-14H2,1-4H3/t19-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146809
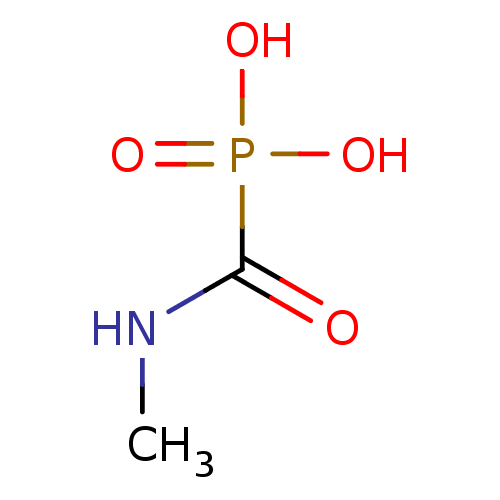
((methylamino)carbonylphosphonic acid | CHEMBL97388)Show InChI InChI=1S/C2H6NO4P/c1-3-2(4)8(5,6)7/h1H3,(H,3,4)(H2,5,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028951
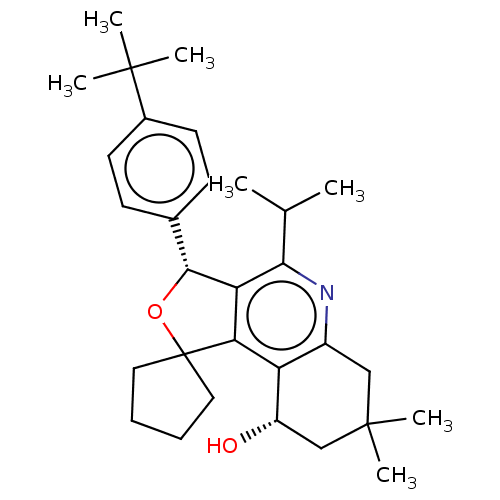
(CHEMBL3359665)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C30H41NO2/c1-18(2)26-24-25(23-21(31-26)16-29(6,7)17-22(23)32)30(14-8-9-15-30)33-27(24)19-10-12-20(13-11-19)28(3,4)5/h10-13,18,22,27,32H,8-9,14-17H2,1-7H3/t22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50243761
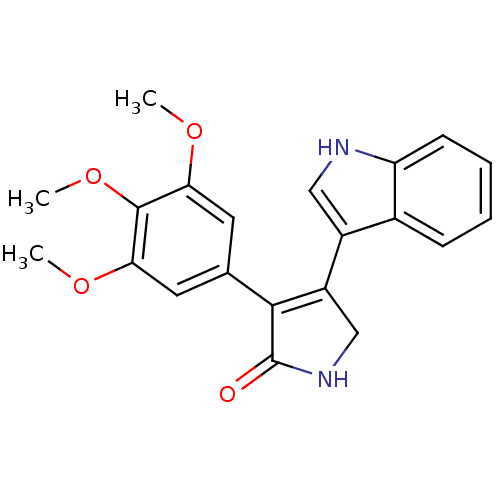
(4-(1H-Indol-3-yl)-3-(3,4,5-trimethoxyphenyl)-1,5-d...)Show SMILES COc1cc(cc(OC)c1OC)C1=C(CNC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H20N2O4/c1-25-17-8-12(9-18(26-2)20(17)27-3)19-15(11-23-21(19)24)14-10-22-16-7-5-4-6-13(14)16/h4-10,22H,11H2,1-3H3,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant VEGFR2 |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028958
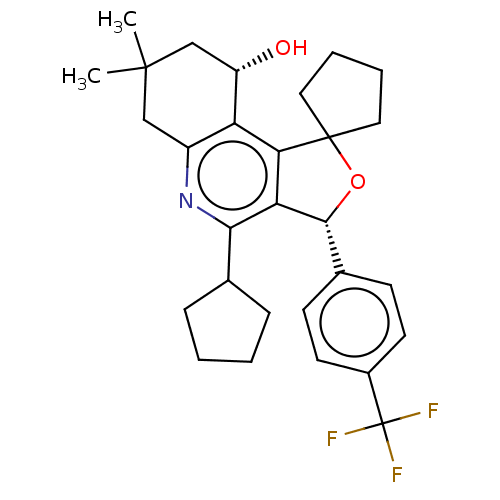
(CHEMBL3360275)Show SMILES CC1(C)C[C@H](O)c2c3c([C@H](OC33CCCC3)c3ccc(cc3)C(F)(F)F)c(nc2C1)C1CCCC1 |r| Show InChI InChI=1S/C29H34F3NO2/c1-27(2)15-20-22(21(34)16-27)24-23(25(33-20)17-7-3-4-8-17)26(35-28(24)13-5-6-14-28)18-9-11-19(12-10-18)29(30,31)32/h9-12,17,21,26,34H,3-8,13-16H2,1-2H3/t21-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM50243761

(4-(1H-Indol-3-yl)-3-(3,4,5-trimethoxyphenyl)-1,5-d...)Show SMILES COc1cc(cc(OC)c1OC)C1=C(CNC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H20N2O4/c1-25-17-8-12(9-18(26-2)20(17)27-3)19-15(11-23-21(19)24)14-10-22-16-7-5-4-6-13(14)16/h4-10,22H,11H2,1-3H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant VEGFR3 |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50146800
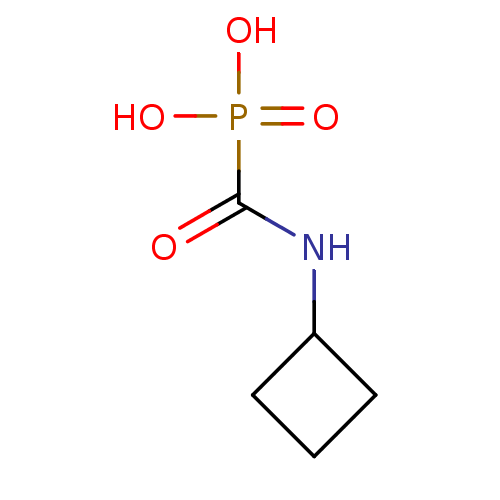
((cyclobutylamino)carbonylphosphonic acid | CHEMBL9...)Show InChI InChI=1S/C5H10NO4P/c7-5(11(8,9)10)6-4-2-1-3-4/h4H,1-3H2,(H,6,7)(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-8 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028959
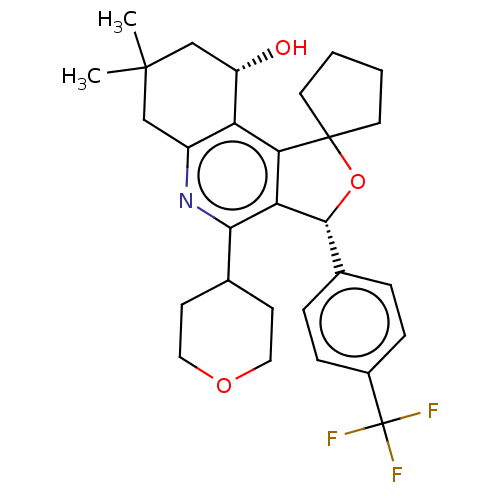
(CHEMBL3360276)Show SMILES CC1(C)C[C@H](O)c2c3c([C@H](OC33CCCC3)c3ccc(cc3)C(F)(F)F)c(nc2C1)C1CCOCC1 |r| Show InChI InChI=1S/C29H34F3NO3/c1-27(2)15-20-22(21(34)16-27)24-23(25(33-20)17-9-13-35-14-10-17)26(36-28(24)11-3-4-12-28)18-5-7-19(8-6-18)29(30,31)32/h5-8,17,21,26,34H,3-4,9-16H2,1-2H3/t21-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028964
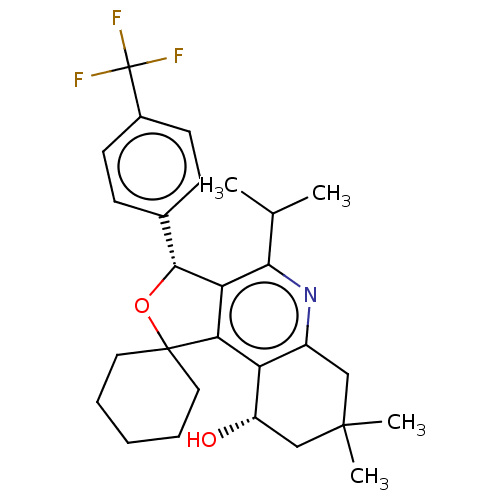
(CHEMBL3360278)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCCC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C28H34F3NO2/c1-16(2)24-22-23(21-19(32-24)14-26(3,4)15-20(21)33)27(12-6-5-7-13-27)34-25(22)17-8-10-18(11-9-17)28(29,30)31/h8-11,16,20,25,33H,5-7,12-15H2,1-4H3/t20-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028950
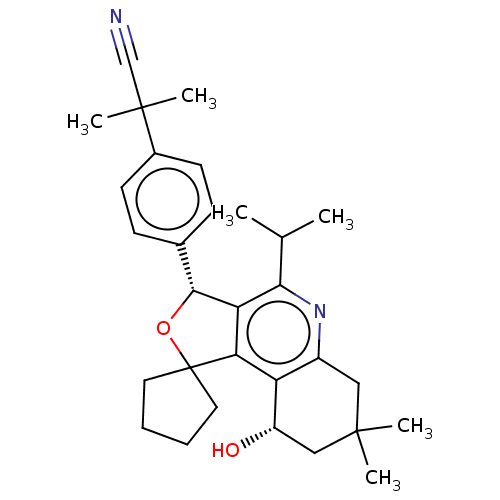
(CHEMBL3359664)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(cc1)C(C)(C)C#N |r| Show InChI InChI=1S/C30H38N2O2/c1-18(2)26-24-25(23-21(32-26)15-28(3,4)16-22(23)33)30(13-7-8-14-30)34-27(24)19-9-11-20(12-10-19)29(5,6)17-31/h9-12,18,22,27,33H,7-8,13-16H2,1-6H3/t22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028952
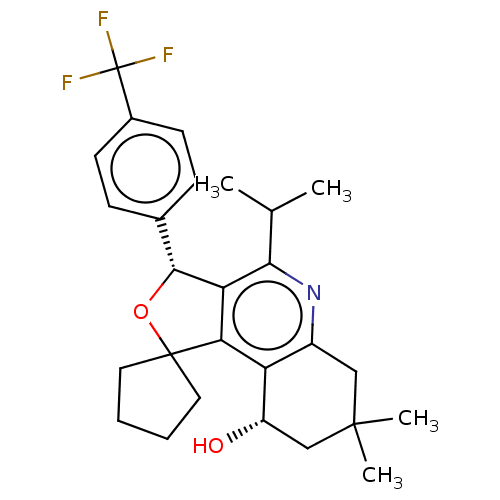
(CHEMBL3360269)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H32F3NO2/c1-15(2)23-21-22(20-18(31-23)13-25(3,4)14-19(20)32)26(11-5-6-12-26)33-24(21)16-7-9-17(10-8-16)27(28,29)30/h7-10,15,19,24,32H,5-6,11-14H2,1-4H3/t19-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146816
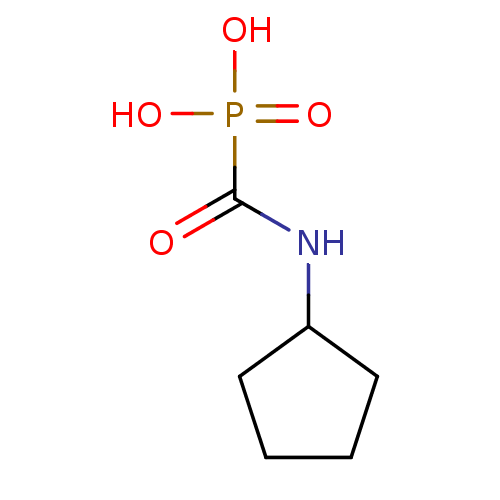
((cyclopentylamino)carbonylphosphonic acid | CHEMBL...)Show InChI InChI=1S/C6H12NO4P/c8-6(12(9,10)11)7-5-3-1-2-4-5/h5H,1-4H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146816

((cyclopentylamino)carbonylphosphonic acid | CHEMBL...)Show InChI InChI=1S/C6H12NO4P/c8-6(12(9,10)11)7-5-3-1-2-4-5/h5H,1-4H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028955
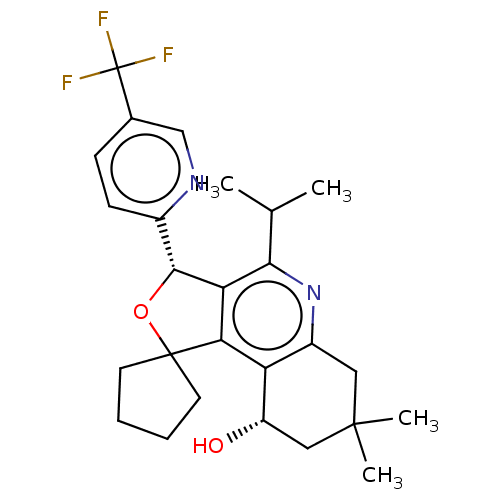
(CHEMBL3360272)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C26H31F3N2O2/c1-14(2)22-20-21(19-17(31-22)11-24(3,4)12-18(19)32)25(9-5-6-10-25)33-23(20)16-8-7-15(13-30-16)26(27,28)29/h7-8,13-14,18,23,32H,5-6,9-12H2,1-4H3/t18-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396791
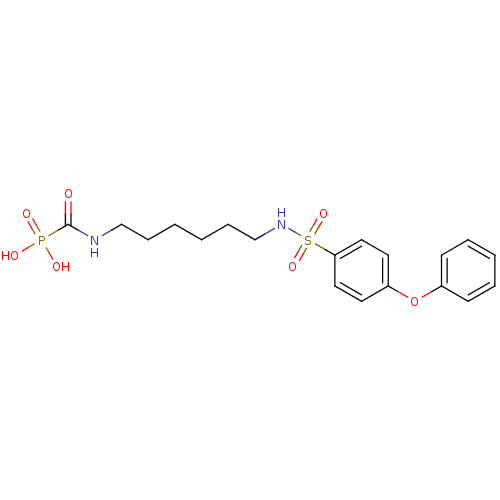
(CHEMBL2172744)Show SMILES OP(O)(=O)C(=O)NCCCCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C19H25N2O7PS/c22-19(29(23,24)25)20-14-6-1-2-7-15-21-30(26,27)18-12-10-17(11-13-18)28-16-8-4-3-5-9-16/h3-5,8-13,21H,1-2,6-7,14-15H2,(H,20,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 2-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396793
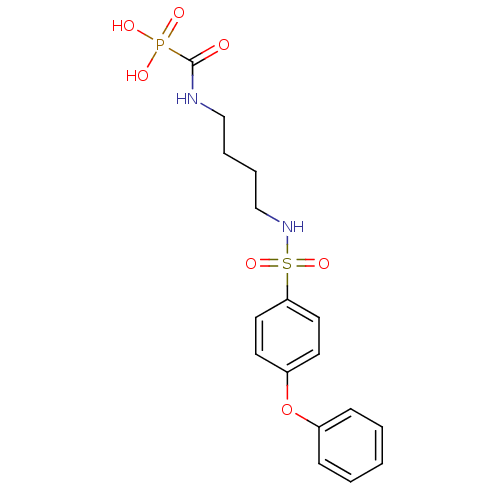
(CHEMBL2170079)Show SMILES OP(O)(=O)C(=O)NCCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C17H21N2O7PS/c20-17(27(21,22)23)18-12-4-5-13-19-28(24,25)16-10-8-15(9-11-16)26-14-6-2-1-3-7-14/h1-3,6-11,19H,4-5,12-13H2,(H,18,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 2-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50146801
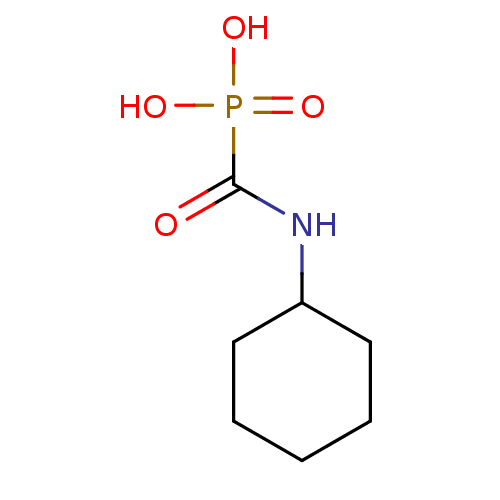
((cyclohexylamino)carbonylphosphonic acid | CHEMBL3...)Show InChI InChI=1S/C7H14NO4P/c9-7(13(10,11)12)8-6-4-2-1-3-5-6/h6H,1-5H2,(H,8,9)(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-1 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028954
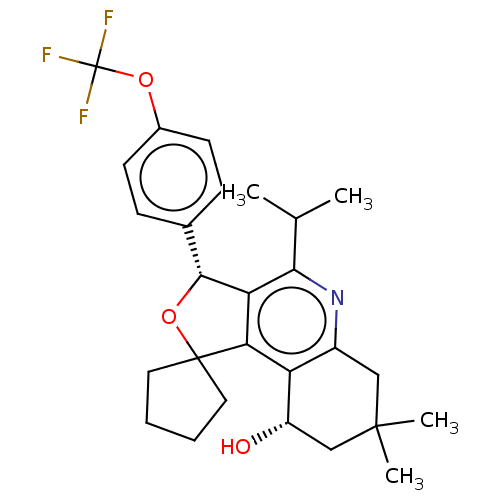
(CHEMBL3360271)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C27H32F3NO3/c1-15(2)23-21-22(20-18(31-23)13-25(3,4)14-19(20)32)26(11-5-6-12-26)34-24(21)16-7-9-17(10-8-16)33-27(28,29)30/h7-10,15,19,24,32H,5-6,11-14H2,1-4H3/t19-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 131 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028956
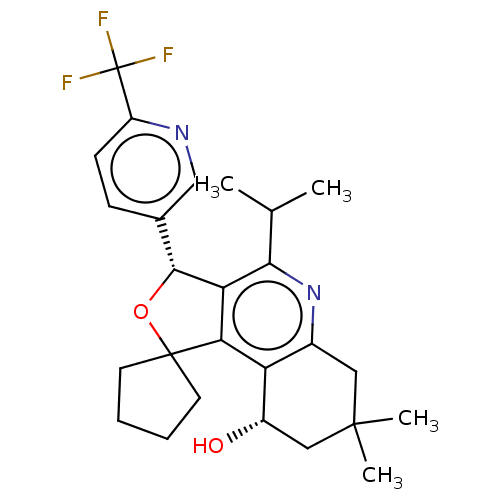
(CHEMBL3360273)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(nc1)C(F)(F)F |r| Show InChI InChI=1S/C26H31F3N2O2/c1-14(2)22-20-21(19-16(31-22)11-24(3,4)12-17(19)32)25(9-5-6-10-25)33-23(20)15-7-8-18(30-13-15)26(27,28)29/h7-8,13-14,17,23,32H,5-6,9-12H2,1-4H3/t17-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 135 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146800

((cyclobutylamino)carbonylphosphonic acid | CHEMBL9...)Show InChI InChI=1S/C5H10NO4P/c7-5(11(8,9)10)6-4-2-1-3-4/h4H,1-3H2,(H,6,7)(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028965

(CHEMBL3360279)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCOCC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H32F3NO3/c1-15(2)23-21-22(20-18(31-23)13-25(3,4)14-19(20)32)26(9-11-33-12-10-26)34-24(21)16-5-7-17(8-6-16)27(28,29)30/h5-8,15,19,24,32H,9-14H2,1-4H3/t19-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 151 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP in presence of 88% human plasma by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50396802
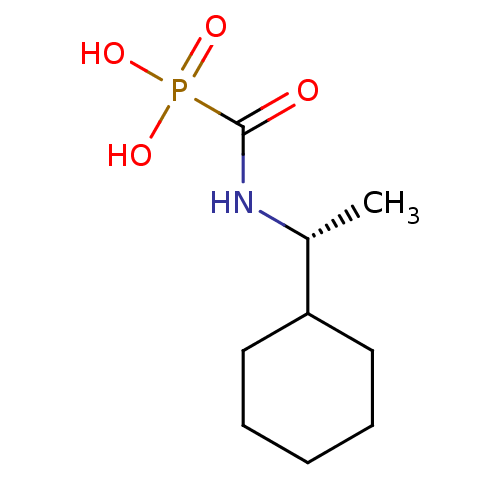
(CHEMBL2172747)Show InChI InChI=1S/C9H18NO4P/c1-7(8-5-3-2-4-6-8)10-9(11)15(12,13)14/h7-8H,2-6H2,1H3,(H,10,11)(H2,12,13,14)/t7-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146805
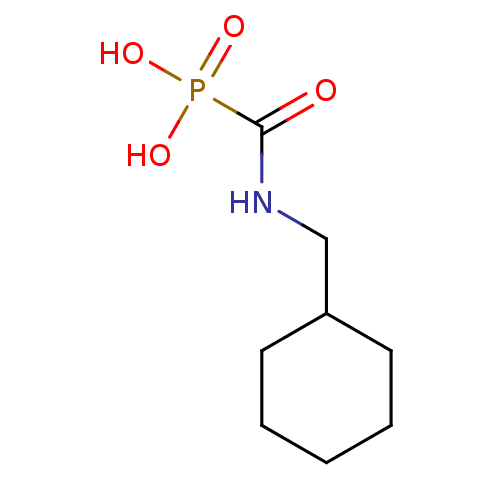
(CHEMBL97951 | [(cyclohexylmethyl)amino]carbonylpho...)Show InChI InChI=1S/C8H16NO4P/c10-8(14(11,12)13)9-6-7-4-2-1-3-5-7/h7H,1-6H2,(H,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of MMP2 |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146805

(CHEMBL97951 | [(cyclohexylmethyl)amino]carbonylpho...)Show InChI InChI=1S/C8H16NO4P/c10-8(14(11,12)13)9-6-7-4-2-1-3-5-7/h7H,1-6H2,(H,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50146815

((cyclopropylamino)carbonylphosphonic acid | CHEMBL...)Show InChI InChI=1S/C4H8NO4P/c6-4(10(7,8)9)5-3-1-2-3/h3H,1-2H2,(H,5,6)(H2,7,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-8 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FLT3 |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146797
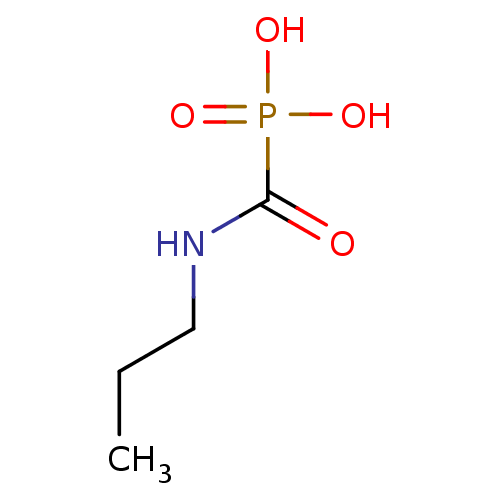
((propylamino)carbonylphosphonic acid | CHEMBL94346)Show InChI InChI=1S/C4H10NO4P/c1-2-3-5-4(6)10(7,8)9/h2-3H2,1H3,(H,5,6)(H2,7,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396792
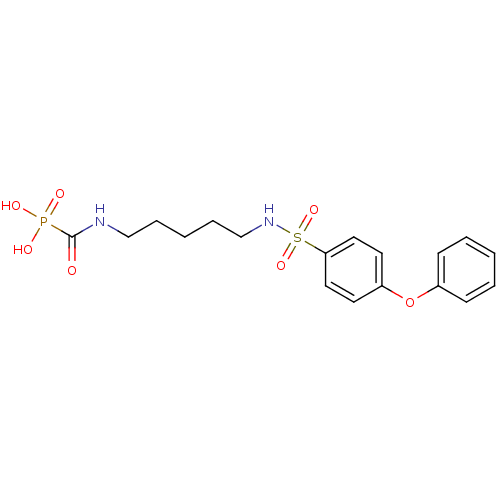
(CHEMBL2170080)Show SMILES OP(O)(=O)C(=O)NCCCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H23N2O7PS/c21-18(28(22,23)24)19-13-5-2-6-14-20-29(25,26)17-11-9-16(10-12-17)27-15-7-3-1-4-8-15/h1,3-4,7-12,20H,2,5-6,13-14H2,(H,19,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 2-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50269746
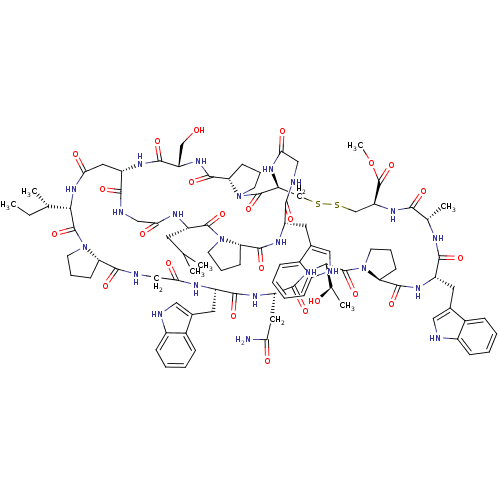
(CHEMBL524883 | methyl (1S,5S,11S,17S,20S,23S,29S,3...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)C[C@@H]2NC(=O)[C@H](CO)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)CNC(=O)[C@@H]3CCCN3C1=O)[C@@H](C)O)C(=O)OC)NC(=O)CNC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)CNC2=O |r| Show InChI InChI=1S/C96H127N23O24S2/c1-8-49(4)79-94(140)118-31-15-25-70(118)88(134)103-44-76(124)105-62(35-53-40-99-59-23-13-10-20-56(53)59)85(131)108-64(37-74(97)122)86(132)115-80(51(6)121)95(141)119-32-18-28-73(119)90(136)111-63(36-54-41-100-60-24-14-11-21-57(54)60)84(130)104-50(5)81(127)113-69(96(142)143-7)47-145-144-46-68-93(139)117-30-17-27-72(117)91(137)112-67(45-120)87(133)109-65(38-75(123)114-79)83(129)102-42-77(125)106-66(33-48(2)3)92(138)116-29-16-26-71(116)89(135)110-61(82(128)101-43-78(126)107-68)34-52-39-98-58-22-12-9-19-55(52)58/h9-14,19-24,39-41,48-51,61-73,79-80,98-100,120-121H,8,15-18,25-38,42-47H2,1-7H3,(H2,97,122)(H,101,128)(H,102,129)(H,103,134)(H,104,130)(H,105,124)(H,106,125)(H,107,126)(H,108,131)(H,109,133)(H,110,135)(H,111,136)(H,112,137)(H,113,127)(H,114,123)(H,115,132)/t49-,50-,51+,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,73-,79-,80-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH and Co. KG
Curated by ChEMBL
| Assay Description
Antagonist activity at human glucagon receptor expressed in BHK21 cells assessed as inhibition of glucagon-induced cAMP elevation by RIA |
J Nat Prod 67: 1528-31 (2004)
Article DOI: 10.1021/np040093o
BindingDB Entry DOI: 10.7270/Q2P84BNR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50396791

(CHEMBL2172744)Show SMILES OP(O)(=O)C(=O)NCCCCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C19H25N2O7PS/c22-19(29(23,24)25)20-14-6-1-2-7-15-21-30(26,27)18-12-10-17(11-13-18)28-16-8-4-3-5-9-16/h3-5,8-13,21H,1-2,6-7,14-15H2,(H,20,22)(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 1-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028957
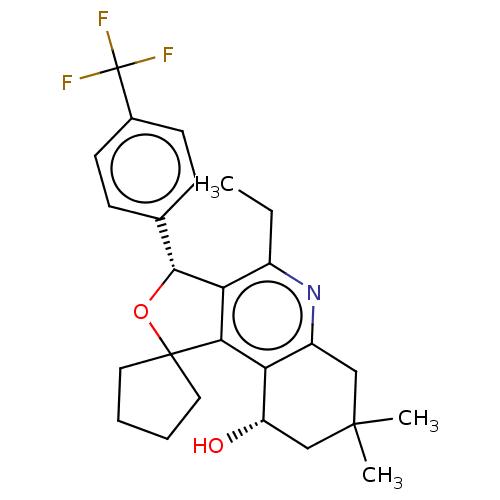
(CHEMBL3360274)Show SMILES CCc1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H30F3NO2/c1-4-17-21-22(20-18(30-17)13-24(2,3)14-19(20)31)25(11-5-6-12-25)32-23(21)15-7-9-16(10-8-15)26(27,28)29/h7-10,19,23,31H,4-6,11-14H2,1-3H3/t19-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Glucagon receptor
(Homo sapiens (Human)) | BDBM50269738
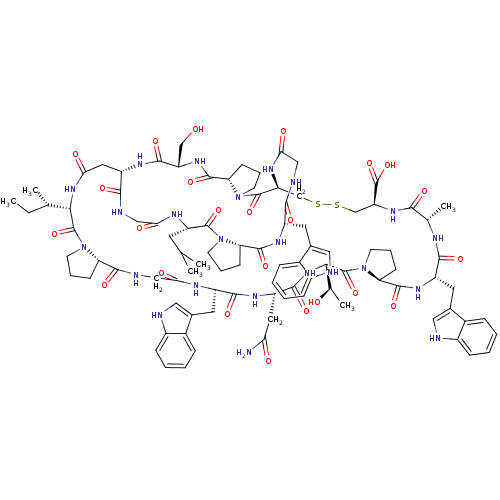
(BI-32169 | CHEMBL526383)Show SMILES CC[C@H](C)[C@@H]1NC(=O)C[C@@H]2NC(=O)[C@H](CO)NC(=O)[C@@H]3CCCN3C(=O)[C@H](CSSC[C@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)[C@@H]3CCCN3C(=O)[C@@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](Cc3c[nH]c4ccccc34)NC(=O)CNC(=O)[C@@H]3CCCN3C1=O)[C@@H](C)O)C(O)=O)NC(=O)CNC(=O)[C@H](Cc1c[nH]c3ccccc13)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)CNC2=O |r| Show InChI InChI=1S/C95H125N23O24S2/c1-7-48(4)78-93(139)117-30-14-24-69(117)87(133)102-43-75(123)104-61(34-52-39-98-58-22-12-9-19-55(52)58)84(130)107-63(36-73(96)121)85(131)114-79(50(6)120)94(140)118-31-17-27-72(118)89(135)110-62(35-53-40-99-59-23-13-10-20-56(53)59)83(129)103-49(5)80(126)112-68(95(141)142)46-144-143-45-67-92(138)116-29-16-26-71(116)90(136)111-66(44-119)86(132)108-64(37-74(122)113-78)82(128)101-41-76(124)105-65(32-47(2)3)91(137)115-28-15-25-70(115)88(134)109-60(81(127)100-42-77(125)106-67)33-51-38-97-57-21-11-8-18-54(51)57/h8-13,18-23,38-40,47-50,60-72,78-79,97-99,119-120H,7,14-17,24-37,41-46H2,1-6H3,(H2,96,121)(H,100,127)(H,101,128)(H,102,133)(H,103,129)(H,104,123)(H,105,124)(H,106,125)(H,107,130)(H,108,132)(H,109,134)(H,110,135)(H,111,136)(H,112,126)(H,113,122)(H,114,131)(H,141,142)/t48-,49-,50+,60-,61-,62-,63-,64-,65-,66-,67-,68-,69-,70-,71-,72-,78-,79-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH and Co. KG
Curated by ChEMBL
| Assay Description
Antagonist activity at human glucagon receptor expressed in BHK21 cells assessed as inhibition of glucagon-induced cAMP elevation by RIA |
J Nat Prod 67: 1528-31 (2004)
Article DOI: 10.1021/np040093o
BindingDB Entry DOI: 10.7270/Q2P84BNR |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50146815

((cyclopropylamino)carbonylphosphonic acid | CHEMBL...)Show InChI InChI=1S/C4H8NO4P/c6-4(10(7,8)9)5-3-1-2-3/h3H,1-2H2,(H,5,6)(H2,7,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-2 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50146816

((cyclopentylamino)carbonylphosphonic acid | CHEMBL...)Show InChI InChI=1S/C6H12NO4P/c8-6(12(9,10)11)7-5-3-1-2-4-5/h5H,1-4H2,(H,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-1 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50146802
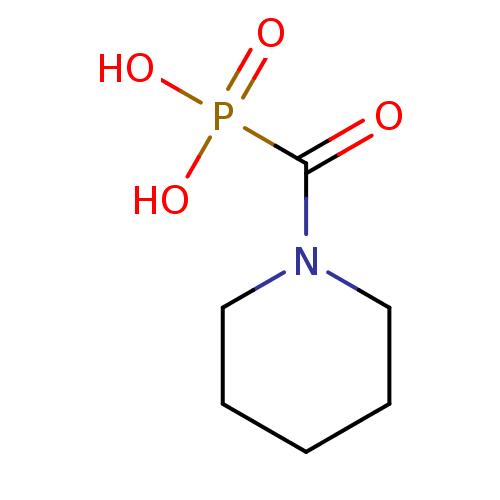
(CHEMBL95068 | piperidin-1-ylcarbonylphosphonic aci...)Show InChI InChI=1S/C6H12NO4P/c8-6(12(9,10)11)7-4-2-1-3-5-7/h1-5H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant matrix metalloprotease-9 was determined |
J Med Chem 47: 2826-32 (2004)
Article DOI: 10.1021/jm030386z
BindingDB Entry DOI: 10.7270/Q2XS5W4C |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50396792

(CHEMBL2170080)Show SMILES OP(O)(=O)C(=O)NCCCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H23N2O7PS/c21-18(28(22,23)24)19-13-5-2-6-14-20-29(25,26)17-11-9-16(10-12-17)27-15-7-3-1-4-8-15/h1,3-4,7-12,20H,2,5-6,13-14H2,(H,19,21)(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 1-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028953
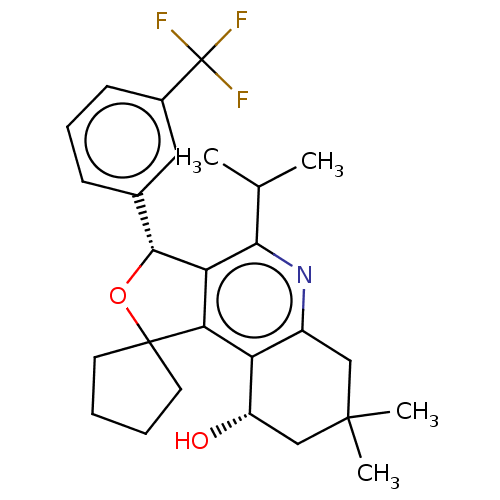
(CHEMBL3360270)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H32F3NO2/c1-15(2)23-21-22(20-18(31-23)13-25(3,4)14-19(20)32)26(10-5-6-11-26)33-24(21)16-8-7-9-17(12-16)27(28,29)30/h7-9,12,15,19,24,32H,5-6,10-11,13-14H2,1-4H3/t19-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028960
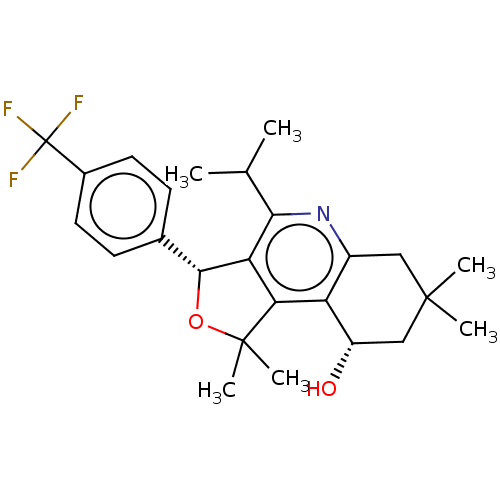
(CHEMBL3360277)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC2(C)C)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C25H30F3NO2/c1-13(2)21-19-20(18-16(29-21)11-23(3,4)12-17(18)30)24(5,6)31-22(19)14-7-9-15(10-8-14)25(26,27)28/h7-10,13,17,22,30H,11-12H2,1-6H3/t17-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 548 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028966
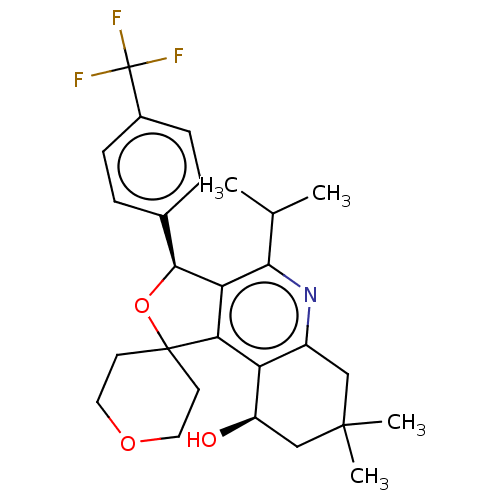
(CHEMBL3360280)Show SMILES CC(C)c1nc2CC(C)(C)C[C@@H](O)c2c2c1[C@@H](OC21CCOCC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H32F3NO3/c1-15(2)23-21-22(20-18(31-23)13-25(3,4)14-19(20)32)26(9-11-33-12-10-26)34-24(21)16-5-7-17(8-6-16)27(28,29)30/h5-8,15,19,24,32H,9-14H2,1-4H3/t19-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 576 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396794
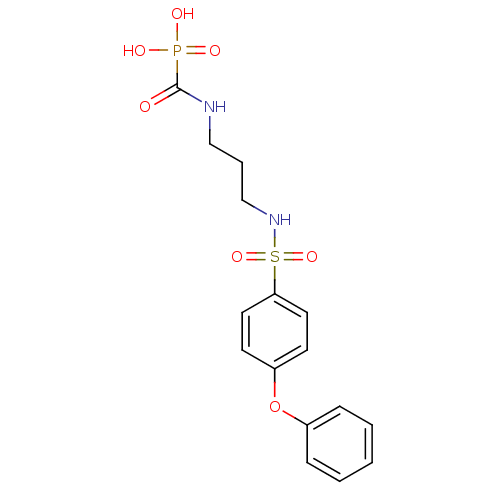
(CHEMBL2170078)Show SMILES OP(O)(=O)C(=O)NCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C16H19N2O7PS/c19-16(26(20,21)22)17-11-4-12-18-27(23,24)15-9-7-14(8-10-15)25-13-5-2-1-3-6-13/h1-3,5-10,18H,4,11-12H2,(H,17,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 2-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50396790
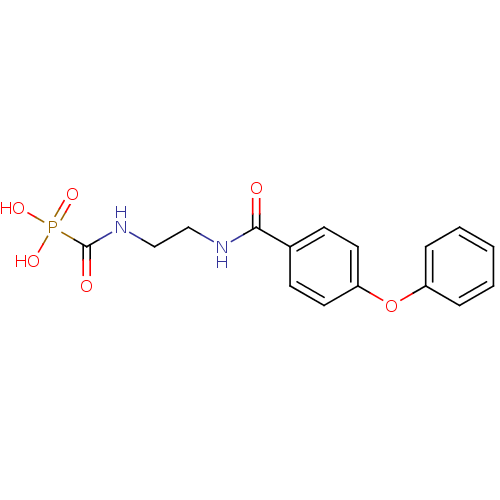
(CHEMBL2172745)Show InChI InChI=1S/C16H17N2O6P/c19-15(17-10-11-18-16(20)25(21,22)23)12-6-8-14(9-7-12)24-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,17,19)(H,18,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 2-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50396790

(CHEMBL2172745)Show InChI InChI=1S/C16H17N2O6P/c19-15(17-10-11-18-16(20)25(21,22)23)12-6-8-14(9-7-12)24-13-4-2-1-3-5-13/h1-9H,10-11H2,(H,17,19)(H,18,20)(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 1-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FAK |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDGFRbeta |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50396794

(CHEMBL2170078)Show SMILES OP(O)(=O)C(=O)NCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C16H19N2O7PS/c19-16(26(20,21)22)17-11-4-12-18-27(23,24)15-9-7-14(8-10-15)25-13-5-2-1-3-6-13/h1-3,5-10,18H,4,11-12H2,(H,17,19)(H2,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 1-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Angiopoietin-1 receptor
(Homo sapiens (Human)) | BDBM47167

(CHEMBL201511 | US8957103, Z1 | US9364459, A)Show SMILES COc1cc(cc(OC)c1OC)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12 |t:13| Show InChI InChI=1S/C21H18N2O5/c1-26-15-8-11(9-16(27-2)19(15)28-3)17-18(21(25)23-20(17)24)13-10-22-14-7-5-4-6-12(13)14/h4-10,22H,1-3H3,(H,23,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant TIE2 |
J Med Chem 51: 3814-24 (2008)
Article DOI: 10.1021/jm8001185
BindingDB Entry DOI: 10.7270/Q2057FQS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50396793

(CHEMBL2170079)Show SMILES OP(O)(=O)C(=O)NCCCCNS(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C17H21N2O7PS/c20-17(27(21,22)23)18-12-4-5-13-19-28(24,25)16-10-8-15(9-11-16)26-14-6-2-1-3-7-14/h1-3,6-11,19H,4-5,12-13H2,(H,18,20)(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
The Hebrew University of Jerusalem
Curated by ChEMBL
| Assay Description
Inhibition of human Carbonic anhydrase 1-mediated CO2 hydration by stopped-flow method |
J Med Chem 55: 7875-82 (2012)
Article DOI: 10.1021/jm300981b
BindingDB Entry DOI: 10.7270/Q23T9JBB |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50028948
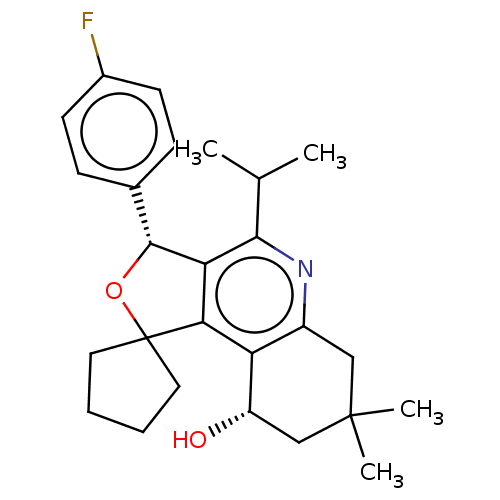
(CHEMBL3359662)Show SMILES CC(C)c1nc2CC(C)(C)C[C@H](O)c2c2c1[C@H](OC21CCCC1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C26H32FNO2/c1-15(2)23-21-22(20-18(28-23)13-25(3,4)14-19(20)29)26(11-5-6-12-26)30-24(21)16-7-9-17(27)10-8-16/h7-10,15,19,24,29H,5-6,11-14H2,1-4H3/t19-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 796 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH& Co. KG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CETP by fluorescence transfer assay |
J Med Chem 57: 8766-76 (2014)
Article DOI: 10.1021/jm500431d
BindingDB Entry DOI: 10.7270/Q2HT2QX3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data