

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479471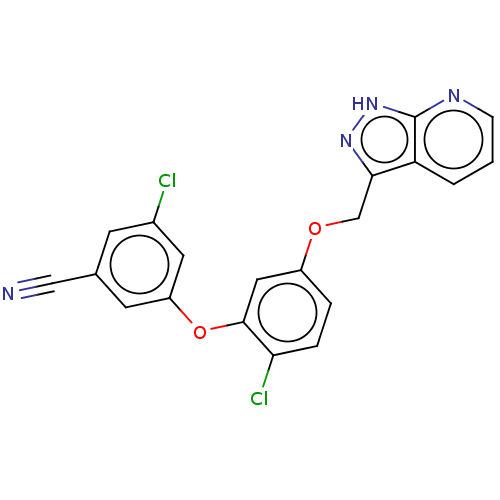 (CHEMBL491019 | MK-1107) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203205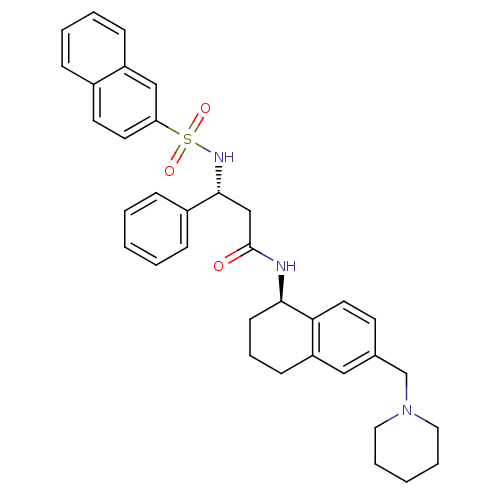 ((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50479470 (CHEMBL489586 | MK-4965) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484635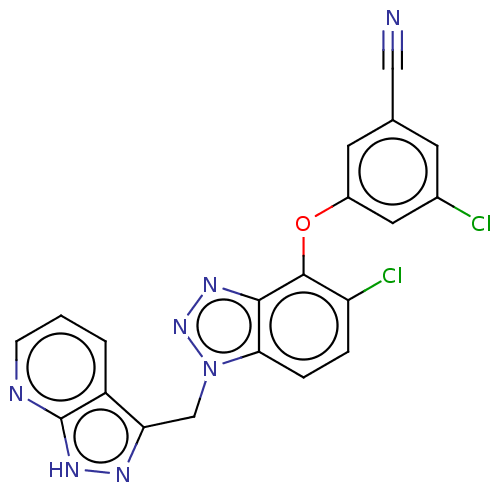 (CHEMBL1939500) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107460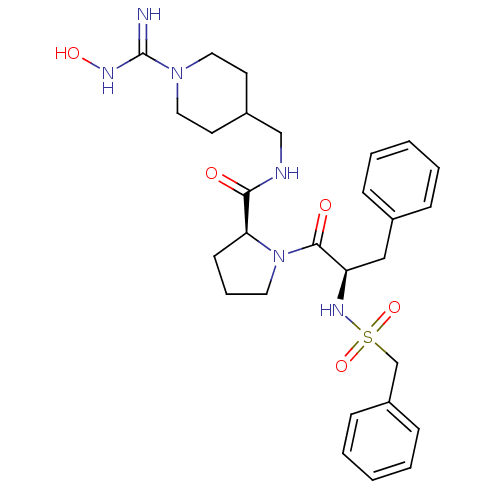 ((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484632 (Mk-6186 | Mk6186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203200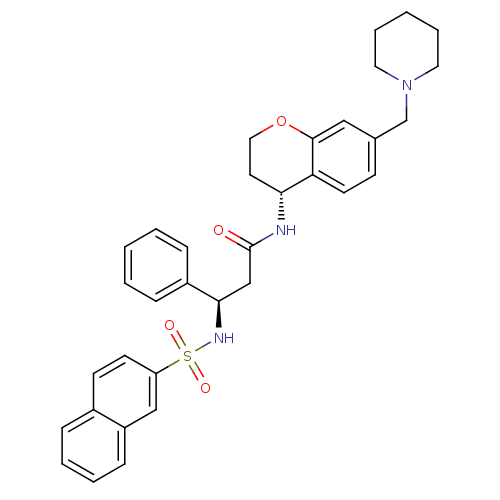 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449917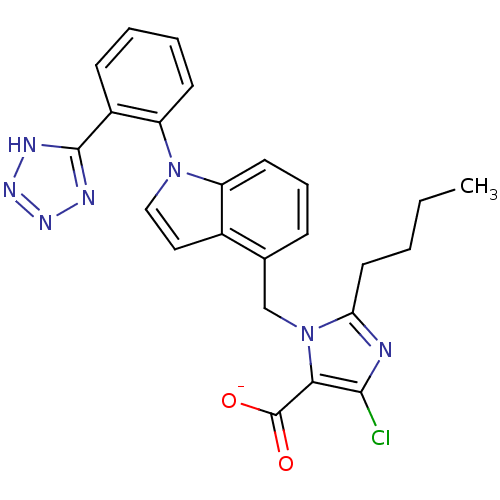 (BMS-180560 | CHEMBL2021417) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484629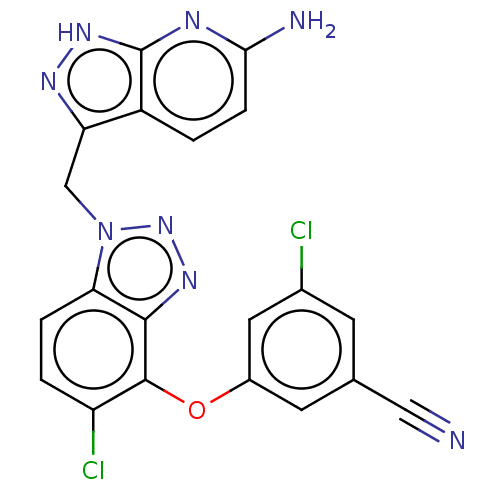 (CHEMBL1939503) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203199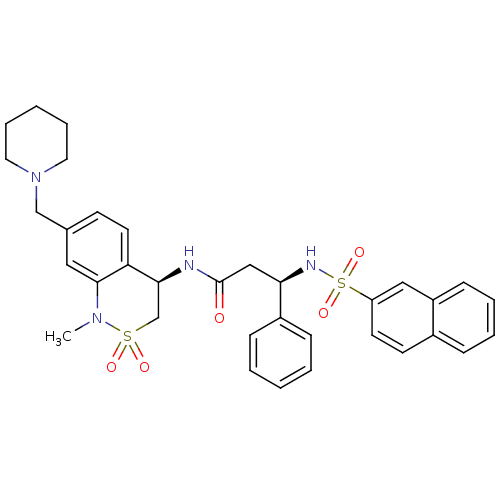 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203211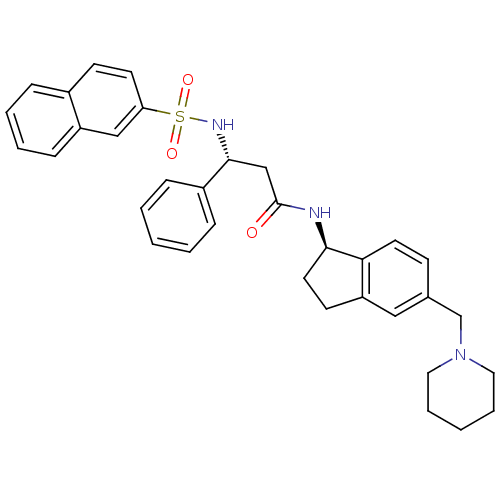 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484630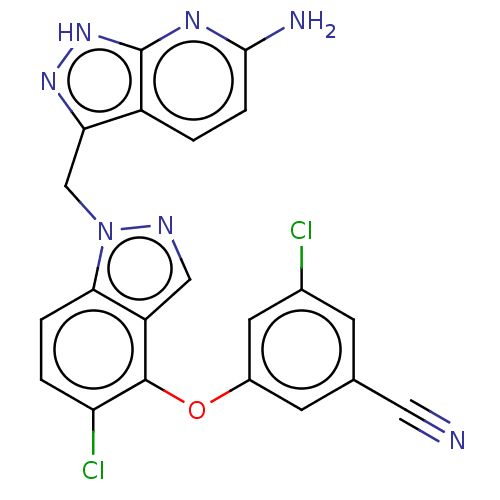 (CHEMBL1939502 | MK-7445) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24772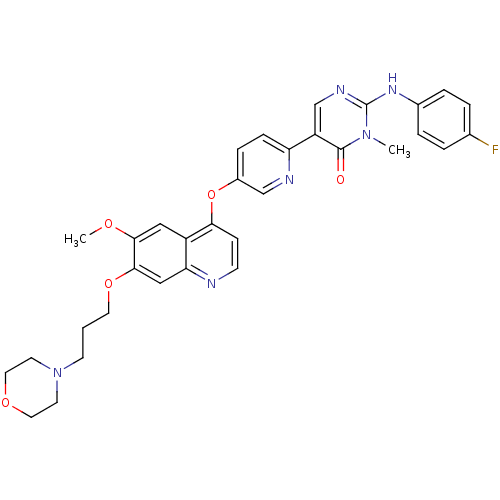 (2-[(4-fluorophenyl)amino]-5-[5-({6-methoxy-7-[3-(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.40 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50366780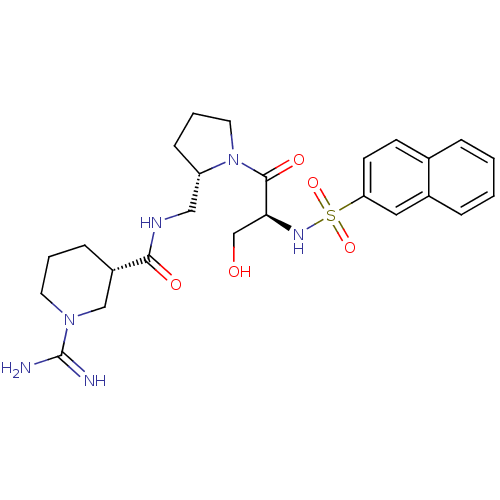 (BMS-189090 | CHEMBL138877) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro reversible inhibition of thrombin catalytic activity | Bioorg Med Chem Lett 12: 41-4 (2001) BindingDB Entry DOI: 10.7270/Q2MP53T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484624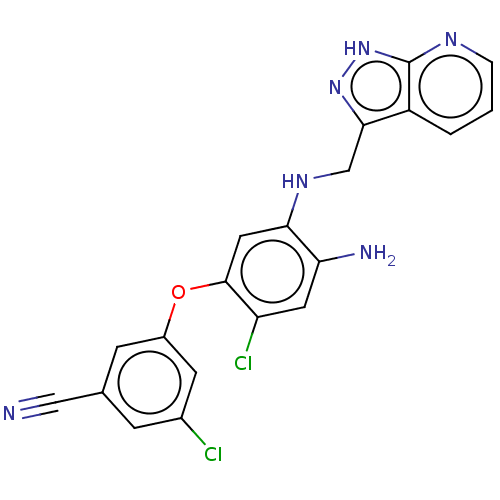 (CHEMBL1939510) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484631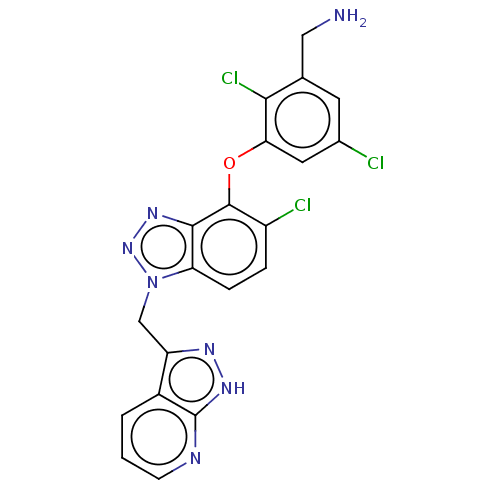 (CHEMBL1939501) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50452209 (CHEMBL4207777 | US10647727, Example 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity to PDE2 (unknown origin) by SPR analysis | Bioorg Med Chem Lett 27: 5167-5171 (2017) Article DOI: 10.1016/j.bmcl.2017.10.054 BindingDB Entry DOI: 10.7270/Q28C9ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449914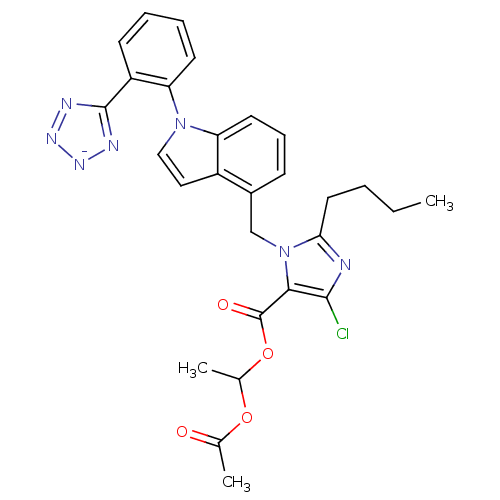 (CHEMBL2079784) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24769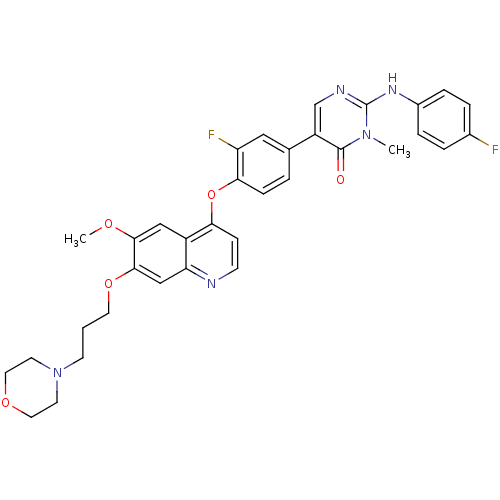 (5-[3-fluoro-4-({6-methoxy-7-[3-(morpholin-4-yl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 4.90 | -47.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484634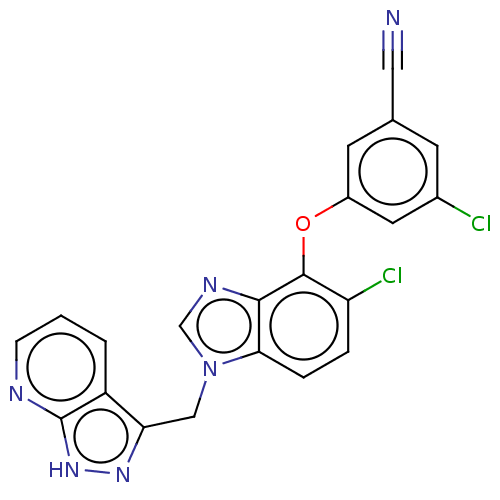 (CHEMBL1939504) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50452200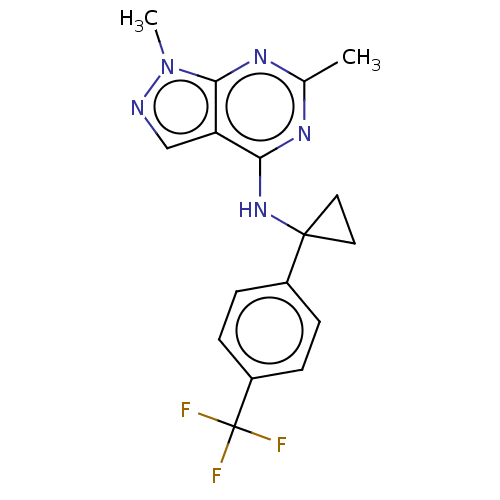 (CHEMBL4212416 | US10647727, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity to PDE2 (unknown origin) by SPR analysis | Bioorg Med Chem Lett 27: 5167-5171 (2017) Article DOI: 10.1016/j.bmcl.2017.10.054 BindingDB Entry DOI: 10.7270/Q28C9ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50366331 (CHEMBL1790055) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50200932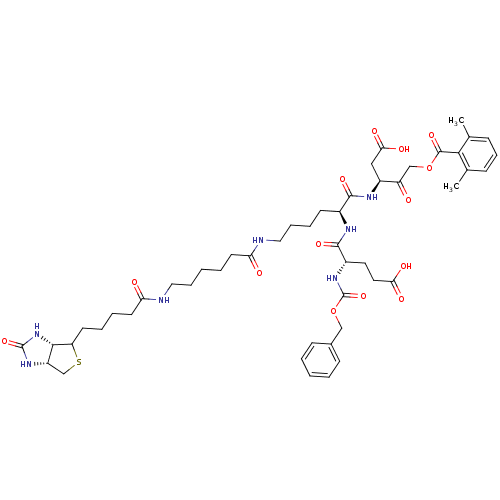 ((3S)-3-[(2S)-6-(6-{5-[(3aS,6aR)-2-oxo-hexahydro-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Edinburgh Curated by ChEMBL | Assay Description Inhibition of human caspase 1 | J Med Chem 49: 7636-45 (2006) Article DOI: 10.1021/jm060385h BindingDB Entry DOI: 10.7270/Q2319VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449919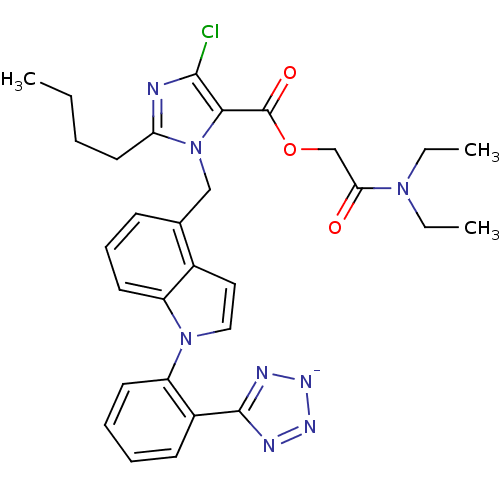 (CHEMBL2021415) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449910 (CHEMBL2079782) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50107463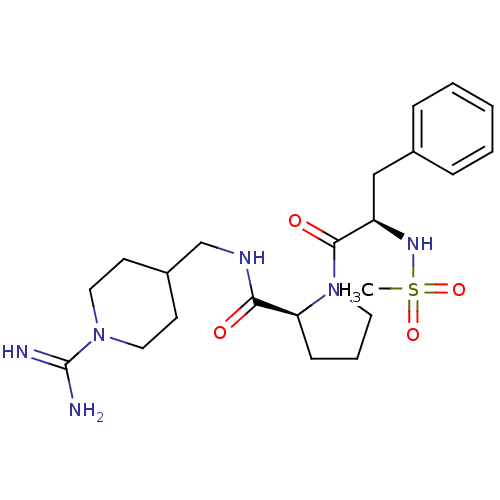 ((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Competitive kinetic for human alpha thrombin inhibition Ki was determined | Bioorg Med Chem Lett 12: 45-9 (2001) BindingDB Entry DOI: 10.7270/Q2GX4C3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24751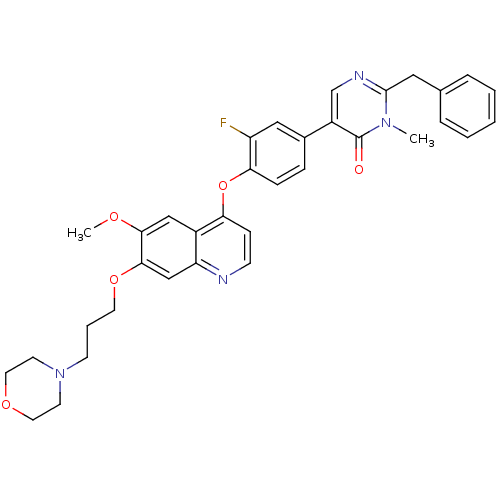 (2-benzyl-5-[3-fluoro-4-({6-methoxy-7-[3-(morpholin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.90 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449912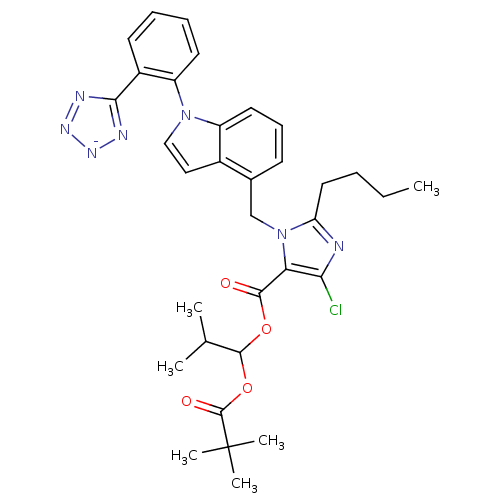 (CHEMBL2079781) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24754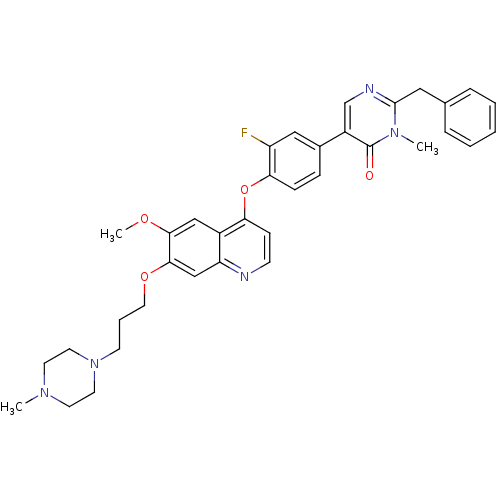 (2-benzyl-5-[3-fluoro-4-({6-methoxy-7-[3-(4-methylp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.20 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24764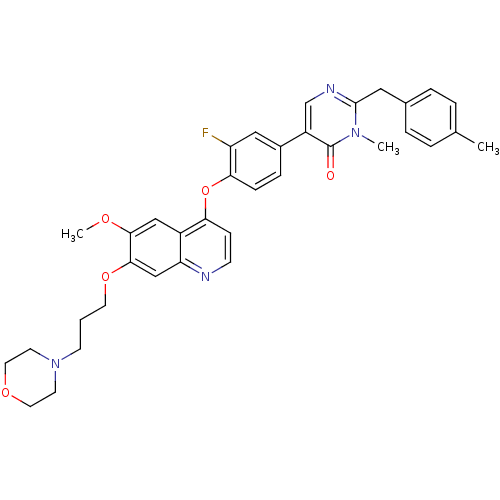 (5-[3-fluoro-4-({6-methoxy-7-[3-(morpholin-4-yl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 9.60 | -45.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24752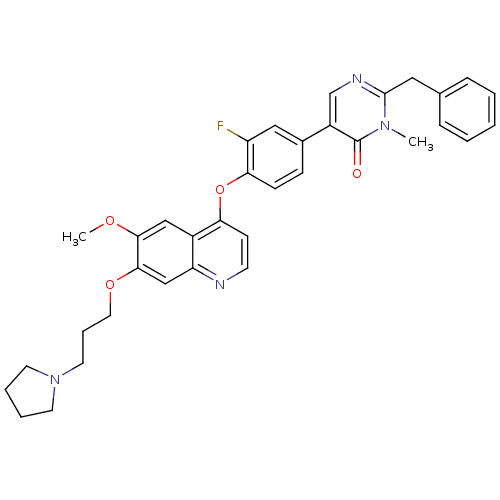 (2-benzyl-5-[3-fluoro-4-({6-methoxy-7-[3-(pyrrolidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -44.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449909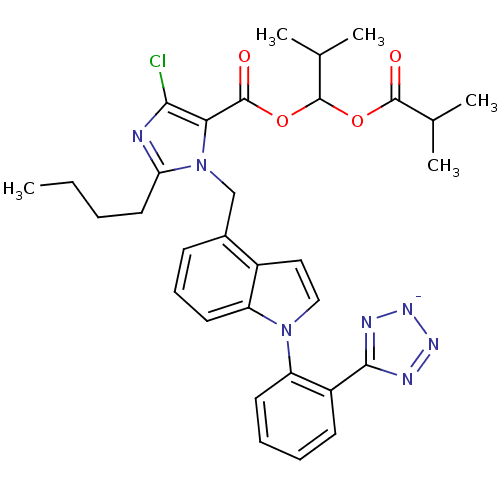 (CHEMBL2079769) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50452211 (CHEMBL4216198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity to PDE2 (unknown origin) by SPR analysis | Bioorg Med Chem Lett 27: 5167-5171 (2017) Article DOI: 10.1016/j.bmcl.2017.10.054 BindingDB Entry DOI: 10.7270/Q28C9ZV3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24768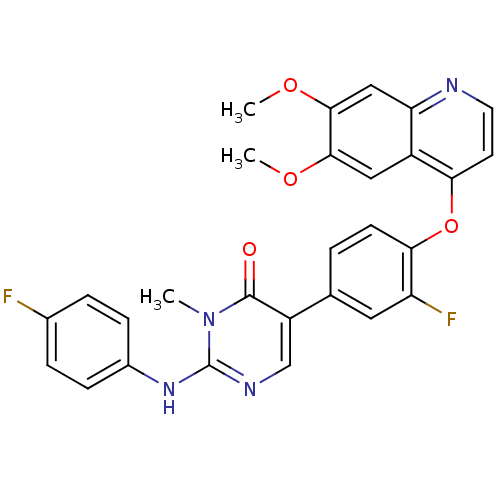 (5-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]-3-fluorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | -44.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203210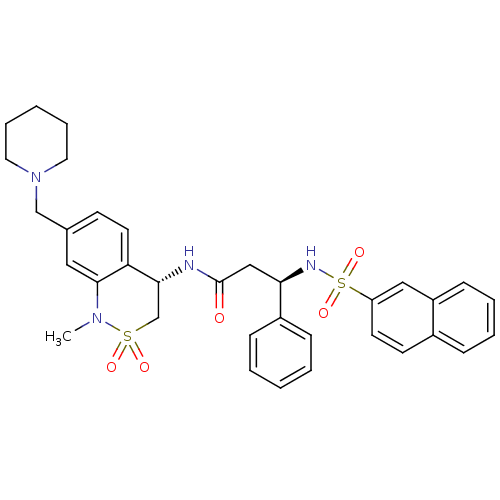 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203206 ((R)-3-(naphthalene-3-sulfonamido)-3-phenyl-N-((R)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24765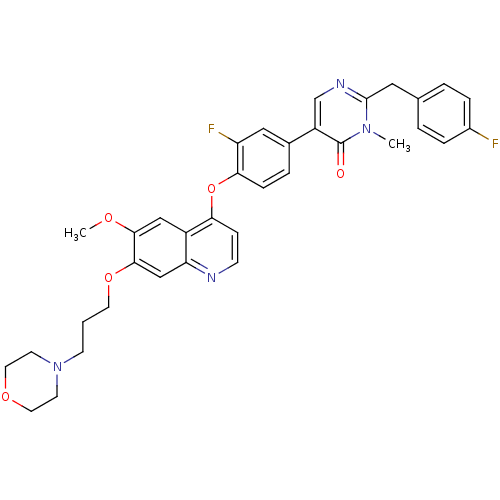 (5-[3-fluoro-4-({6-methoxy-7-[3-(morpholin-4-yl)pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203197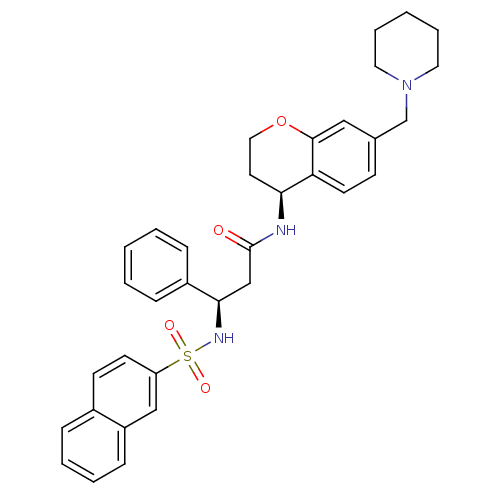 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50452216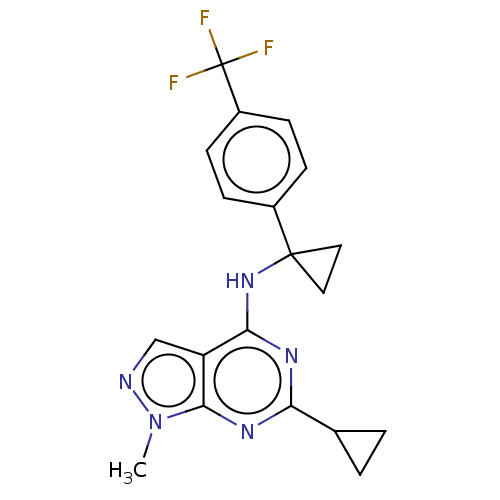 (CHEMBL4215537 | US10647727, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity to PDE2 (unknown origin) by SPR analysis | Bioorg Med Chem Lett 27: 5167-5171 (2017) Article DOI: 10.1016/j.bmcl.2017.10.054 BindingDB Entry DOI: 10.7270/Q28C9ZV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24755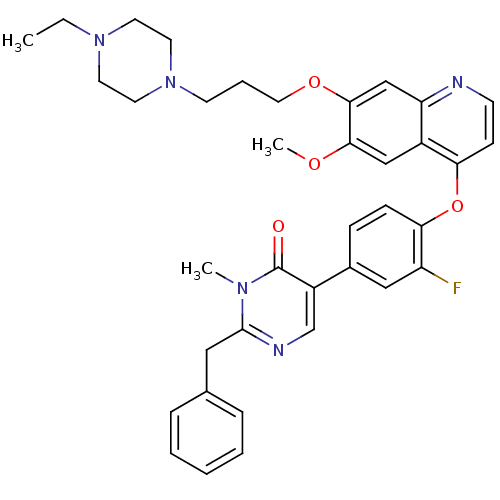 (2-benzyl-5-[4-({7-[3-(4-ethylpiperazin-1-yl)propox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449918 (BMS-181688 | CHEMBL2021416) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484633 (CHEMBL1939507) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Co. Curated by ChEMBL | Assay Description Inhibition of Human immunodeficiency virus reverse transcriptase K103N mutant by SPA assay | J Med Chem 54: 7920-33 (2011) Article DOI: 10.1021/jm2010173 BindingDB Entry DOI: 10.7270/Q2W66PMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24753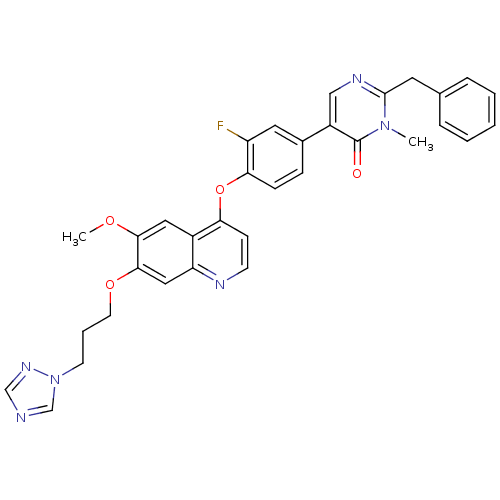 (2-benzyl-5-[3-fluoro-4-({6-methoxy-7-[3-(1H-1,2,4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24760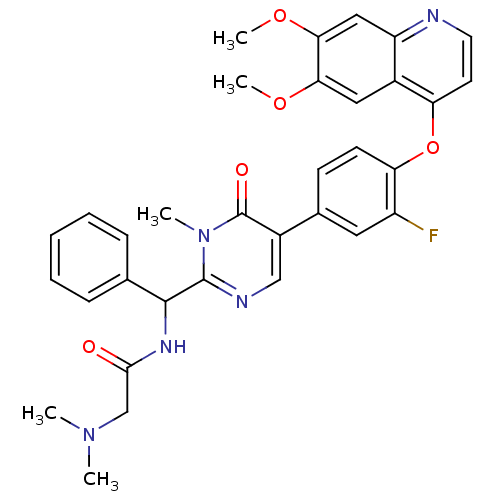 (N-[(5-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]-3-fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449920 (CHEMBL2079768) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50203208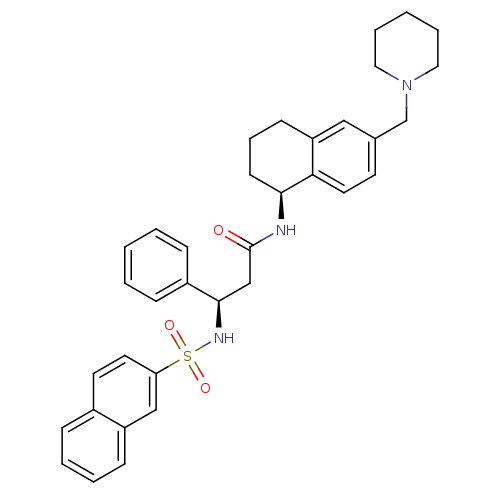 ((R)-3-(naphthalene-7-sulfonamido)-3-phenyl-N-((S)-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells | J Med Chem 50: 607-10 (2007) Article DOI: 10.1021/jm061224g BindingDB Entry DOI: 10.7270/Q2KP81T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24756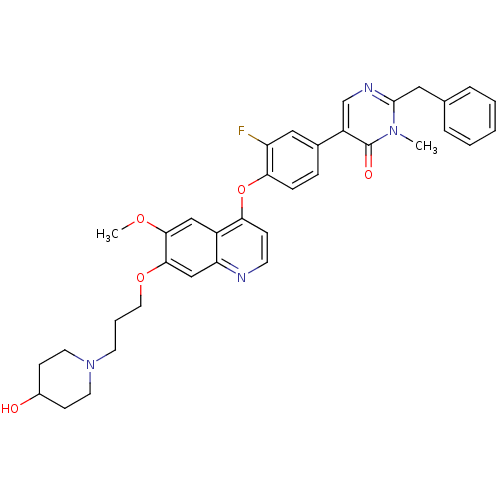 (2-benzyl-5-[3-fluoro-4-({7-[3-(4-hydroxypiperidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 33 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24770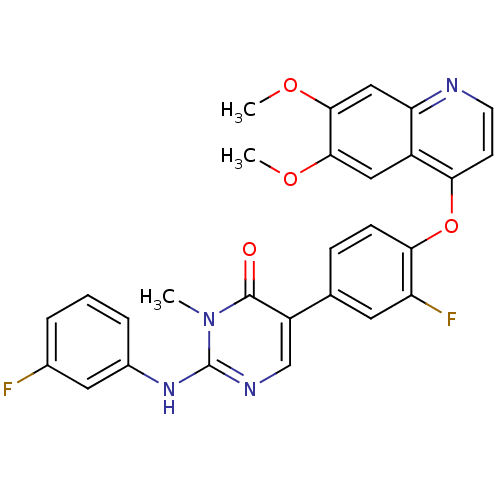 (5-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]-3-fluorophe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 38 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM24750 (2-benzyl-5-{4-[(6,7-dimethoxyquinolin-4-yl)oxy]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 39 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Amgen | Assay Description In vitro kinase assays were done to establish IC50 values against recombinant enzymes using homogeneous time-resolved fluorescence (HTRF) assay. For ... | J Med Chem 51: 5766-79 (2008) Article DOI: 10.1021/jm8006189 BindingDB Entry DOI: 10.7270/Q23X84XN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50449911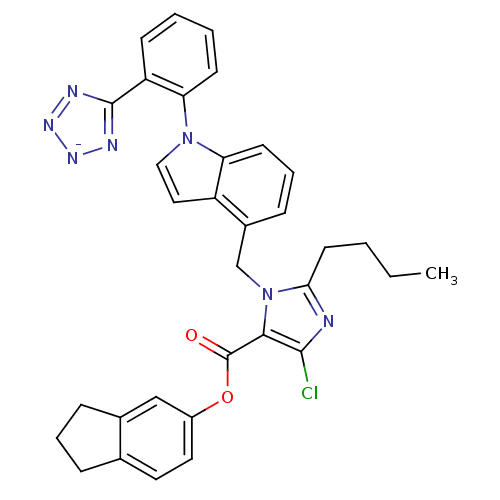 (CHEMBL2079770) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Angiotensin II receptor, type 1 determined using [125I]- labeled [Sar1,Ile8] angiotensin II in rat adrenocortical membranes | Bioorg Med Chem Lett 4: 145-150 (1994) Article DOI: 10.1016/S0960-894X(01)81137-9 BindingDB Entry DOI: 10.7270/Q2V988KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 366 total ) | Next | Last >> |