

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50495454 (CHEMBL3108923) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50495447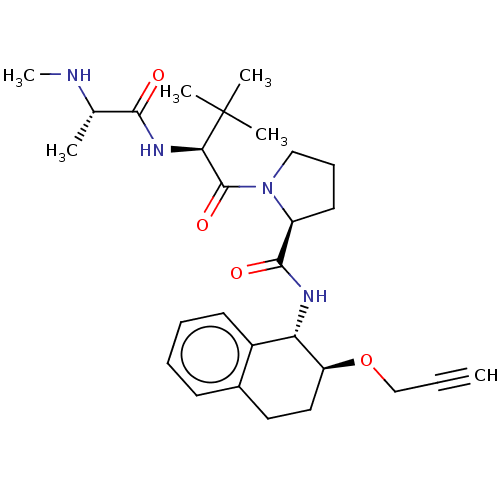 (CHEMBL3108925) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50495451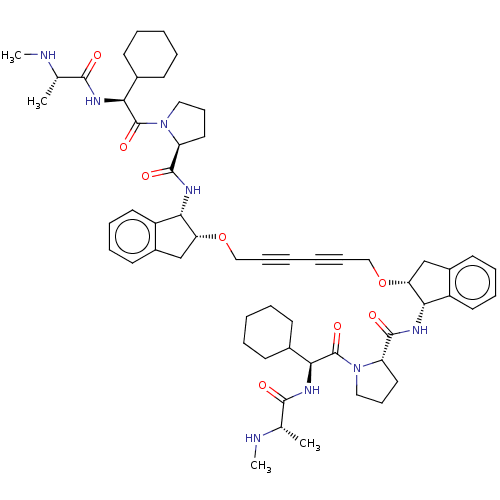 (CHEMBL3108827) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50495451 (CHEMBL3108827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50495454 (CHEMBL3108923) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50495451 (CHEMBL3108827) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged cIAP2 BIR3 domain (I235 to A336) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50495451 (CHEMBL3108827) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR2 domain (R214 to P260) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50113978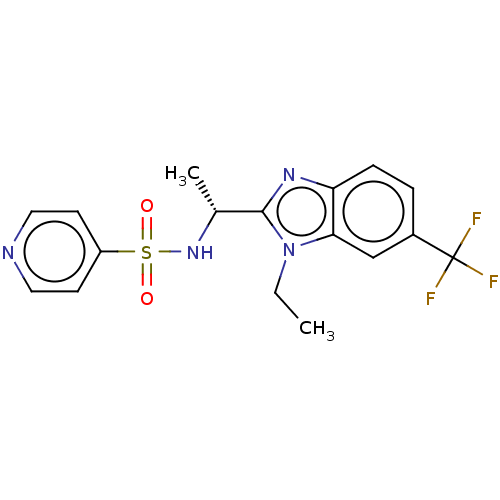 (CHEMBL3605543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50495446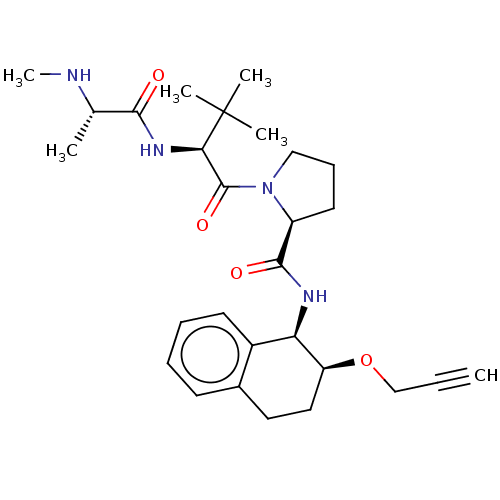 (CHEMBL3108924) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50495447 (CHEMBL3108925) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50114058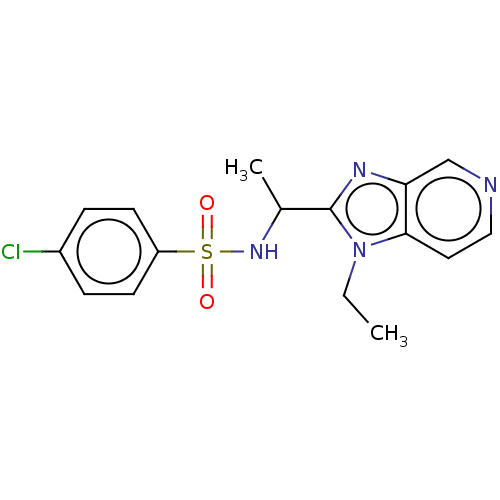 (CHEMBL3605528) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50495441 (CHEMBL3108926) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged cIAP1 BIR3 domain (L250 to G350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50239422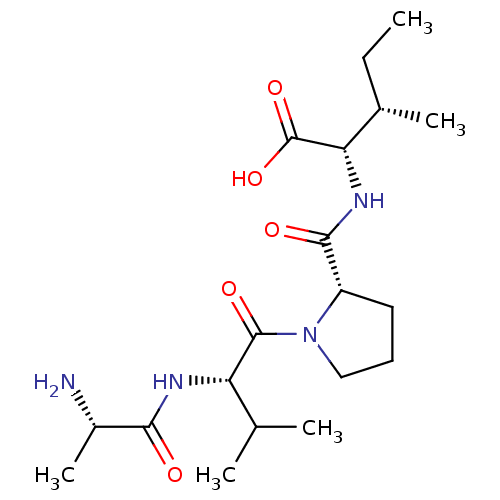 (CHEMBL234346) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of GST-tagged XIAP BIR3 domain (unknown origin)-biotinylated Smac 7-mer peptide interaction by fluorescent microvolume assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50495446 (CHEMBL3108924) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50113995 (CHEMBL3605542) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50113995 (CHEMBL3605542) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50114058 (CHEMBL3605528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50495441 (CHEMBL3108926) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of AbuRPFK-5FAM from GST-tagged XIAP BIR3 domain (N252 to E350) (unknown origin) after 20 mins by fluorescence polarization assay | J Med Chem 56: 9897-919 (2013) Article DOI: 10.1021/jm401075x BindingDB Entry DOI: 10.7270/Q23T9M6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50113979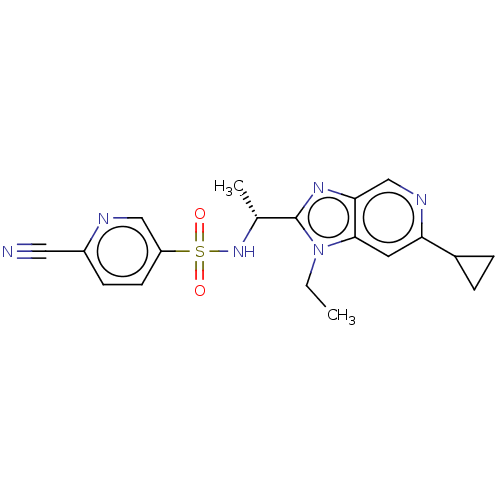 (CHEMBL3605558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50113979 (CHEMBL3605558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50113980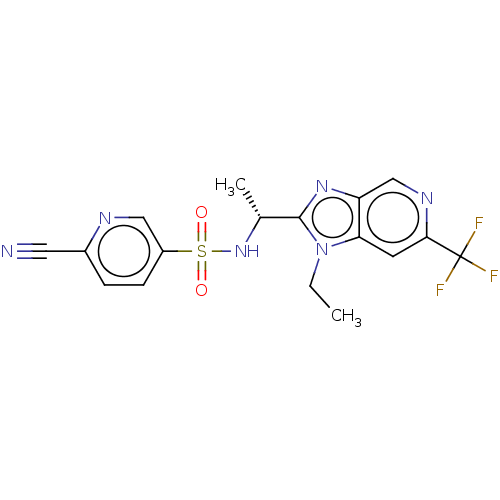 (CHEMBL3605557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50113979 (CHEMBL3605558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50113980 (CHEMBL3605557) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP3A5 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50113980 (CHEMBL3605557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50113979 (CHEMBL3605558) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50113979 (CHEMBL3605558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50113979 (CHEMBL3605558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50113980 (CHEMBL3605557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50113980 (CHEMBL3605557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50113980 (CHEMBL3605557) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes incubated for 5 mins in presence of NADPH and specific substrates by LC/MS/MS method | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50113980 (CHEMBL3605557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50113979 (CHEMBL3605558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human ERG | J Med Chem 58: 7057-75 (2015) Article DOI: 10.1021/acs.jmedchem.5b01078 BindingDB Entry DOI: 10.7270/Q2Z89F6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069633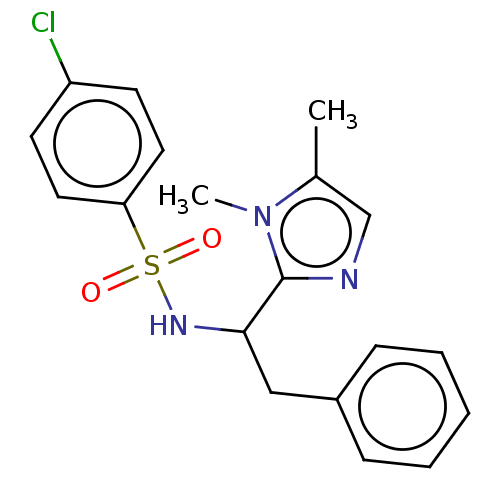 (CHEMBL3402541) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069634 (CHEMBL3402542) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069635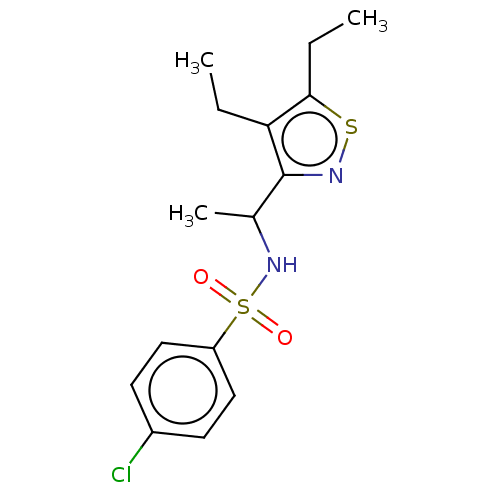 (CHEMBL3402543) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069636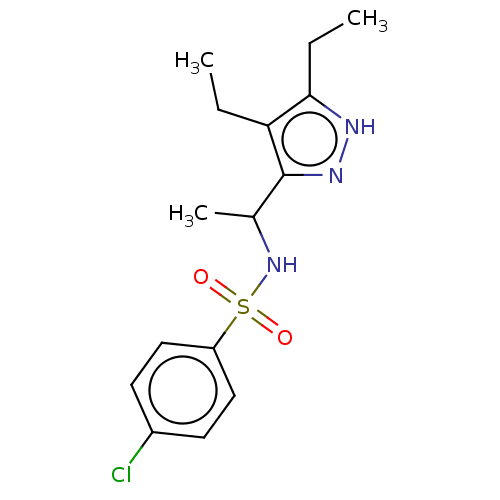 (CHEMBL3402544) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 310 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069637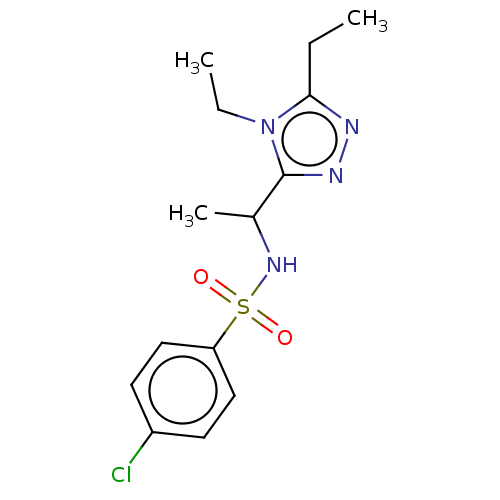 (CHEMBL3402545) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069638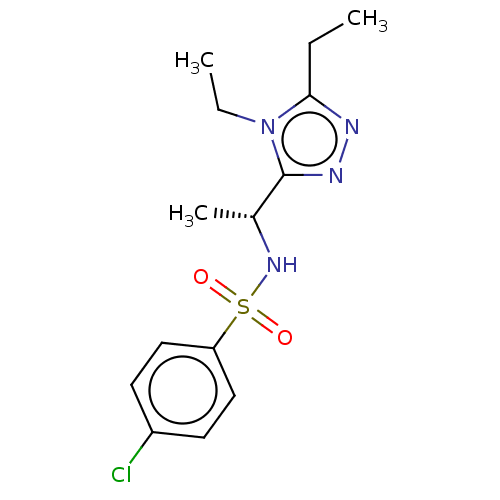 (CHEMBL3402546) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 250 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069639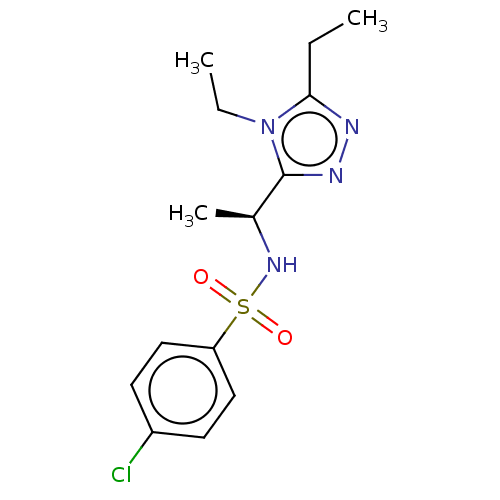 (CHEMBL3402547) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069640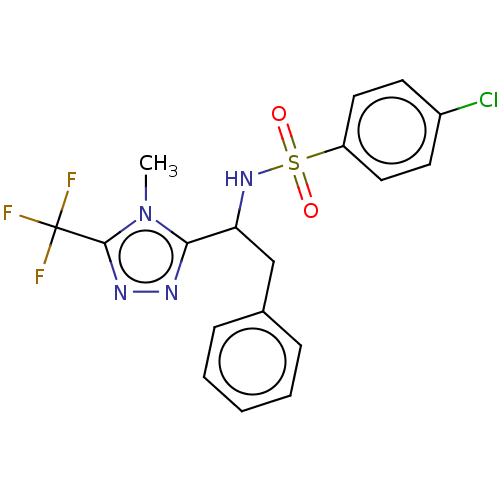 (CHEMBL3402527) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.17E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069641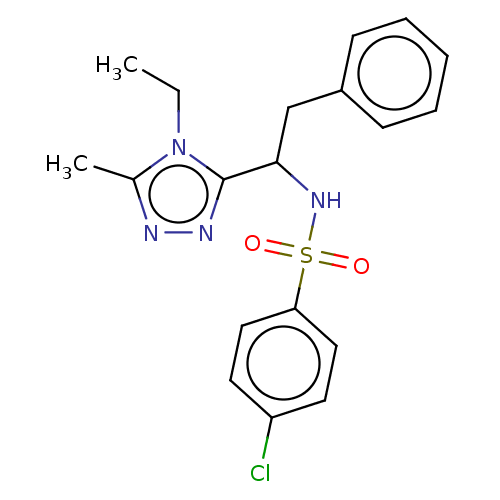 (CHEMBL3402528) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 460 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069660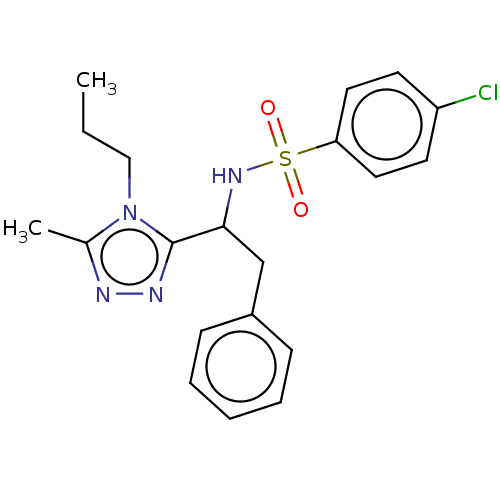 (CHEMBL3402529) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069661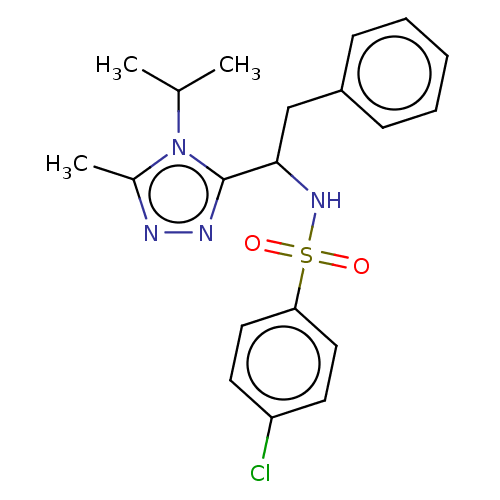 (CHEMBL3402530) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069662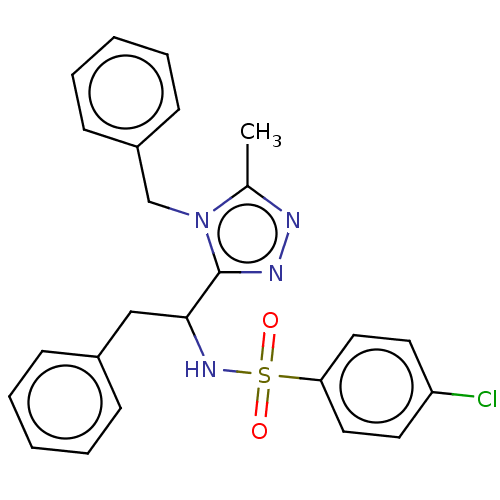 (CHEMBL3402531) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069663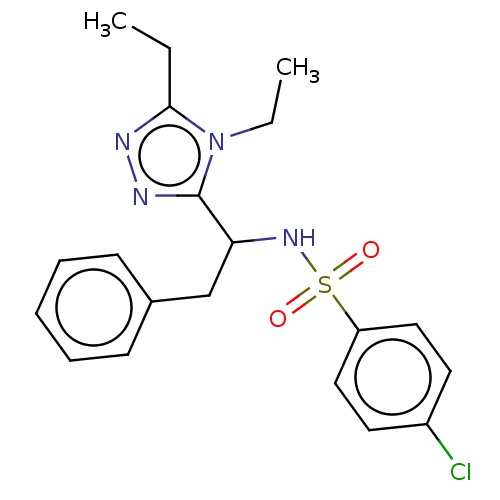 (CHEMBL3402532) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069669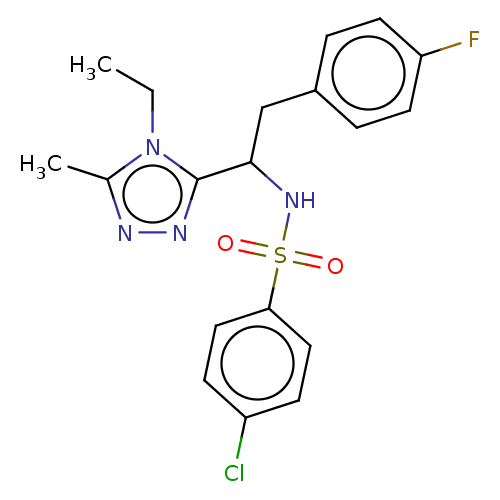 (CHEMBL3402533) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069670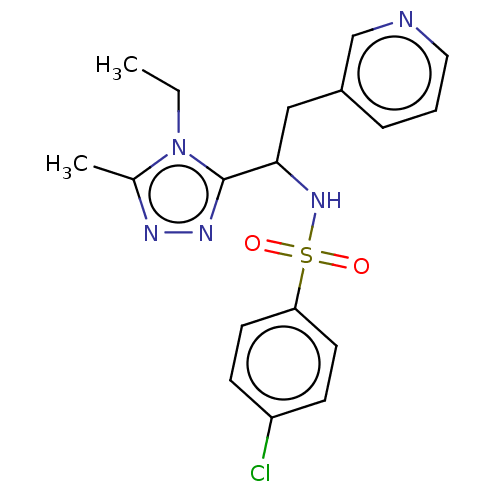 (CHEMBL3402534) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.37E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069671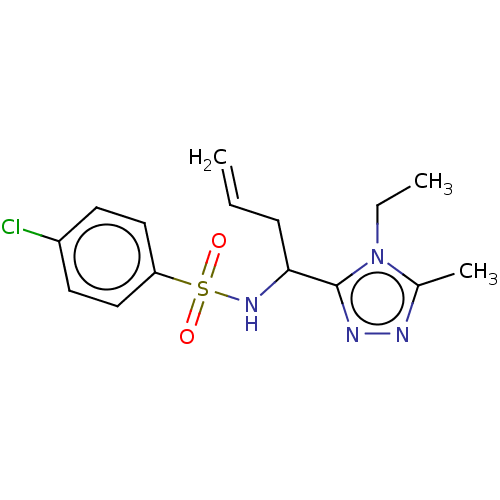 (CHEMBL3402535) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069672 (CHEMBL3402536) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.19E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50069674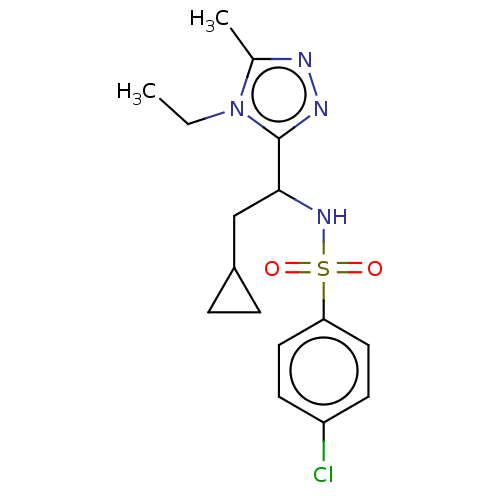 (CHEMBL3402537) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.69E+3 | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity against S1P1R in human U2OS cells expressing beta-arrestin/green fluorescent protein assessed as effect on S1P-induced beta-arres... | Bioorg Med Chem Lett 25: 2041-5 (2015) Article DOI: 10.1016/j.bmcl.2015.03.095 BindingDB Entry DOI: 10.7270/Q22F7Q3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 133 total ) | Next | Last >> |