

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000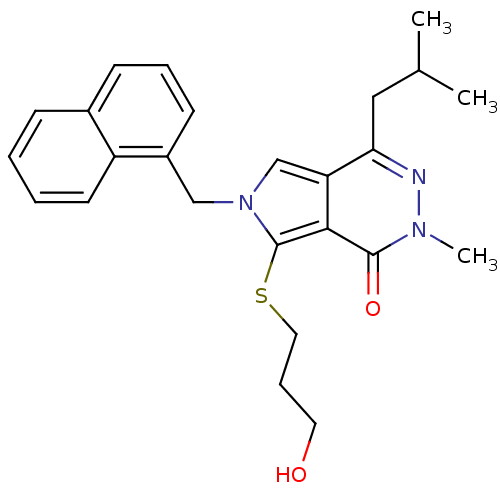 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001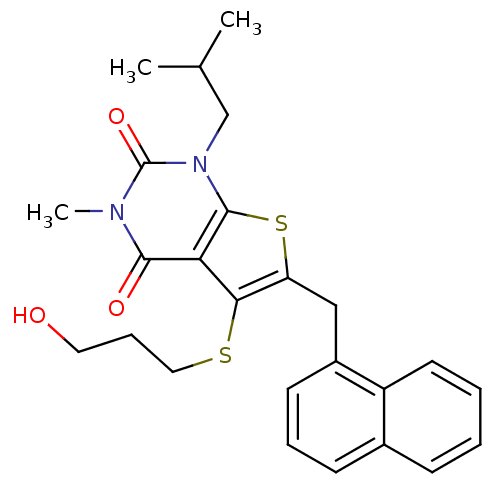 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.275 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22001 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | PubMed | 0.302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002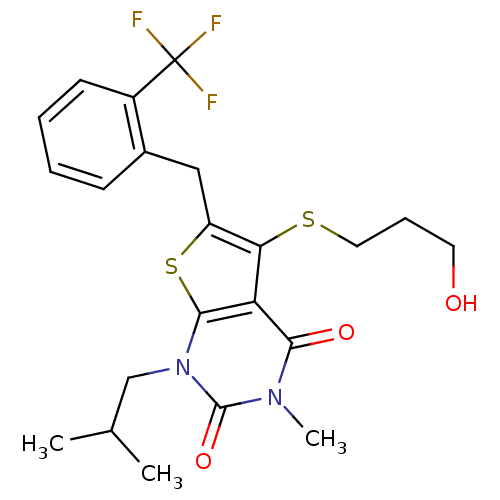 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22002 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22000 (7-[(3-hydroxypropyl)sulfanyl]-2-methyl-4-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21986 (5-[(3-hydroxypropyl)sulfanyl]-3-methyl-1-(2-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416571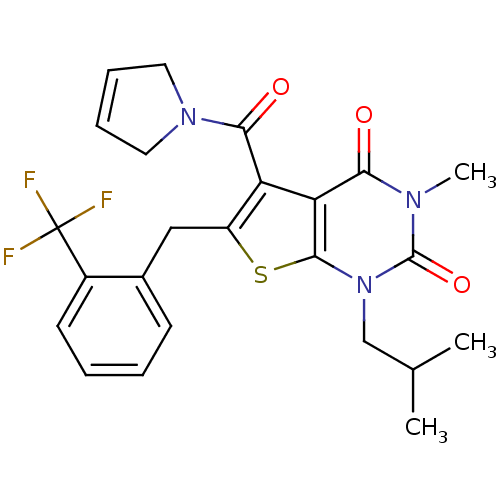 (CHEMBL1221551) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416571 (CHEMBL1221551) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22017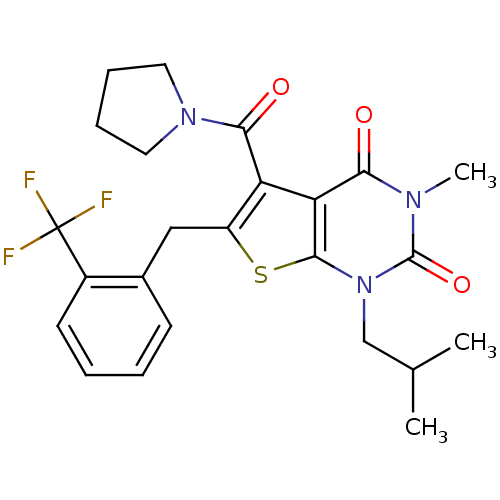 (3-methyl-1-(2-methylpropyl)-5-(pyrrolidin-1-ylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22017 (3-methyl-1-(2-methylpropyl)-5-(pyrrolidin-1-ylcarb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM22025 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-6-(1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50031739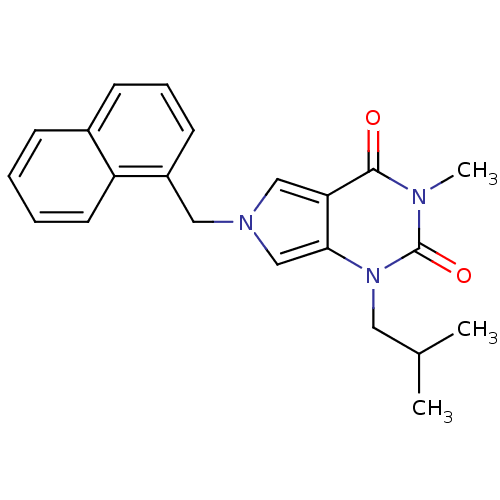 (1-Isobutyl-3-methyl-6-naphthalen-1-ylmethyl-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50324668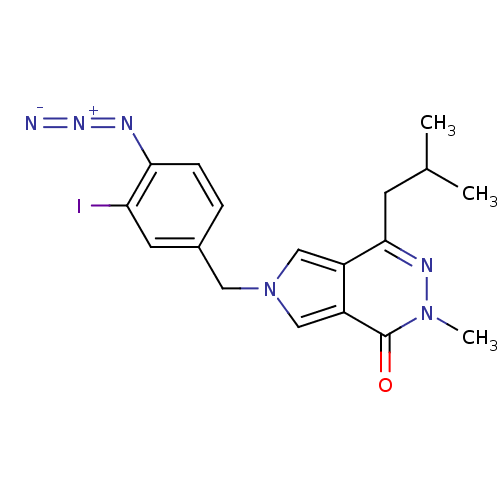 (6-[[4-azido-3-(iodo-125I)phenyl]methyl]-2,6-dihydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21985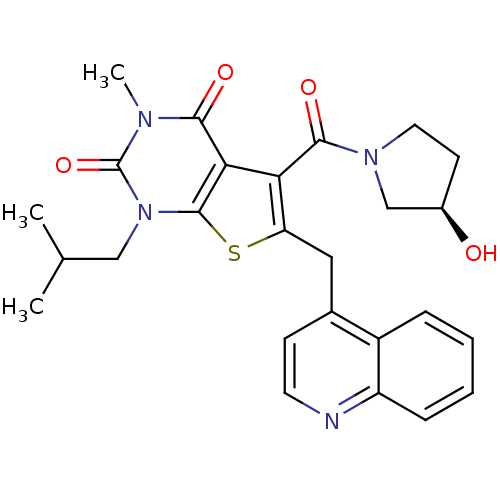 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 30.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM21985 (5-{[(3R)-3-hydroxypyrrolidin-1-yl]carbonyl}-3-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50031739 (1-Isobutyl-3-methyl-6-naphthalen-1-ylmethyl-1,6-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monocarboxylate transporter 1 (Homo sapiens (Human)) | BDBM50416572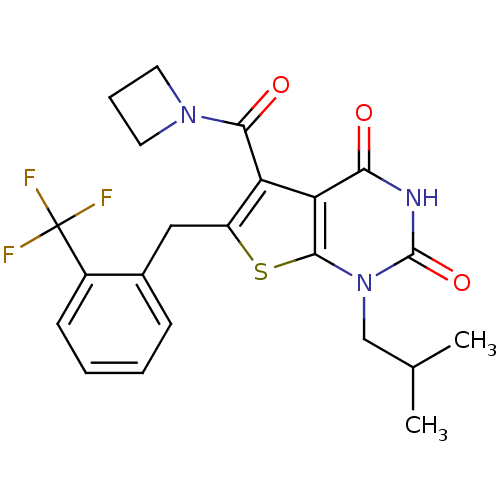 (CHEMBL1221510) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | <200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]5-[(3-hydroxypropyl)thio]-3-methyl-1-[2-(methyl-t)propyl-2,3-t2]-6-(1-naphthalenylmethyl)-1H-pyrrolo[3,4-d]pyrimidine-2,4(3H,6H)-... | Nat Chem Biol 1: 371-6 (2005) BindingDB Entry DOI: 10.7270/Q2MC9063 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443545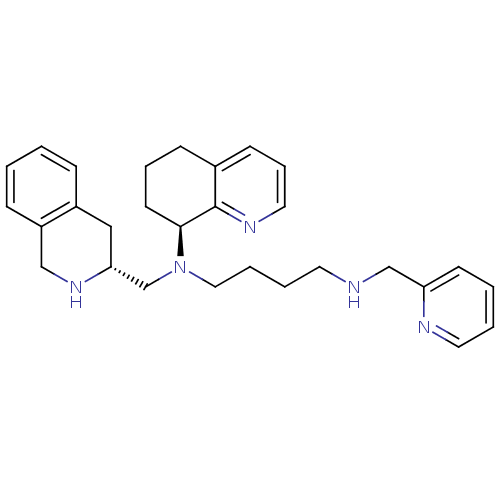 (CHEMBL3091683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125954 (CHEMBL3627858) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125950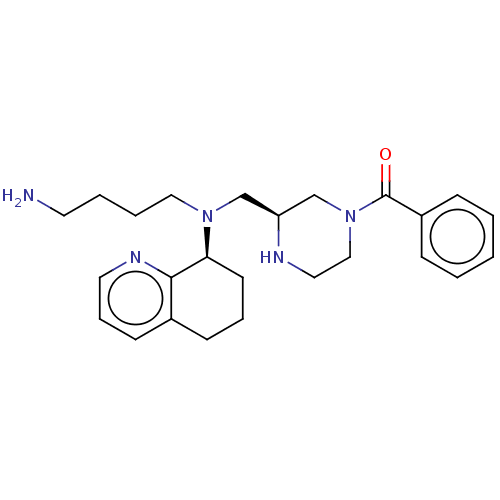 (CHEMBL3627793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125958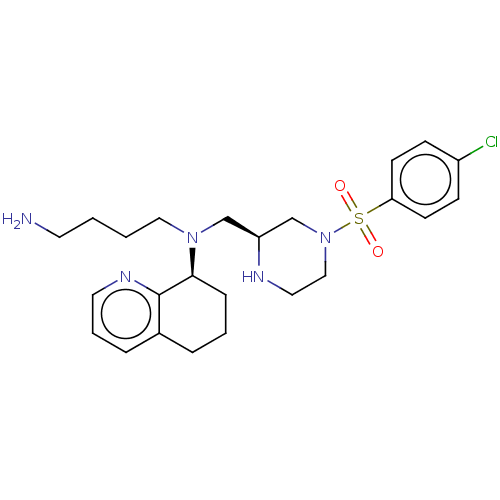 (CHEMBL3627862) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541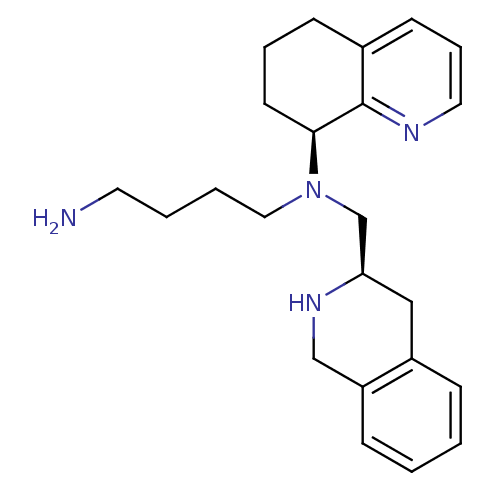 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443543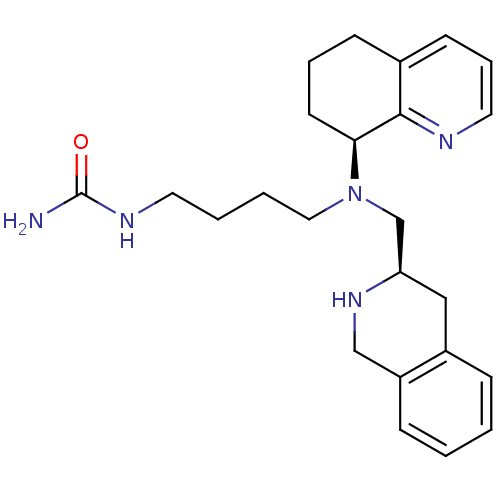 (CHEMBL3091685) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443544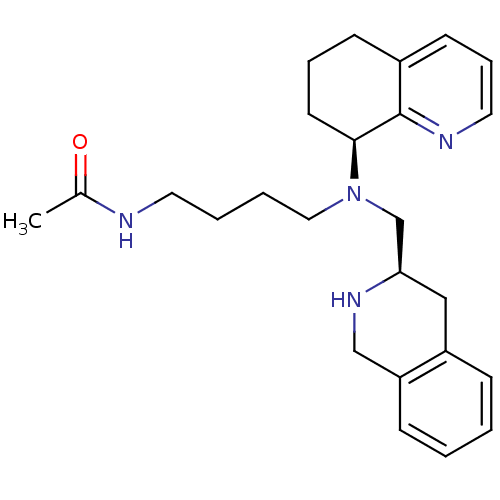 (CHEMBL3091684) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125951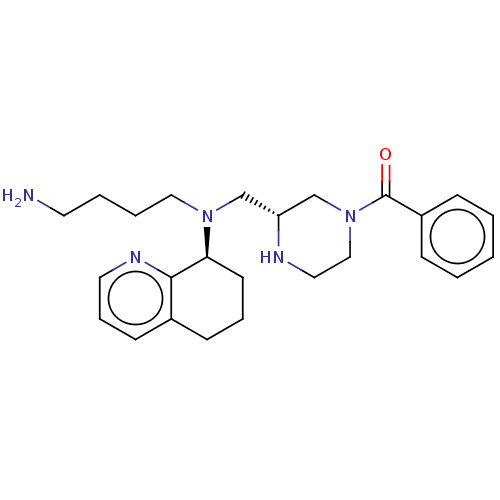 (CHEMBL3627794) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443546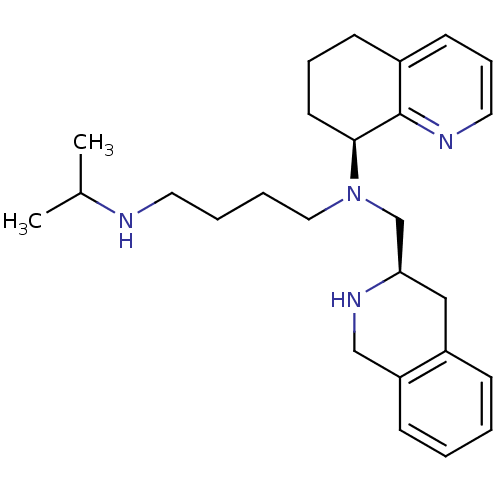 (CHEMBL3091682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443547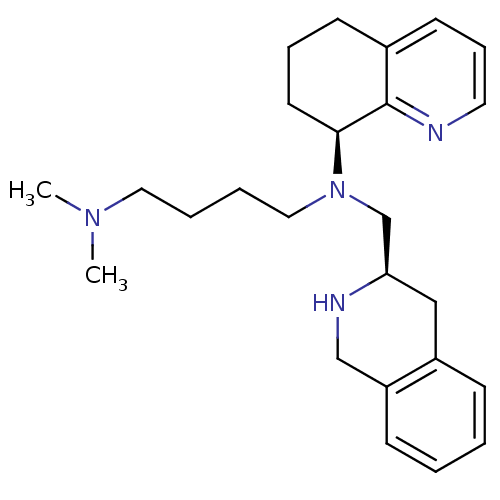 (CHEMBL3091681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) assessed as inhibition of SDF-1-induced beta-arrestin recruitment incubated for 30 mins prior to SDF-1 ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125953 (CHEMBL3627799) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 (unknown origin) expressed in CHO-K1 cells assessed as inhibition of SDF-1alpha/forskolin-induced cAMP production | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443549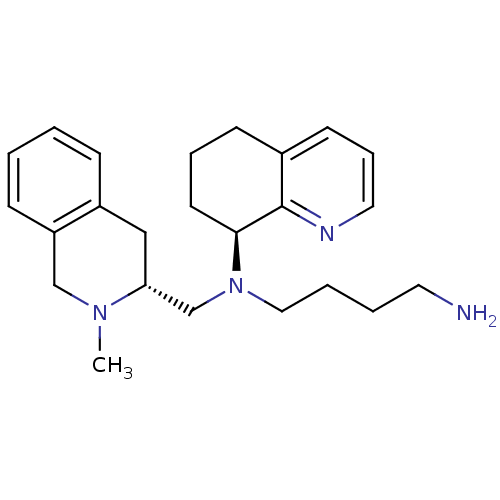 (CHEMBL3091693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443548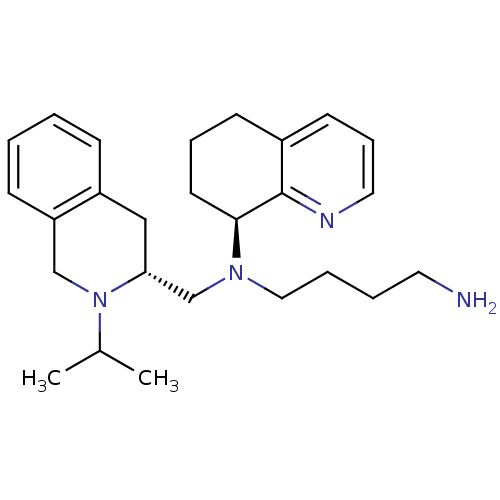 (CHEMBL3091694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125955 (CHEMBL3627859) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50035696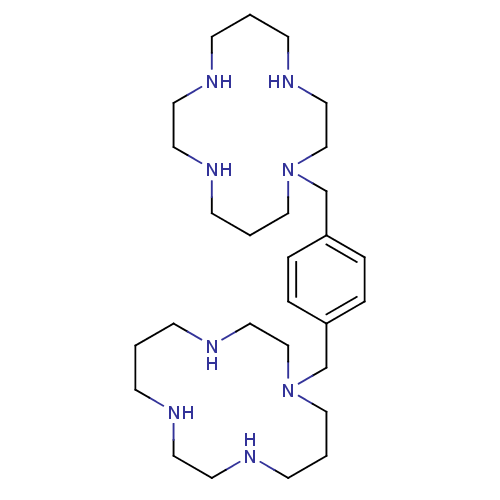 (1,1''-{1,4-phenylenebis(methylene)}-bis{1,4,8,11-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infection | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50315305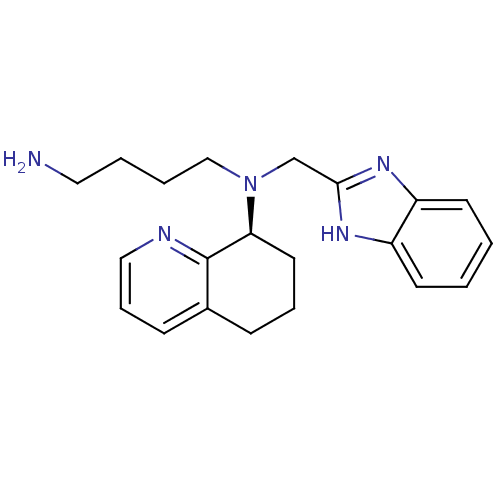 ((S)-N-((1H-benzo[d]imidazol-2-yl)methyl)-N-(4-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infection | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443547 (CHEMBL3091681) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443541 (CHEMBL3091687) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human PBMC assessed as inhibition of HIV-1 3B infection | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125952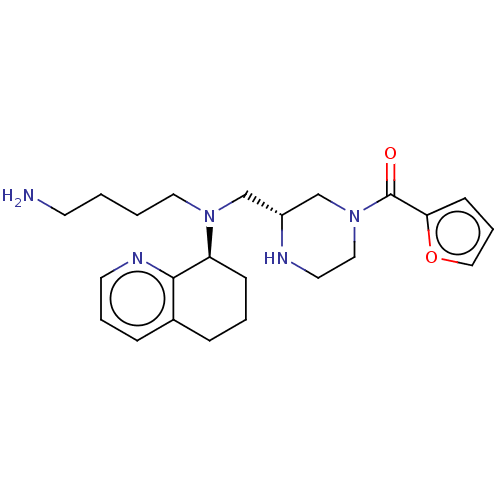 (CHEMBL3627798) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50125957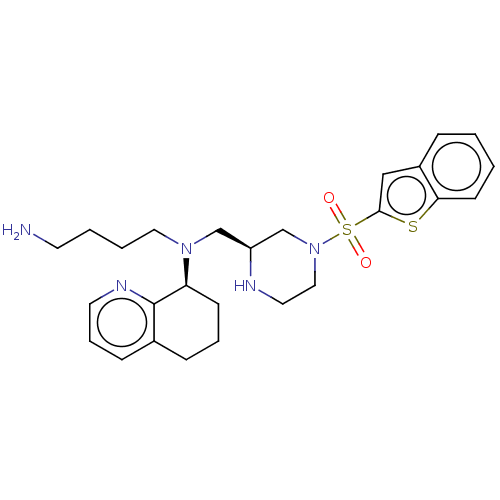 (CHEMBL3627861) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory Institute for Drug Development Curated by ChEMBL | Assay Description Displacement of [125I]-SDF-1 from CXCR4 (unknown origin) | Bioorg Med Chem Lett 25: 4950-5 (2015) Article DOI: 10.1016/j.bmcl.2015.04.036 BindingDB Entry DOI: 10.7270/Q24Q7WST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443549 (CHEMBL3091693) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443551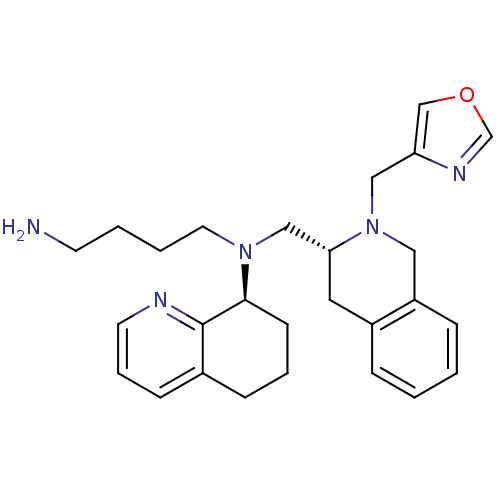 (CHEMBL3091691) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443546 (CHEMBL3091682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443554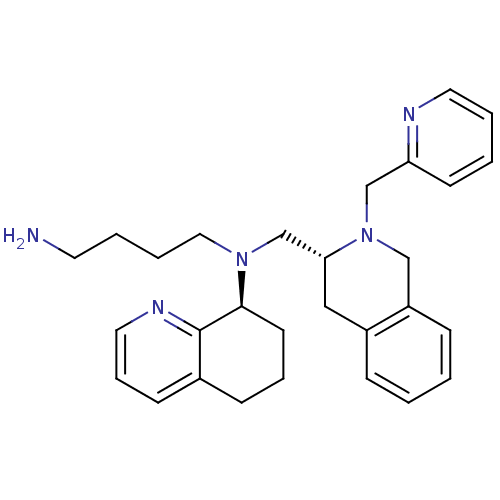 (CHEMBL3091688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human Chem-1 cells assessed as inhibition of SDF-1alpha-mediated calcium flux preincubated for 10 mins by FLIPR assay | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443552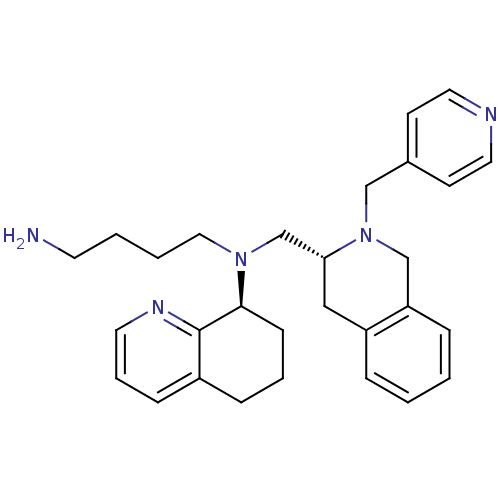 (CHEMBL3091690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50443554 (CHEMBL3091688) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human MAGI-CCR5 cells assessed as inhibition of HIV-1 3B entry after 2 to 6 days by beta-galactosidase reporter gene ... | ACS Med Chem Lett 4: 1025-30 (2013) Article DOI: 10.1021/ml400183q BindingDB Entry DOI: 10.7270/Q2ZS2Z0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 97 total ) | Next | Last >> |