 Found 133 hits with Last Name = 'ross' and Initial = 'bc'
Found 133 hits with Last Name = 'ross' and Initial = 'bc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469937
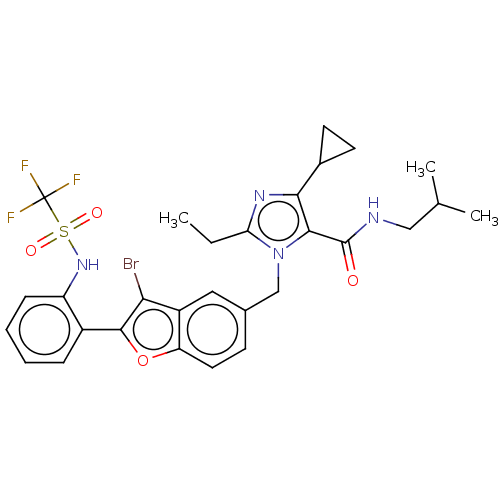
(CHEMBL104493)Show SMILES CCc1nc(C2CC2)c(C(=O)NCC(C)C)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C29H30BrF3N4O4S/c1-4-23-35-25(18-10-11-18)26(28(38)34-14-16(2)3)37(23)15-17-9-12-22-20(13-17)24(30)27(41-22)19-7-5-6-8-21(19)36-42(39,40)29(31,32)33/h5-9,12-13,16,18,36H,4,10-11,14-15H2,1-3H3,(H,34,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469896
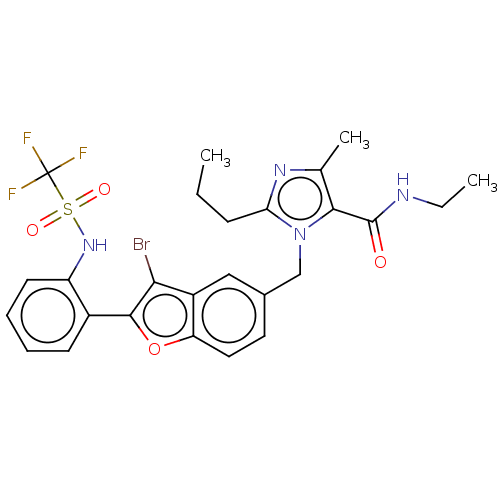
(CHEMBL326910)Show SMILES CCCc1nc(C)c(C(=O)NCC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H26BrF3N4O4S/c1-4-8-21-32-15(3)23(25(35)31-5-2)34(21)14-16-11-12-20-18(13-16)22(27)24(38-20)17-9-6-7-10-19(17)33-39(36,37)26(28,29)30/h6-7,9-13,33H,4-5,8,14H2,1-3H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469890

(CHEMBL63072)Show SMILES CCc1nc(C2CC2)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H24BrF3N4O4S/c1-3-20-32-22(15-9-10-15)23(25(35)31-2)34(20)13-14-8-11-19-17(12-14)21(27)24(38-19)16-6-4-5-7-18(16)33-39(36,37)26(28,29)30/h4-8,11-12,15,33H,3,9-10,13H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469939
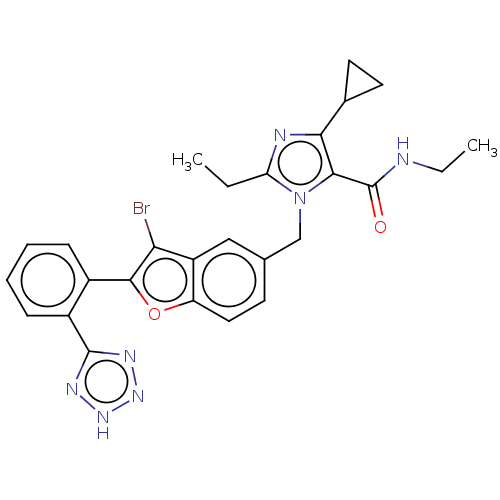
(CHEMBL323125)Show SMILES CCNC(=O)c1c(nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1-c1nn[nH]n1)C1CC1 Show InChI InChI=1S/C27H26BrN7O2/c1-3-21-30-23(16-10-11-16)24(27(36)29-4-2)35(21)14-15-9-12-20-19(13-15)22(28)25(37-20)17-7-5-6-8-18(17)26-31-33-34-32-26/h5-9,12-13,16H,3-4,10-11,14H2,1-2H3,(H,29,36)(H,31,32,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469901

(GR-138950 | SAPRISARTAN)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H22BrF3N4O4S/c1-2-19-31-21(14-8-9-14)22(24(30)34)33(19)12-13-7-10-18-16(11-13)20(26)23(37-18)15-5-3-4-6-17(15)32-38(35,36)25(27,28)29/h3-7,10-11,14,32H,2,8-9,12H2,1H3,(H2,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469888
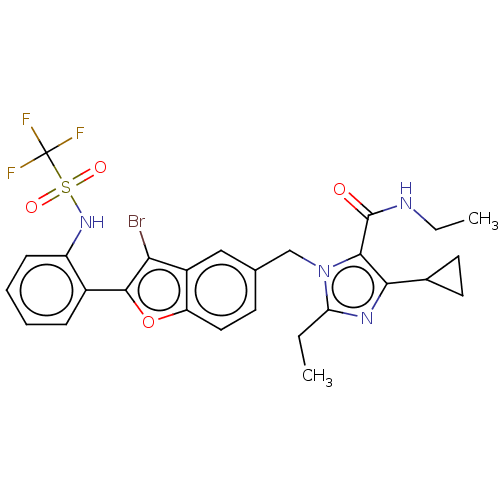
(CHEMBL104667)Show SMILES CCNC(=O)c1c(nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F)C1CC1 Show InChI InChI=1S/C27H26BrF3N4O4S/c1-3-21-33-23(16-10-11-16)24(26(36)32-4-2)35(21)14-15-9-12-20-18(13-15)22(28)25(39-20)17-7-5-6-8-19(17)34-40(37,38)27(29,30)31/h5-9,12-13,16,34H,3-4,10-11,14H2,1-2H3,(H,32,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469892

(CHEMBL107081)Show SMILES CCCc1nc(C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H22BrF3N4O4S/c1-3-6-19-30-13(2)21(23(29)33)32(19)12-14-9-10-18-16(11-14)20(25)22(36-18)15-7-4-5-8-17(15)31-37(34,35)24(26,27)28/h4-5,7-11,31H,3,6,12H2,1-2H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469886

(CHEMBL107006)Show SMILES CCc1nc(CC)n(Cc2ccc3oc(c(Br)c3c2)-c2ccccc2NS(=O)(=O)C(F)(F)F)c1C(N)=O Show InChI InChI=1S/C24H22BrF3N4O4S/c1-3-16-21(23(29)33)32(19(4-2)30-16)12-13-9-10-18-15(11-13)20(25)22(36-18)14-7-5-6-8-17(14)31-37(34,35)24(26,27)28/h5-11,31H,3-4,12H2,1-2H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469893

(CHEMBL104418)Show SMILES CCCc1nc(Cl)c(C(=O)NCC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H23BrClF3N4O4S/c1-3-7-19-32-23(27)21(24(35)31-4-2)34(19)13-14-10-11-18-16(12-14)20(26)22(38-18)15-8-5-6-9-17(15)33-39(36,37)25(28,29)30/h5-6,8-12,33H,3-4,7,13H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469897

(CHEMBL104281)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(c2c1)C(F)(F)F)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H22F6N4O4S/c1-2-19-34-21(14-8-9-14)22(24(33)37)36(19)12-13-7-10-18-16(11-13)20(25(27,28)29)23(40-18)15-5-3-4-6-17(15)35-41(38,39)26(30,31)32/h3-7,10-11,14,35H,2,8-9,12H2,1H3,(H2,33,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469940
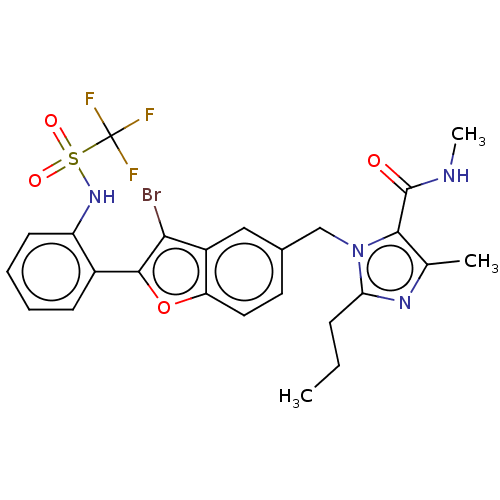
(CHEMBL105887)Show SMILES CCCc1nc(C)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-4-7-20-31-14(2)22(24(34)30-3)33(20)13-15-10-11-19-17(12-15)21(26)23(37-19)16-8-5-6-9-18(16)32-38(35,36)25(27,28)29/h5-6,8-12,32H,4,7,13H2,1-3H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469935
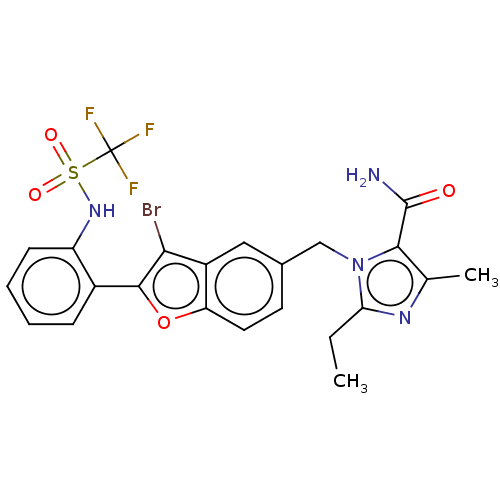
(CHEMBL62909)Show SMILES CCc1nc(C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H20BrF3N4O4S/c1-3-18-29-12(2)20(22(28)32)31(18)11-13-8-9-17-15(10-13)19(24)21(35-17)14-6-4-5-7-16(14)30-36(33,34)23(25,26)27/h4-10,30H,3,11H2,1-2H3,(H2,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469938
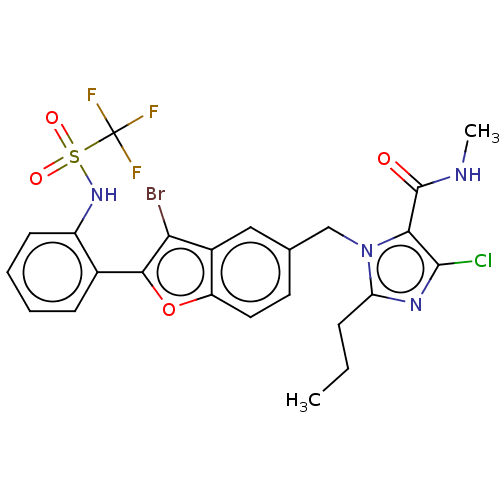
(CHEMBL322989)Show SMILES CCCc1nc(Cl)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H21BrClF3N4O4S/c1-3-6-18-31-22(26)20(23(34)30-2)33(18)12-13-9-10-17-15(11-13)19(25)21(37-17)14-7-4-5-8-16(14)32-38(35,36)24(27,28)29/h4-5,7-11,32H,3,6,12H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469934

(CHEMBL324196)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(Cl)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H22ClF3N4O4S/c1-2-19-31-21(14-8-9-14)22(24(30)34)33(19)12-13-7-10-18-16(11-13)20(26)23(37-18)15-5-3-4-6-17(15)32-38(35,36)25(27,28)29/h3-7,10-11,14,32H,2,8-9,12H2,1H3,(H2,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469889

(CHEMBL62553)Show SMILES CCNC(=O)c1c(C)nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-4-20-31-14(3)22(24(34)30-5-2)33(20)13-15-10-11-19-17(12-15)21(26)23(37-19)16-8-6-7-9-18(16)32-38(35,36)25(27,28)29/h6-12,32H,4-5,13H2,1-3H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469887

(CHEMBL107043)Show SMILES CCCCc1nc(Cl)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H23BrClF3N4O4S/c1-3-4-9-19-32-23(27)21(24(35)31-2)34(19)13-14-10-11-18-16(12-14)20(26)22(38-18)15-7-5-6-8-17(15)33-39(36,37)25(28,29)30/h5-8,10-12,33H,3-4,9,13H2,1-2H3,(H,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469899

(CHEMBL107967)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(c(CC)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C27H27F3N4O4S/c1-3-17-19-13-15(14-34-22(4-2)32-23(16-10-11-16)24(34)26(31)35)9-12-21(19)38-25(17)18-7-5-6-8-20(18)33-39(36,37)27(28,29)30/h5-9,12-13,16,33H,3-4,10-11,14H2,1-2H3,(H2,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469891

(CHEMBL322516)Show SMILES CCCc1nc(Cl)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H19BrClF3N4O4S/c1-2-5-17-30-21(25)19(22(29)33)32(17)11-12-8-9-16-14(10-12)18(24)20(36-16)13-6-3-4-7-15(13)31-37(34,35)23(26,27)28/h3-4,6-10,31H,2,5,11H2,1H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469900

(CHEMBL104252)Show SMILES CCNC(=O)c1c(Cl)nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H21BrClF3N4O4S/c1-3-18-31-22(26)20(23(34)30-4-2)33(18)12-13-9-10-17-15(11-13)19(25)21(37-17)14-7-5-6-8-16(14)32-38(35,36)24(27,28)29/h5-11,32H,3-4,12H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469884

(CHEMBL293447)Show SMILES CCc1nc(C)c(C(=O)N(C)C)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-5-20-30-14(2)22(24(34)32(3)4)33(20)13-15-10-11-19-17(12-15)21(26)23(37-19)16-8-6-7-9-18(16)31-38(35,36)25(27,28)29/h6-12,31H,5,13H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469933
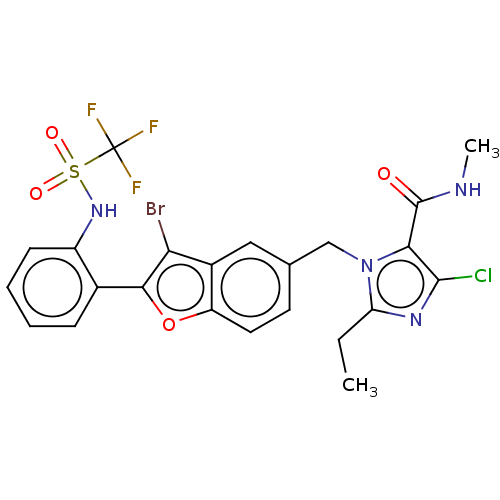
(CHEMBL323378)Show SMILES CCc1nc(Cl)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H19BrClF3N4O4S/c1-3-17-30-21(25)19(22(33)29-2)32(17)11-12-8-9-16-14(10-12)18(24)20(36-16)13-6-4-5-7-15(13)31-37(34,35)23(26,27)28/h4-10,31H,3,11H2,1-2H3,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469894
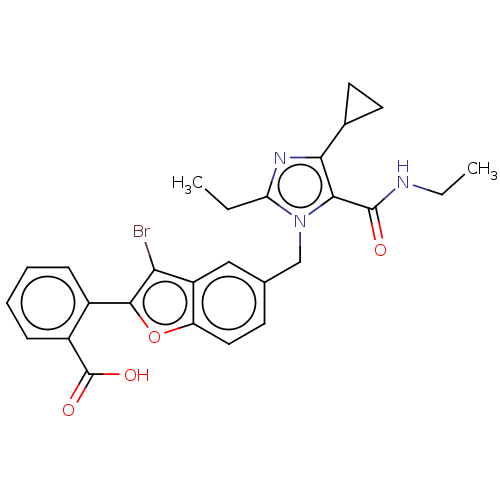
(CHEMBL323315)Show SMILES CCNC(=O)c1c(nc(CC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1C(O)=O)C1CC1 Show InChI InChI=1S/C27H26BrN3O4/c1-3-21-30-23(16-10-11-16)24(26(32)29-4-2)31(21)14-15-9-12-20-19(13-15)22(28)25(35-20)17-7-5-6-8-18(17)27(33)34/h5-9,12-13,16H,3-4,10-11,14H2,1-2H3,(H,29,32)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469941
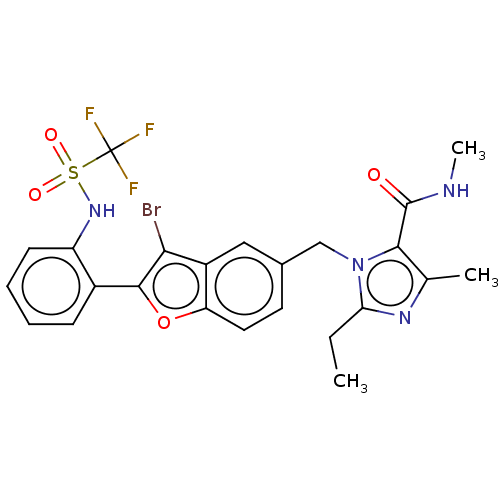
(CHEMBL292040)Show SMILES CCc1nc(C)c(C(=O)NC)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C24H22BrF3N4O4S/c1-4-19-30-13(2)21(23(33)29-3)32(19)12-14-9-10-18-16(11-14)20(25)22(36-18)15-7-5-6-8-17(15)31-37(34,35)24(26,27)28/h5-11,31H,4,12H2,1-3H3,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469895
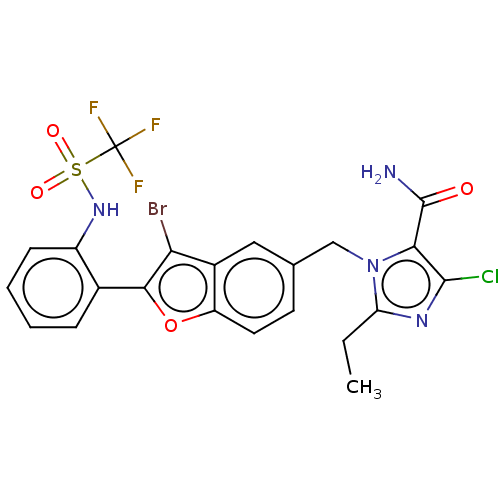
(CHEMBL104626)Show SMILES CCc1nc(Cl)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C22H17BrClF3N4O4S/c1-2-16-29-20(24)18(21(28)32)31(16)10-11-7-8-15-13(9-11)17(23)19(35-15)12-5-3-4-6-14(12)30-36(33,34)22(25,26)27/h3-9,30H,2,10H2,1H3,(H2,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469936
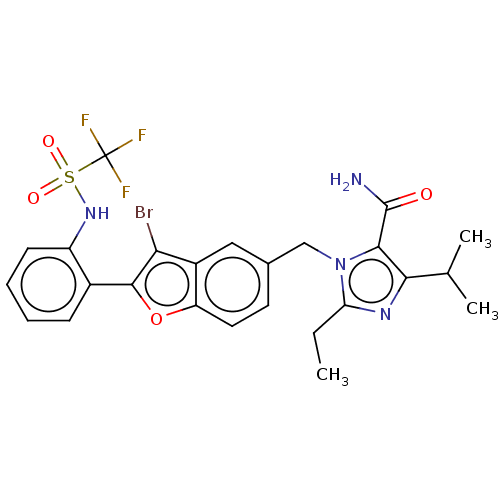
(CHEMBL108115)Show SMILES CCc1nc(C(C)C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H24BrF3N4O4S/c1-4-19-31-21(13(2)3)22(24(30)34)33(19)12-14-9-10-18-16(11-14)20(26)23(37-18)15-7-5-6-8-17(15)32-38(35,36)25(27,28)29/h5-11,13,32H,4,12H2,1-3H3,(H2,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469902
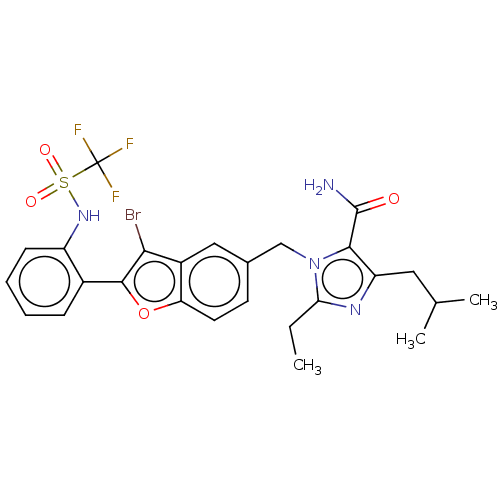
(CHEMBL320326)Show SMILES CCc1nc(CC(C)C)c(C(N)=O)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C26H26BrF3N4O4S/c1-4-21-32-19(11-14(2)3)23(25(31)35)34(21)13-15-9-10-20-17(12-15)22(27)24(38-20)16-7-5-6-8-18(16)33-39(36,37)26(28,29)30/h5-10,12,14,33H,4,11,13H2,1-3H3,(H2,31,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469885

(CHEMBL418811)Show SMILES CNC(=O)c1c(C)nc(C)n1Cc1ccc2oc(c(Br)c2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C23H20BrF3N4O4S/c1-12-20(22(32)28-3)31(13(2)29-12)11-14-8-9-18-16(10-14)19(24)21(35-18)15-6-4-5-7-17(15)30-36(33,34)23(25,26)27/h4-10,30H,11H2,1-3H3,(H,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM82258
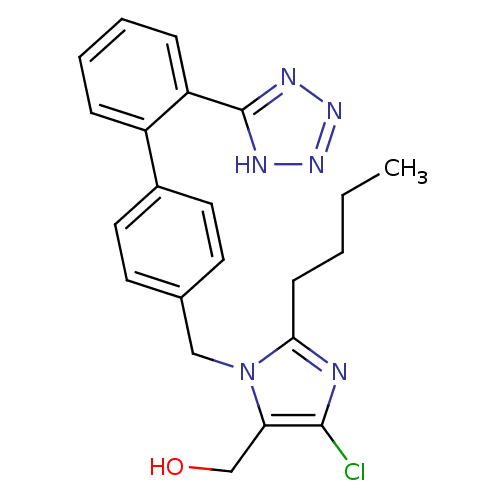
(CAS_114798-26-4 | Losartan | NSC_3961)Show SMILES CCCCc1nc(Cl)c(CO)n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C22H23ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,30H,2-3,8,13-14H2,1H3,(H,25,26,27,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor A/Type-1 angiotensin II receptor B/Type-2 angiotensin II receptor
(RAT) | BDBM50469898
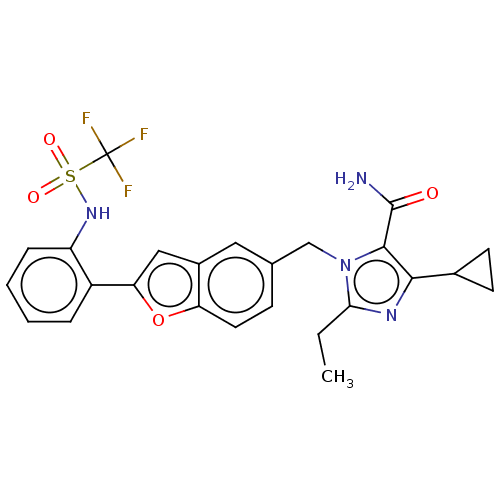
(CHEMBL322828)Show SMILES CCc1nc(C2CC2)c(C(N)=O)n1Cc1ccc2oc(cc2c1)-c1ccccc1NS(=O)(=O)C(F)(F)F Show InChI InChI=1S/C25H23F3N4O4S/c1-2-21-30-22(15-8-9-15)23(24(29)33)32(21)13-14-7-10-19-16(11-14)12-20(36-19)17-5-3-4-6-18(17)31-37(34,35)25(26,27)28/h3-7,10-12,15,31H,2,8-9,13H2,1H3,(H2,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd.
Curated by ChEMBL
| Assay Description
Tested for in vitro binding affinity against angiotensin II receptor of rat liver |
J Med Chem 37: 3108-20 (1994)
Article DOI: 10.1021/jm00045a016
BindingDB Entry DOI: 10.7270/Q21R6T7V |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072292

((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072285
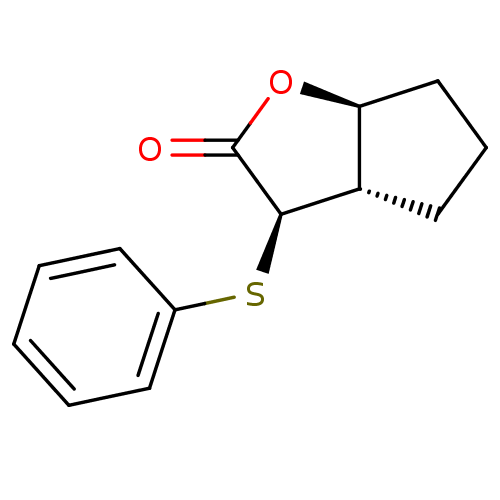
((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042653

(7-[3-Bromo-4,5-bis-(4-fluoro-phenyl)-2-isopropyl-p...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(C(C)C)c(Br)c(c1-c1ccc(F)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C27H28BrF2NO4/c1-16(2)26-25(28)24(17-4-8-19(29)9-5-17)27(18-6-10-20(30)11-7-18)31(26)13-12-21(32)14-22(33)15-23(34)35-3/h4-13,16,21-22,32-33H,14-15H2,1-3H3/b13-12+/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042654
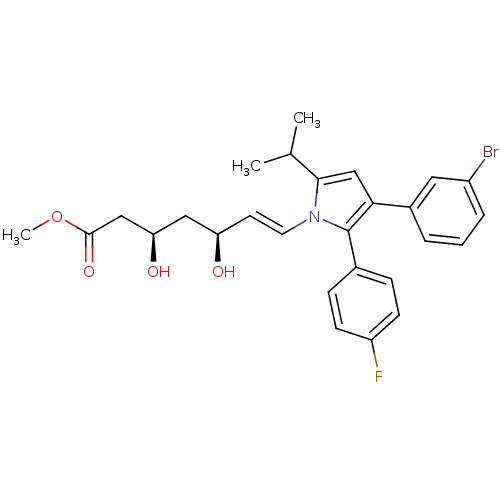
(7-[3-(3-Bromo-phenyl)-2-(4-fluoro-phenyl)-5-isopro...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccc(F)cc1)-c1cccc(Br)c1)C(C)C Show InChI InChI=1S/C27H29BrFNO4/c1-17(2)25-16-24(19-5-4-6-20(28)13-19)27(18-7-9-21(29)10-8-18)30(25)12-11-22(31)14-23(32)15-26(33)34-3/h4-13,16-17,22-23,31-32H,14-15H2,1-3H3/b12-11+/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072289

((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10-,11+,12+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072283
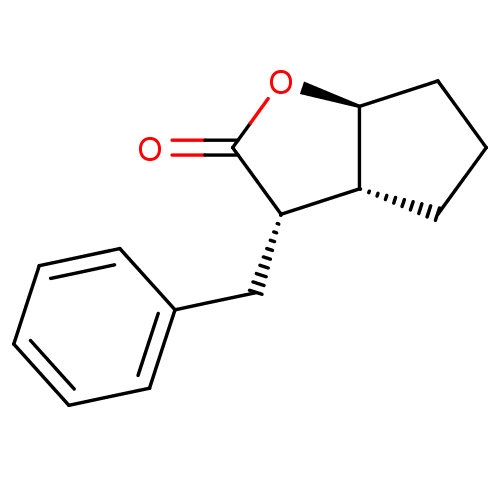
((3S,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12+,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042650
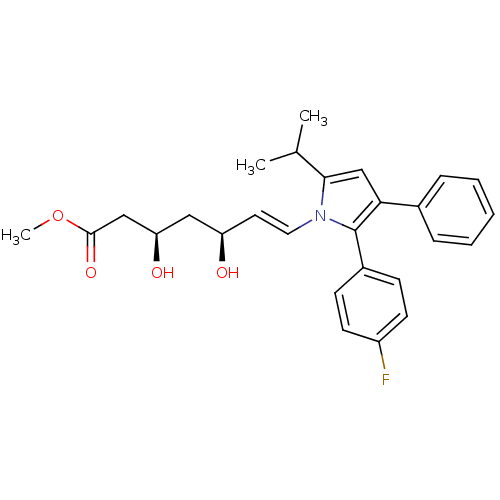
(7-[2-(4-Fluoro-phenyl)-5-isopropyl-3-phenyl-pyrrol...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccc(F)cc1)-c1ccccc1)C(C)C Show InChI InChI=1S/C27H30FNO4/c1-18(2)25-17-24(19-7-5-4-6-8-19)27(20-9-11-21(28)12-10-20)29(25)14-13-22(30)15-23(31)16-26(32)33-3/h4-14,17-18,22-23,30-31H,15-16H2,1-3H3/b14-13+/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042652

(7-[2-(4-Fluoro-phenyl)-5-isopropyl-3-pyridin-2-yl-...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccc(F)cc1)-c1ccccn1)C(C)C Show InChI InChI=1S/C26H29FN2O4/c1-17(2)24-16-22(23-6-4-5-12-28-23)26(18-7-9-19(27)10-8-18)29(24)13-11-20(30)14-21(31)15-25(32)33-3/h4-13,16-17,20-21,30-31H,14-15H2,1-3H3/b13-11+/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042656

(7-[2,3-Bis-(4-fluoro-phenyl)-5-isopropyl-pyrrol-1-...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccc(F)cc1)-c1ccc(F)cc1)C(C)C Show InChI InChI=1S/C27H29F2NO4/c1-17(2)25-16-24(18-4-8-20(28)9-5-18)27(19-6-10-21(29)11-7-19)30(25)13-12-22(31)14-23(32)15-26(33)34-3/h4-13,16-17,22-23,31-32H,14-15H2,1-3H3/b13-12+/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50072286
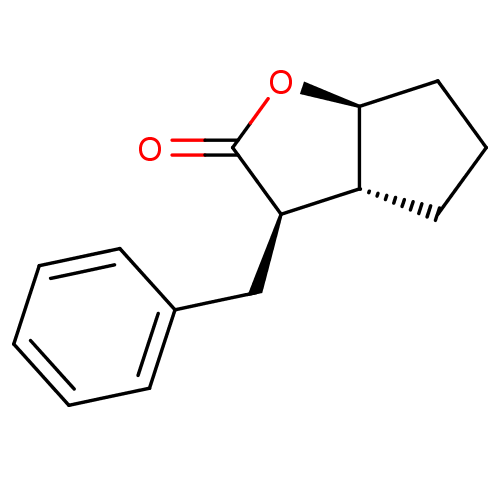
((3R,3aR,6aS)-3-Benzyl-hexahydro-cyclopenta[b]furan...)Show InChI InChI=1S/C14H16O2/c15-14-12(9-10-5-2-1-3-6-10)11-7-4-8-13(11)16-14/h1-3,5-6,11-13H,4,7-9H2/t11-,12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042646
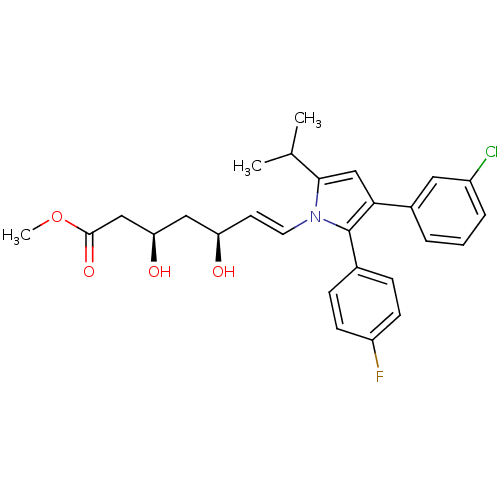
(7-[3-(3-Chloro-phenyl)-2-(4-fluoro-phenyl)-5-isopr...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccc(F)cc1)-c1cccc(Cl)c1)C(C)C Show InChI InChI=1S/C27H29ClFNO4/c1-17(2)25-16-24(19-5-4-6-20(28)13-19)27(18-7-9-21(29)10-8-18)30(25)12-11-22(31)14-23(32)15-26(33)34-3/h4-13,16-17,22-23,31-32H,14-15H2,1-3H3/b12-11+/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50004774
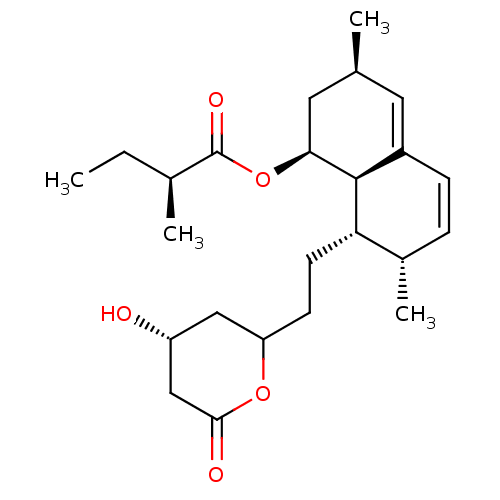
((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072284

((1S,2R,4aR,6aS,6bS,7R,9aS,10aS)-2-Acetoxy-1,4a,6a,...)Show SMILES [H][C@@]12[#6]-[#6][C@]3([#6])[C@@]4([H])[#6]-[#6][C@@]5([#6])[C@]6([H])[#6@@H](-[#6]-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#6](=O)-[#8][C@@]6([H])[#6][C@]5([#6])[#6]4=[#6]-[#6][C@@]3([H])[C@]1([#6])[#6](=O)-[#8]2 |r,c:33| Show InChI InChI=1S/C30H40O5/c1-16(2)13-17(31)14-18-24-21(34-25(18)32)15-29(5)20-7-8-22-27(3,19(20)9-12-28(24,29)4)11-10-23-30(22,6)26(33)35-23/h7,13,18-19,21-24H,8-12,14-15H2,1-6H3/t18-,19+,21+,22-,23-,24-,27-,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of trypsin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042647

(7-[2-(4-Fluoro-phenyl)-5-isopropyl-3-pyridin-4-yl-...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccc(F)cc1)-c1ccncc1)C(C)C Show InChI InChI=1S/C26H29FN2O4/c1-17(2)24-16-23(18-8-11-28-12-9-18)26(19-4-6-20(27)7-5-19)29(24)13-10-21(30)14-22(31)15-25(32)33-3/h4-13,16-17,21-22,30-31H,14-15H2,1-3H3/b13-10+/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50006412
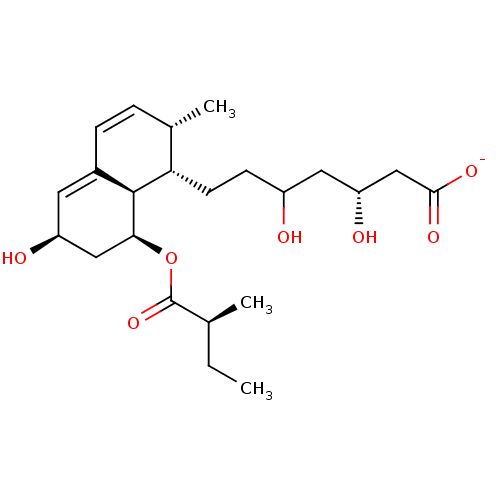
((3R,5R)-3,5-dihydroxy-7-((1S,2S,6S,8S,8aR)-6-hydro...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](O)C=C2C=C[C@H](C)[C@H](CCC(O)C[C@@H](O)CC([O-])=O)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C23H36O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,9,13-14,16-20,22,24-26H,4,7-8,10-12H2,1-3H3,(H,27,28)/p-1/t13-,14-,16?,17-,18+,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072285

((3R,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Chymotrypsin-C
(Homo sapiens (Human)) | BDBM50072290

((3S,3aR,6aS)-3-Allyl-hexahydro-cyclopenta[b]furan-...)Show InChI InChI=1S/C10H14O2/c1-2-4-8-7-5-3-6-9(7)12-10(8)11/h2,7-9H,1,3-6H2/t7-,8+,9+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Chymotrypsinogen |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072292

((3S,3aS,6aS)-3-Phenylsulfanyl-hexahydro-cyclopenta...)Show InChI InChI=1S/C13H14O2S/c14-13-12(10-7-4-8-11(10)15-13)16-9-5-2-1-3-6-9/h1-3,5-6,10-12H,4,7-8H2/t10-,11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072287

((3S,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahydro-...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10?,11-,12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50072289

((3S,3aR,6aS)-3-(4-Methyl-2-oxo-pent-3-enyl)-hexahy...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6](=O)-[#6]-[#6@H]-1-[#6@H]-2-[#6]-[#6]-[#6]-[#6@@H]-2-[#8]-[#6]-1=O Show InChI InChI=1S/C13H18O3/c1-8(2)6-9(14)7-11-10-4-3-5-12(10)16-13(11)15/h6,10-12H,3-5,7H2,1-2H3/t10-,11+,12+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 8: 2955-60 (1999)
Article DOI: 10.1016/S0960-894X(98)00531-9
BindingDB Entry DOI: 10.7270/Q2JQ105F |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50042645
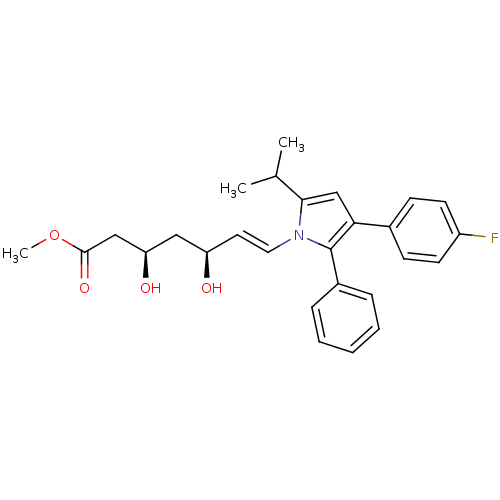
(7-[3-(4-Fluoro-phenyl)-5-isopropyl-2-phenyl-pyrrol...)Show SMILES COC(=O)C[C@H](O)C[C@H](O)\C=C\n1c(cc(c1-c1ccccc1)-c1ccc(F)cc1)C(C)C Show InChI InChI=1S/C27H30FNO4/c1-18(2)25-17-24(19-9-11-21(28)12-10-19)27(20-7-5-4-6-8-20)29(25)14-13-22(30)15-23(31)16-26(32)33-3/h4-14,17-18,22-23,30-31H,15-16H2,1-3H3/b14-13+/t22-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of rat liver microsomal HMG-CoA reductase |
J Med Chem 36: 3658-62 (1994)
BindingDB Entry DOI: 10.7270/Q2SJ1JP7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data