

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50477152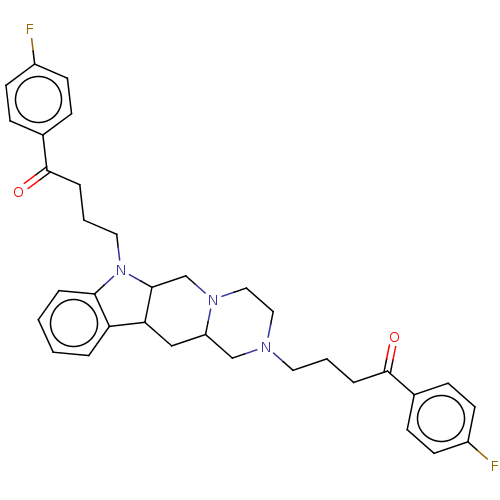 (CHEMBL393466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.643 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50003020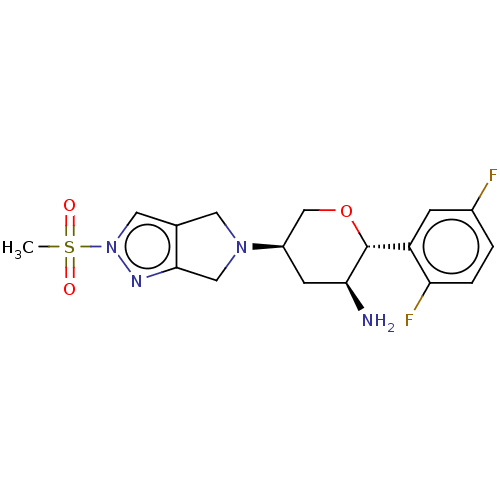 (MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc. Curated by ChEMBL | Assay Description Competitive reversible inhibition of DPP4 (unknown origin) | J Med Chem 57: 3205-12 (2014) Article DOI: 10.1021/jm401992e BindingDB Entry DOI: 10.7270/Q2WD423H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304627 ((2S,3S,4R,5R,6S)-6-methylpiperidine-2,3,4,5-tetrao...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50065258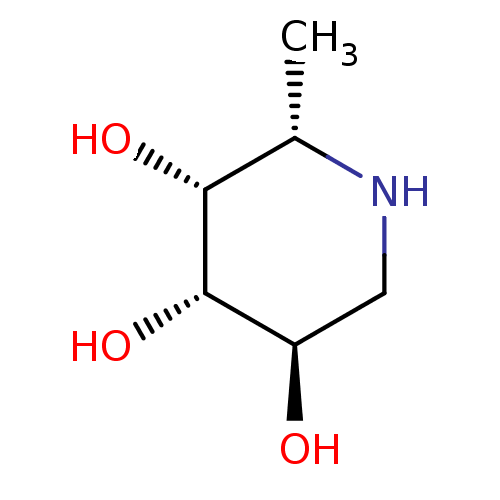 ((2S,3R,4S,5R)-2-Methyl-piperidine-3,4,5-triol | (2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50477152 (CHEMBL393466) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304628 ((2S,3R,4S)-2-methyl-3,4-dihydro-2H-pyrrole-3,4-dio...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50133931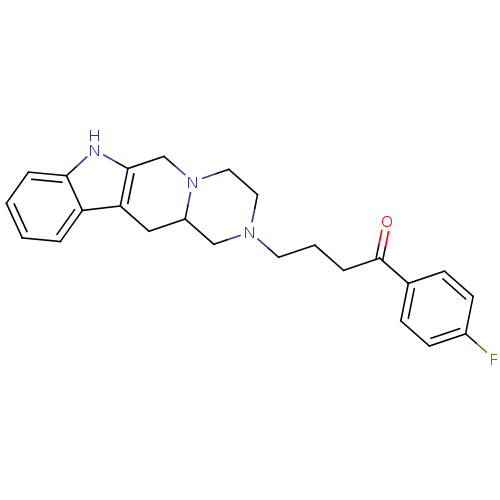 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50133931 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066974 (7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50477151 (CHEMBL393622) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50477151 (CHEMBL393622) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066979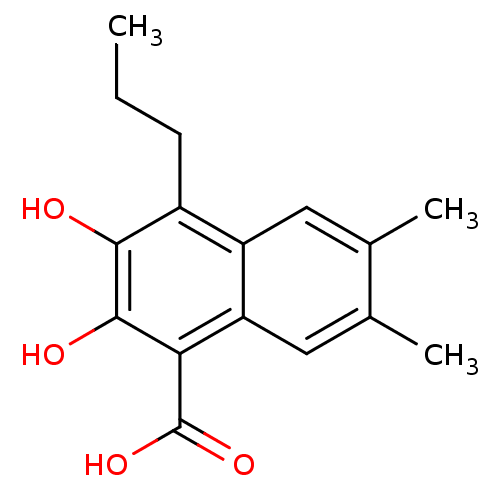 (2,3-Dihydroxy-6,7-dimethyl-4-propyl-naphthalene-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066979 (2,3-Dihydroxy-6,7-dimethyl-4-propyl-naphthalene-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010449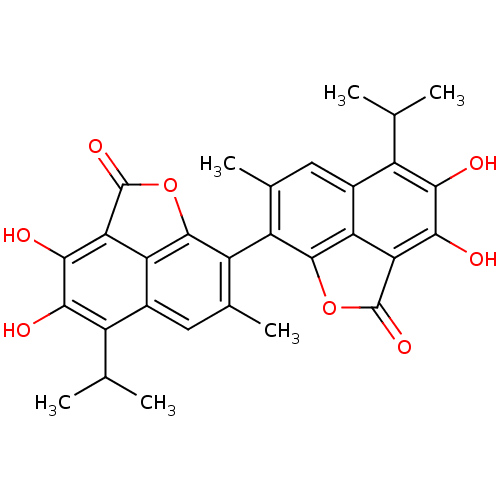 (3,4,3',4'-Tetrahydroxy-5,5'-diisopropyl-7,7'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066975 (7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066973 (2,3-Dihydroxy-4-isopropyl-6-methyl-7-(4-trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066974 (7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010436 (6,7,6',7'-Tetrahydroxy-5,5'-diisopropyl-1,1'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50133931 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066976 (7-Benzyl-2,3-dihydroxy-4,6-dimethyl-naphthalene-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM23223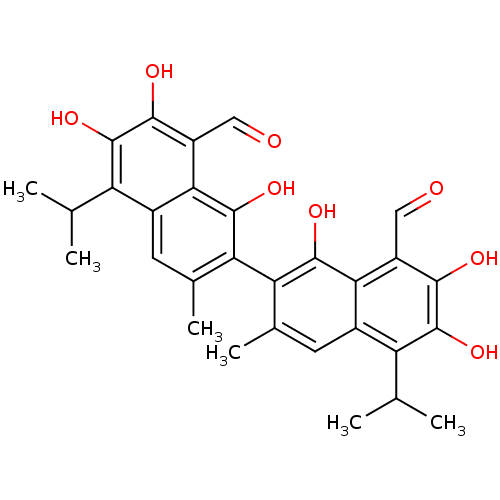 (7-[8-formyl-1,6,7-trihydroxy-3-methyl-5-(propan-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066975 (7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue alpha-L-fucosidase (Bos taurus) | BDBM50304626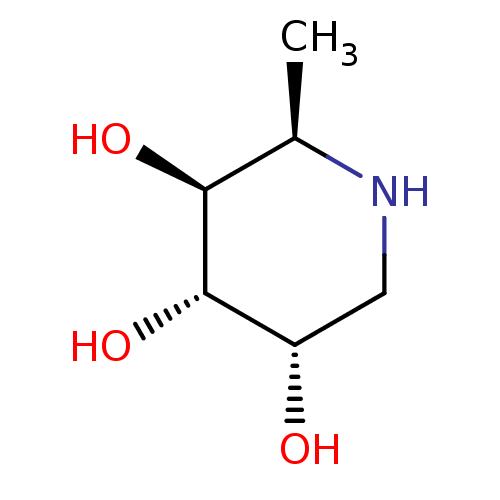 ((2R,3S,4S,5S)-2-methylpiperidine-3,4,5-triol | CHE...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Reims Champagne-Ardenne Curated by ChEMBL | Assay Description Inhibition of bovine kidney alpha-L-fucosidase by para-nitrophenolate release assay | Bioorg Med Chem 17: 8020-6 (2009) Article DOI: 10.1016/j.bmc.2009.10.010 BindingDB Entry DOI: 10.7270/Q2FB5307 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066974 (7-Benzyl-2,3-dihydroxy-6-methyl-4-propyl-naphthale...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-H) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066981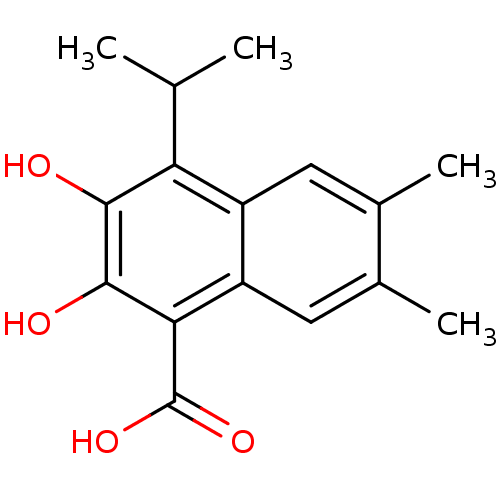 (2,3-Dihydroxy-4-isopropyl-6,7-dimethyl-naphthalene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066977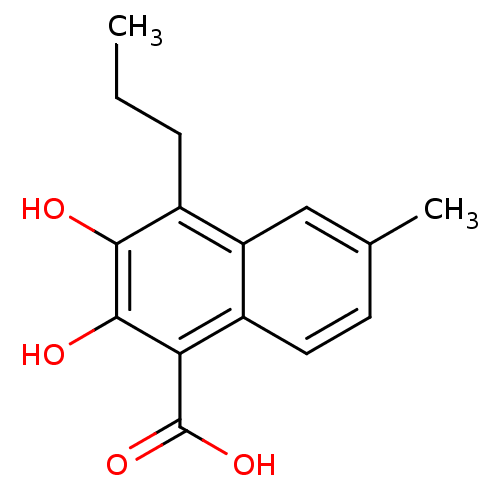 (2,3-Dihydroxy-6-methyl-4-propyl-naphthalene-1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010451 (But-2-enoic acid 1'-but-2-enoyloxy-8,8'-dicyano-6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010446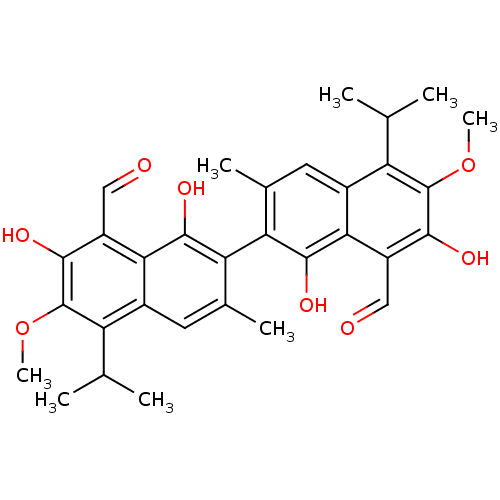 (1,7,1',7'-Tetrahydroxy-5,5'-diisopropyl-6,6'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066981 (2,3-Dihydroxy-4-isopropyl-6,7-dimethyl-naphthalene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50031638 (2,3-Dihydroxy-4-isopropyl-6-methyl-naphthalene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50477152 (CHEMBL393466) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010437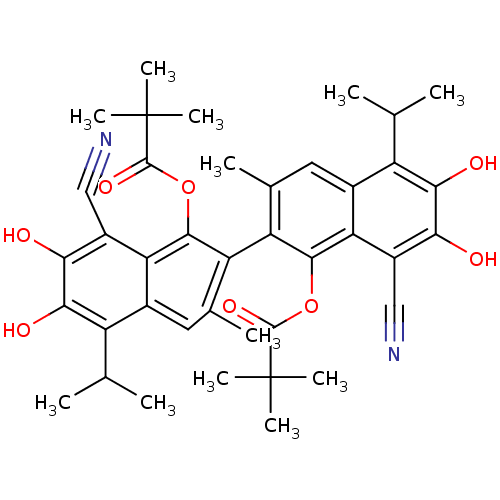 (2,2-Dimethyl-propionic acid 8,8'-dicyano-1'-(2,2-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010450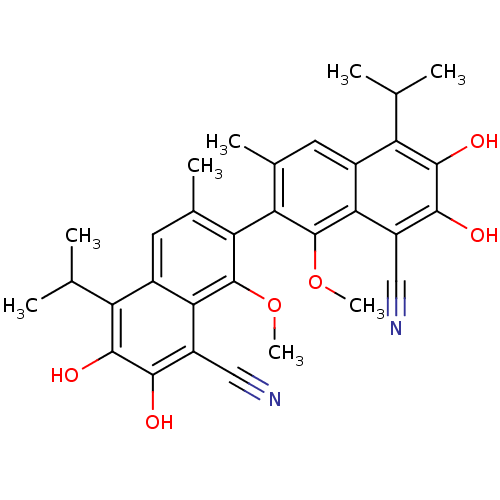 (6,7,6',7'-Tetrahydroxy-5,5'-diisopropyl-1,1'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50031638 (2,3-Dihydroxy-4-isopropyl-6-methyl-naphthalene-1-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010440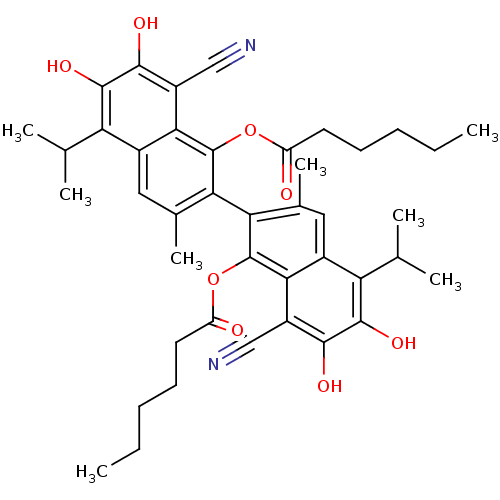 (CHEMBL326383 | Hexanoic acid 8,8'-dicyano-1'-hexan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066980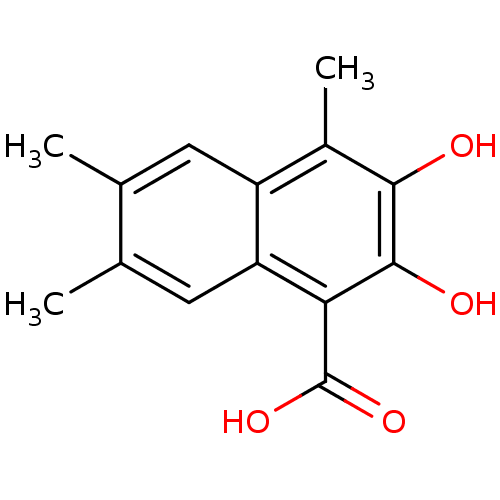 (2,3-Dihydroxy-4,6,7-trimethyl-naphthalene-1-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-M) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010447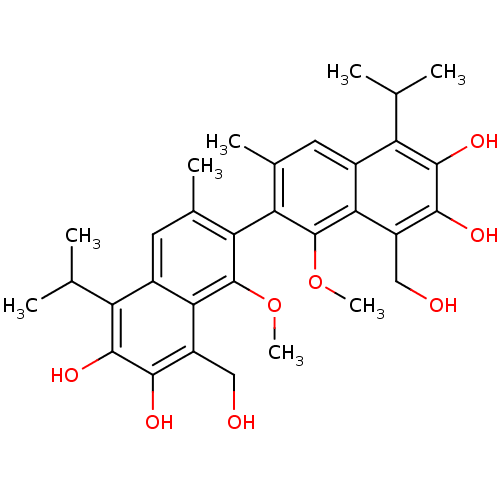 (8,8'-Bis-hydroxymethyl-5,5'-diisopropyl-1,1'-dimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010444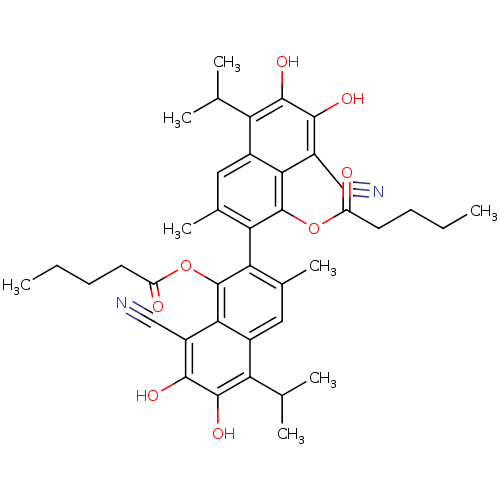 (CHEMBL52639 | Pentanoic acid 8,8'-dicyano-6,7,6',7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010435 (Butyric acid 1'-butyryloxy-8,8'-dicyano-6,7,6',7'-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066977 (2,3-Dihydroxy-6-methyl-4-propyl-naphthalene-1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010439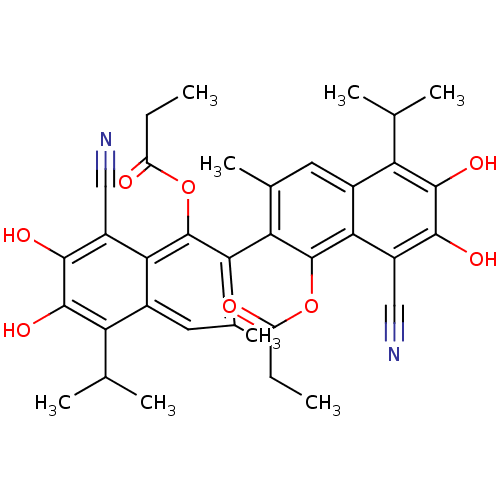 (CHEMBL297483 | Propionic acid 8,8'-dicyano-6,7,6',...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase B chain (Homo sapiens (Human)) | BDBM50066975 (7-Benzyl-2,3-dihydroxy-4-isopropyl-6-methyl-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against Human Lactate Dehydrogenase (LDH-H) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50010438 (Acetic acid 1'-acetoxy-8,8'-dicyano-6,7,6',7'-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM50229024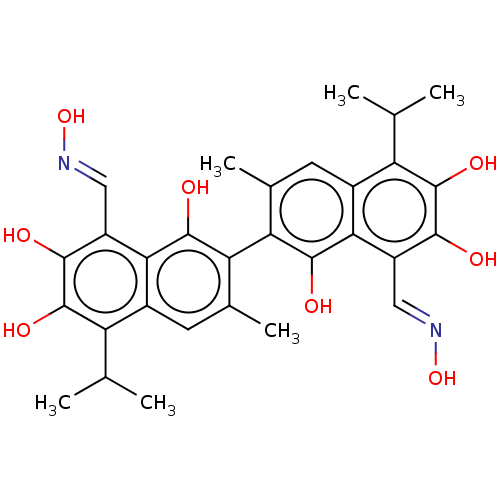 (CHEMBL3349372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description The compound was tested for inhibitory activity against Aldose reductase from human placenta | J Med Chem 34: 3301-5 (1991) BindingDB Entry DOI: 10.7270/Q2VM4B7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| L-lactate dehydrogenase A chain (Homo sapiens (Human)) | BDBM50066976 (7-Benzyl-2,3-dihydroxy-4,6-dimethyl-naphthalene-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New Mexico School of Medicine Curated by ChEMBL | Assay Description inhibitory activity against P. falciparum lactate dehydrogenase (pLDH) | J Med Chem 41: 3879-87 (1998) Article DOI: 10.1021/jm980334n BindingDB Entry DOI: 10.7270/Q298864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2162 total ) | Next | Last >> |