

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448167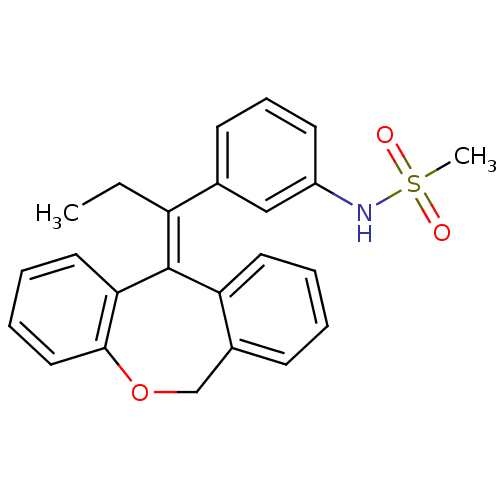 (CHEMBL3120318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448165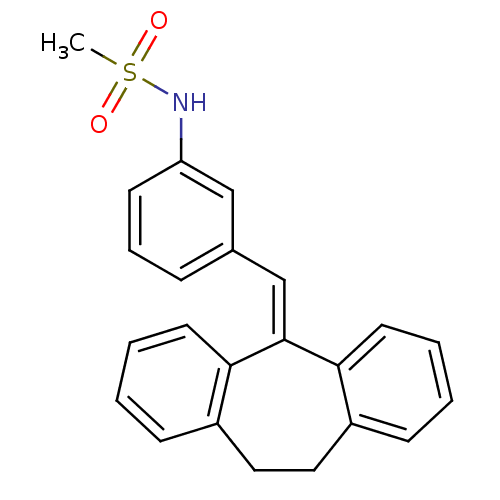 (CHEMBL3120320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448166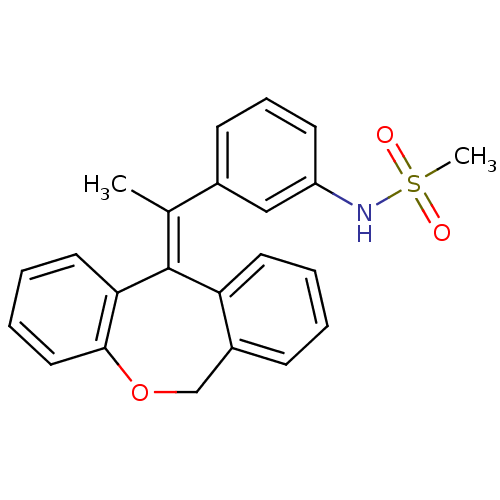 (CHEMBL3120319) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448168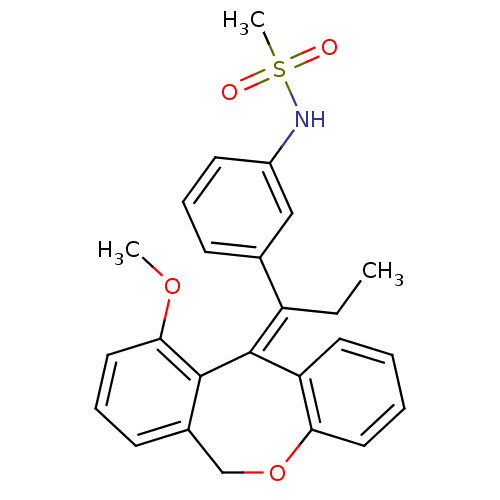 (CHEMBL3120317) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.268 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50448166 (CHEMBL3120319) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.342 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM18207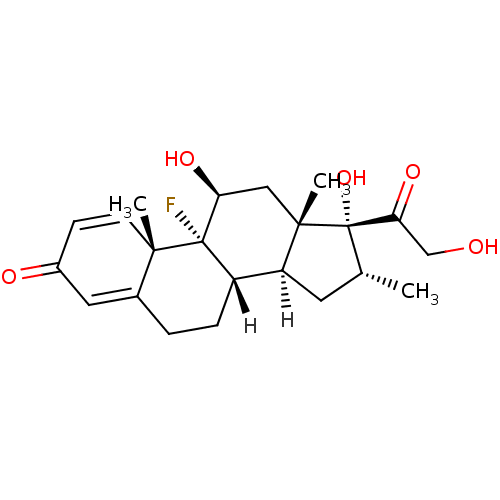 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50448167 (CHEMBL3120318) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.967 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50448165 (CHEMBL3120320) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50448166 (CHEMBL3120319) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50448168 (CHEMBL3120317) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human mineralocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50448166 (CHEMBL3120319) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50448167 (CHEMBL3120318) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50448167 (CHEMBL3120318) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50448168 (CHEMBL3120317) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50448165 (CHEMBL3120320) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448169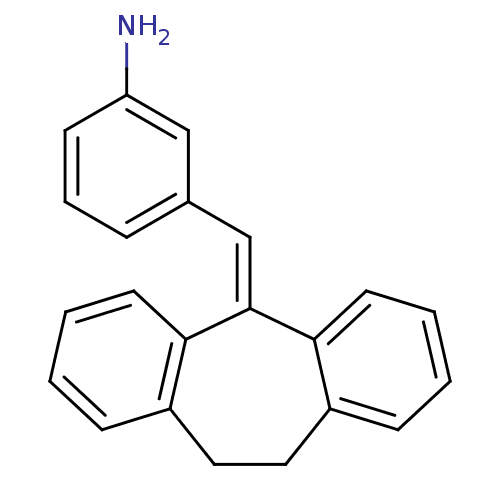 (CHEMBL3120316) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50185243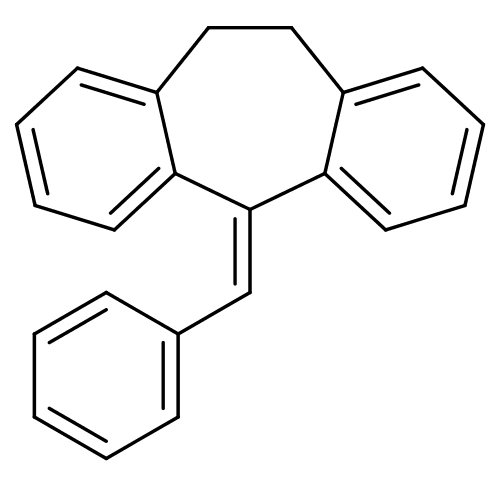 (5-benzylidene-10,11-dihydro-5H-dibenzo[a,d]cyclohe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448170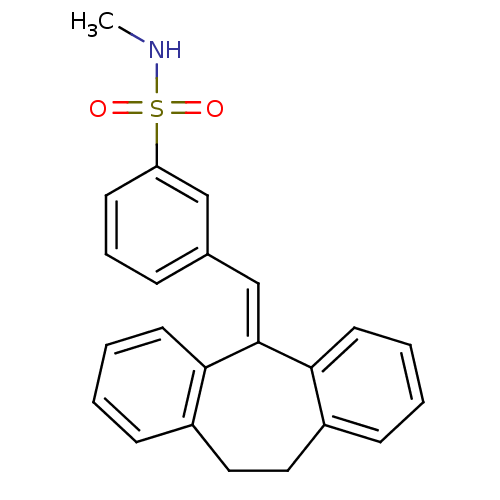 (CHEMBL3120315) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448171 (CHEMBL3120323) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448172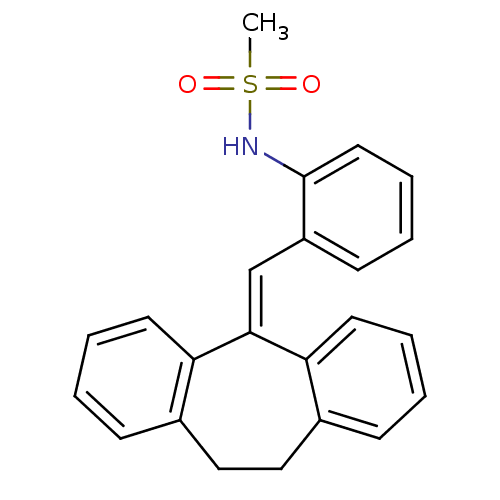 (CHEMBL3120322) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50448173 (CHEMBL3120321) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from human glucocorticoid receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50448168 (CHEMBL3120317) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50448165 (CHEMBL3120320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 418 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 561 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human androgen receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from human progesterone receptor expressed in HEK293 cells | J Med Chem 57: 849-60 (2014) Article DOI: 10.1021/jm401616g BindingDB Entry DOI: 10.7270/Q23X885F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065118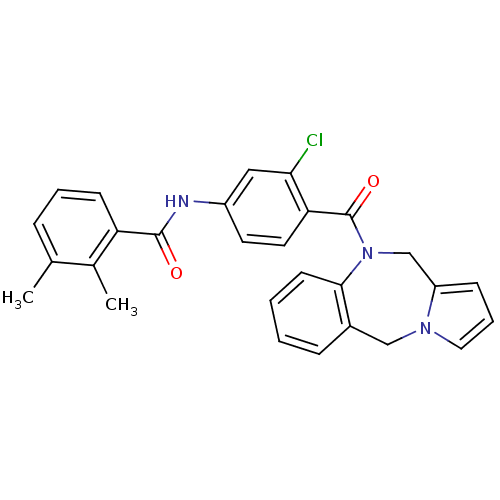 (CHEMBL311931 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50078646 (CHEMBL49322 | N-[4-(4,10-Dihydro-1-thia-9-aza-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065123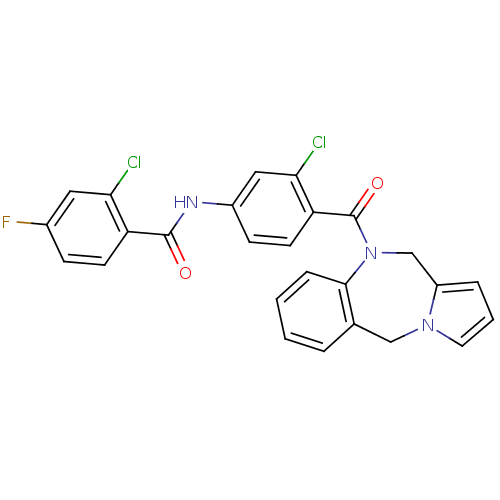 (CHEMBL420031 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human V2 receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065121 (CHEMBL310416 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078656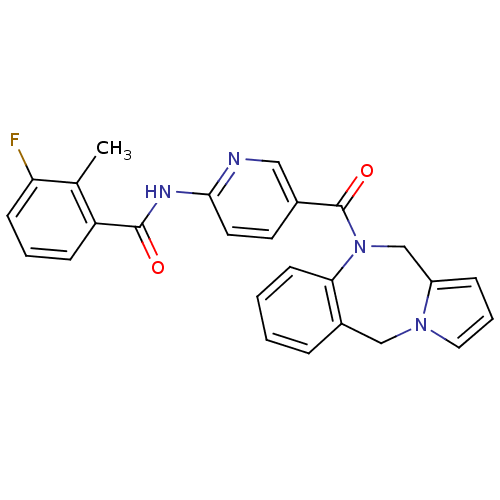 (CHEMBL46295 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 (Homo sapiens (Human)) | BDBM16314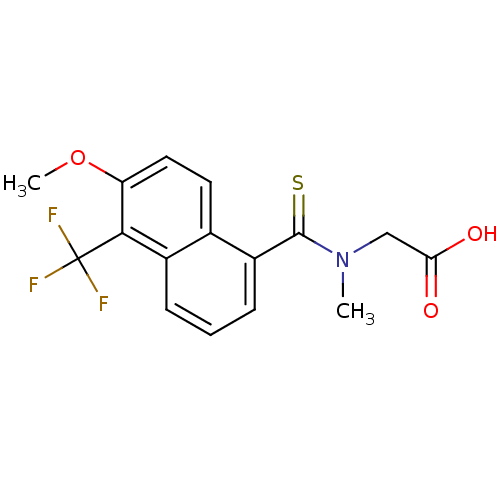 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078652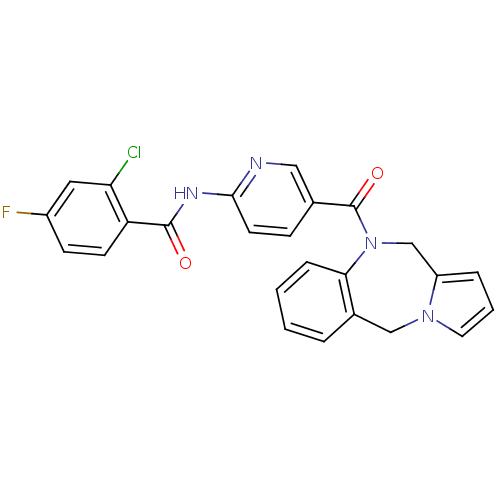 (CHEMBL301788 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B10 (Homo sapiens (Human)) | BDBM16314 (2-{[6-methoxy-5-(trifluoromethyl)naphthalen-1-yl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Institut de Ge´ne´tique et de Biologie Mole´culaire et Cellulaire | Assay Description The IC50-activity assays were carried out on the basis of the quantification of the NADPH consumption that takes place when the enzyme catalyzes the ... | ACS Chem Biol 11: 2693-2705 (2016) Article DOI: 10.1021/acschembio.6b00382 BindingDB Entry DOI: 10.7270/Q2NG4PFZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50087550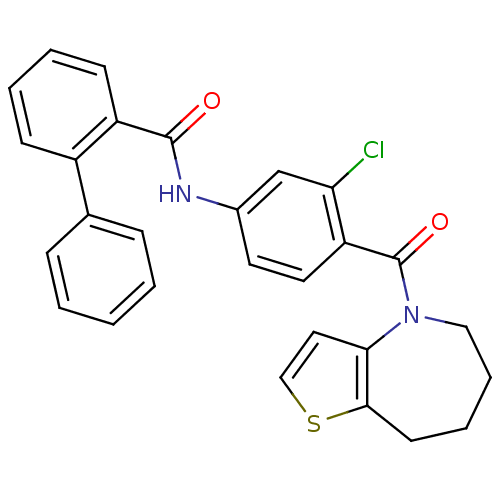 (Biphenyl-2-carboxylic acid [3-chloro-4-(5,6,7,8-te...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065124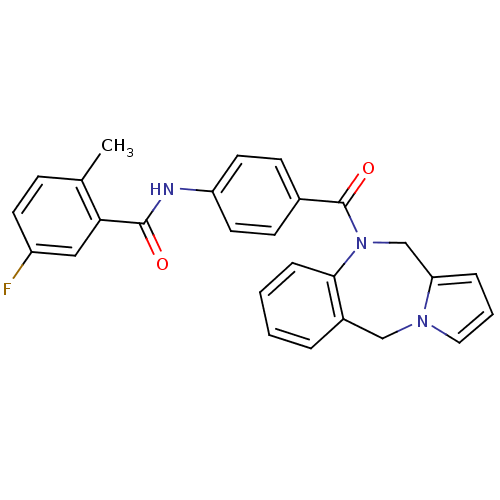 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of [3H]-AVP from human V2 receptor expressed in murine fibroblast cell line (LV2) membranes | J Med Chem 41: 2442-4 (1998) Article DOI: 10.1021/jm980179c BindingDB Entry DOI: 10.7270/Q2CC0ZT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50087541 (Biphenyl-2-carboxylic acid [4-(5H,11H-benzo[e]pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Binding affinity to rat V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065124 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078660 (CHEMBL299532 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50087551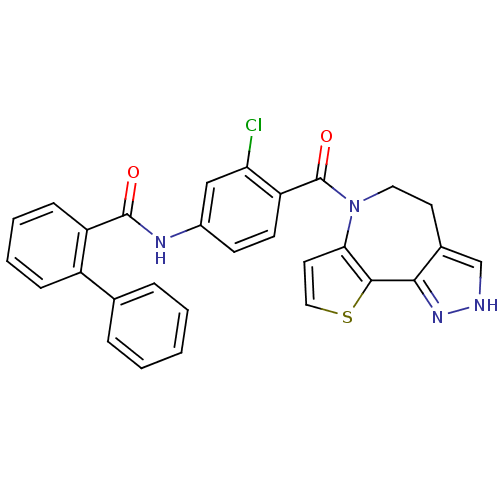 (Biphenyl-2-carboxylic acid [3-chloro-4-(4,5-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro binding affinity to human V2 receptor | Bioorg Med Chem Lett 10: 695-8 (2000) BindingDB Entry DOI: 10.7270/Q2X34WP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078651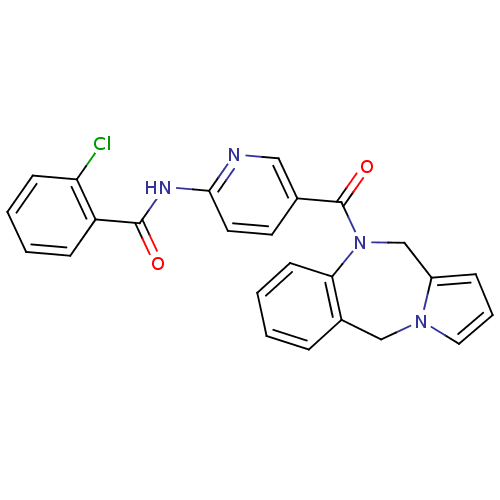 (CHEMBL300963 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 583 total ) | Next | Last >> |