

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127501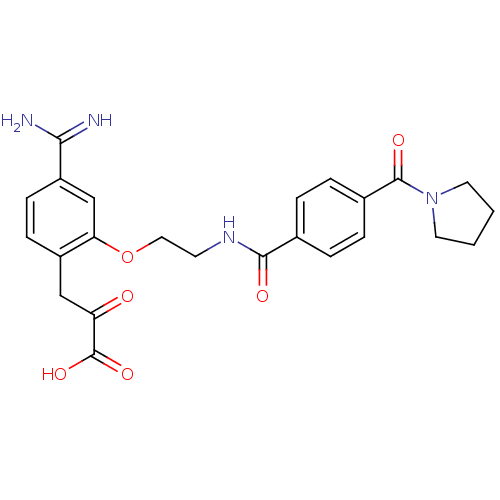 (3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127492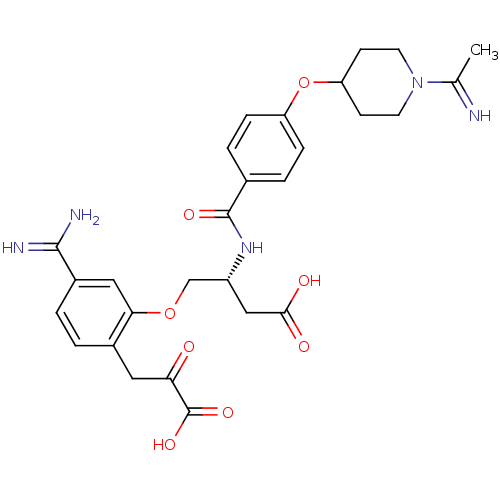 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127502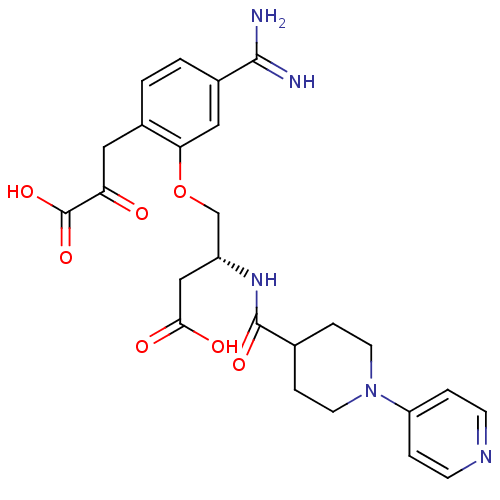 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127495 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127504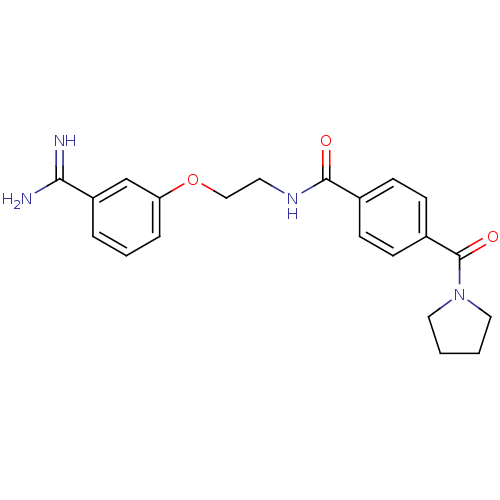 (CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127494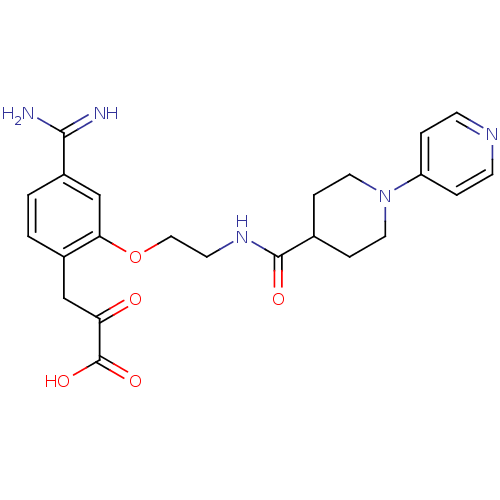 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127498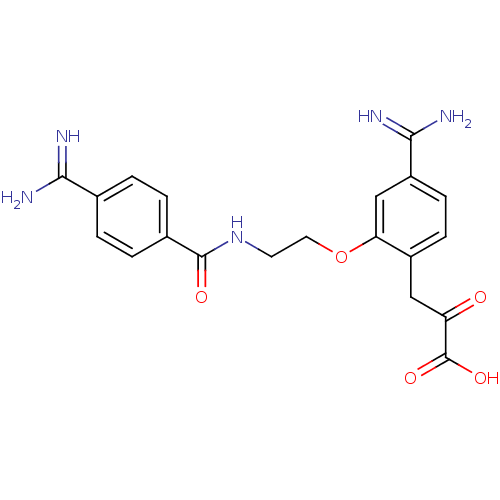 (3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127503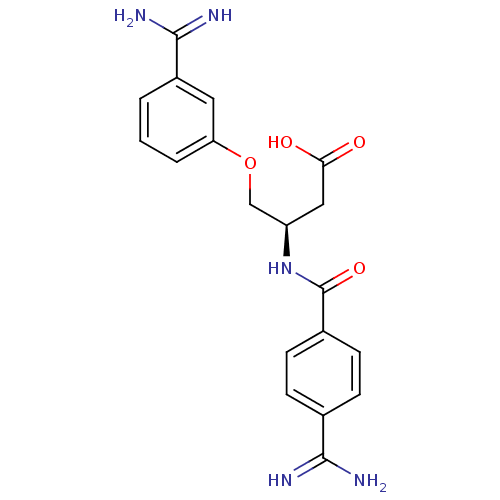 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127493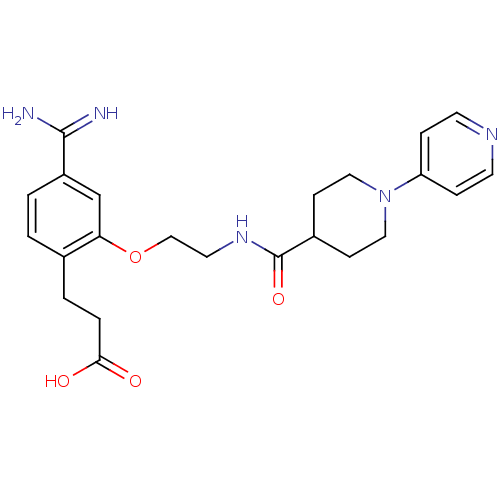 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127496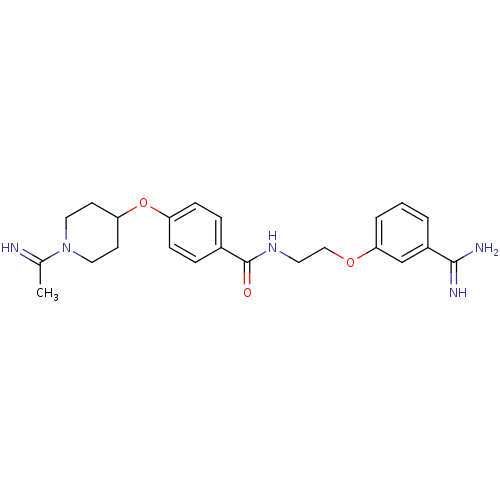 (CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127506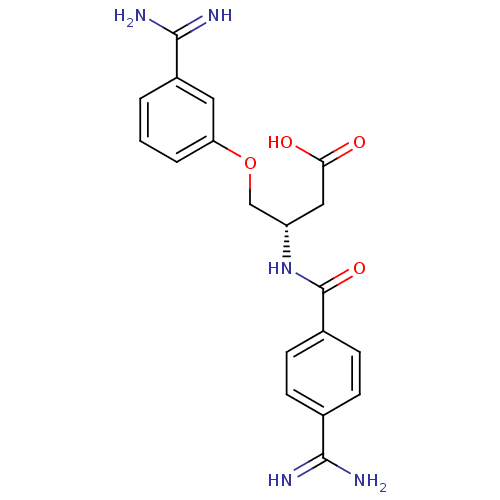 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127497 (4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127500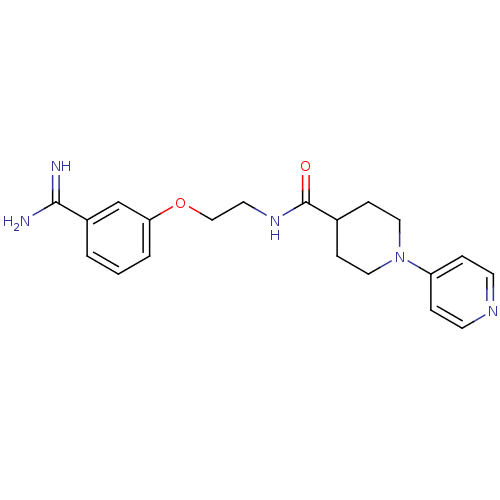 (3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127499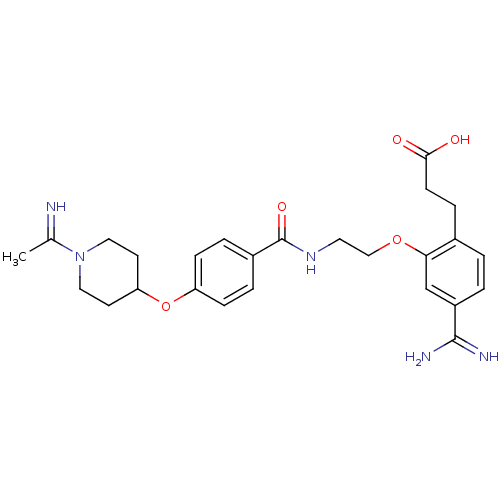 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50127505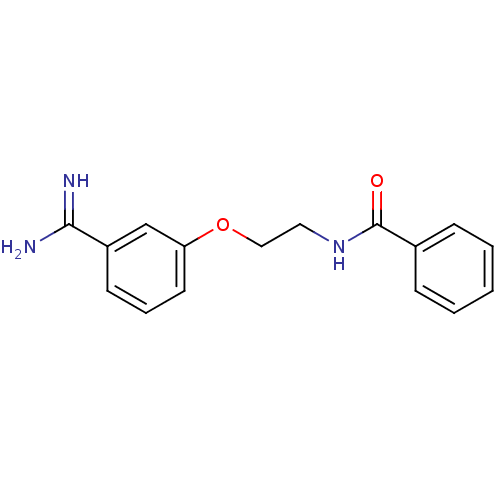 (CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Coagulation factor X (fXa) | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127501 (3-(4-Carbamimidoyl-2-{2-[4-(pyrrolidine-1-carbonyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127498 (3-{4-Carbamimidoyl-2-[2-(4-carbamimidoyl-benzoylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127495 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127504 (CHEMBL55770 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127494 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127496 (CHEMBL51796 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127492 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127502 (4-[5-Carbamimidoyl-2-(2-carboxy-2-oxo-ethyl)-pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127497 (4-Carbamimidoyl-N-[2-(3-carbamimidoyl-phenoxy)-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127505 (CHEMBL55364 | N-[2-(3-Carbamimidoyl-phenoxy)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127500 (3,4,5,6-Tetrahydro-2H-[1,4']bipyridinyl-4-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127493 (3-(4-Carbamimidoyl-2-{2-[(3,4,5,6-tetrahydro-2H-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127503 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127499 (3-[4-Carbamimidoyl-2-(2-{4-[1-(1-imino-ethyl)-pipe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50127506 (3-(4-Carbamimidoyl-benzoylamino)-4-(3-carbamimidoy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ajinomoto Company Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | J Med Chem 46: 1845-57 (2003) Article DOI: 10.1021/jm020485x BindingDB Entry DOI: 10.7270/Q2PZ586C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Homo sapiens (Human)) | BDBM640486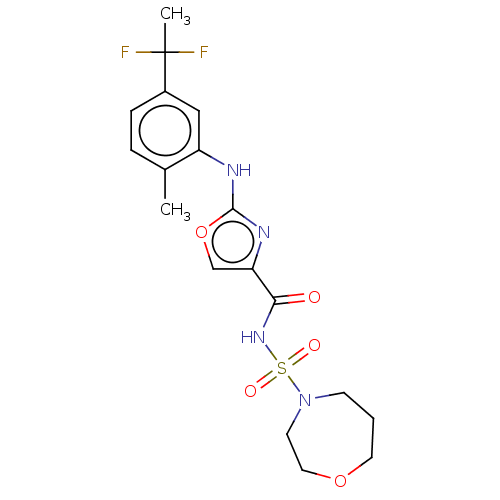 (US20230399319, Example 2-031) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493926 (US10988462, Example 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493906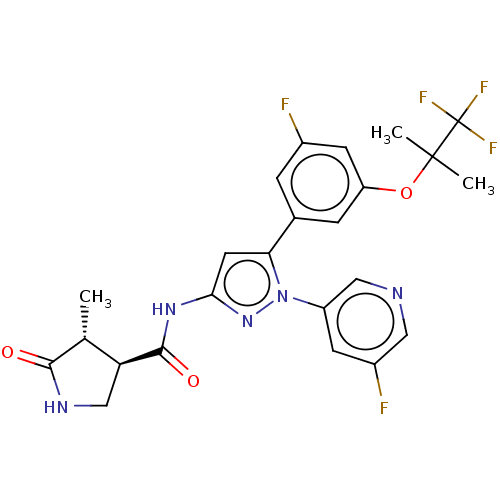 (US10988462, Example 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493905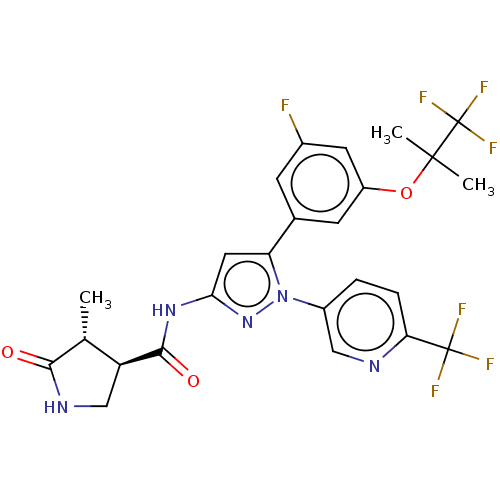 (US10988462, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493894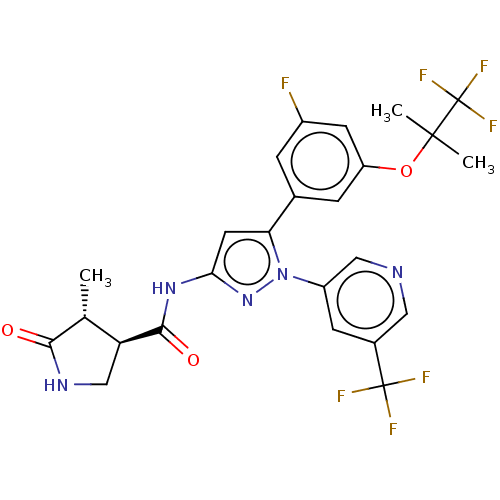 (US10988462, Example 10) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493901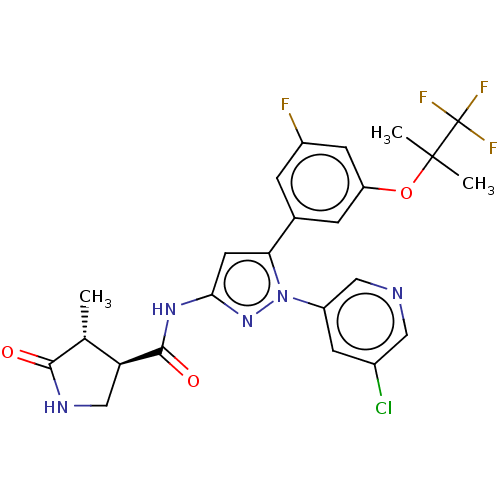 (US10988462, Example 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493925 (US10988462, Example 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493879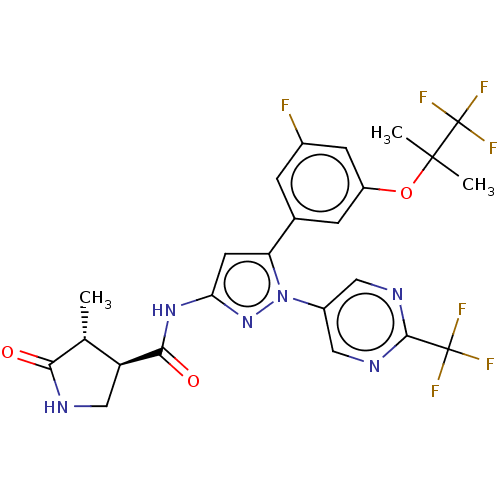 (US10988462, Example 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493917 (US10988462, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493897 (US10988462, Example 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Short transient receptor potential channel 6 (Homo sapiens (Human)) | BDBM50273013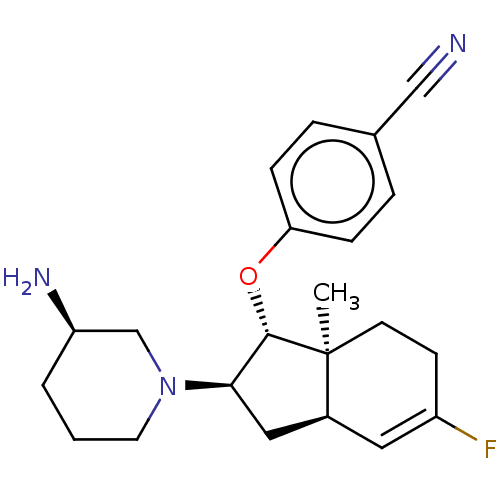 (CHEMBL4129456) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human TRPC6 expressed in HEK293 cells assessed as decrease in intracellular calcium level after 24 hrs by Fluo-4 dye-based FLIPR assay | Bioorg Med Chem Lett 28: 2222-2227 (2018) Article DOI: 10.1016/j.bmcl.2018.03.056 BindingDB Entry DOI: 10.7270/Q2CF9SMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493907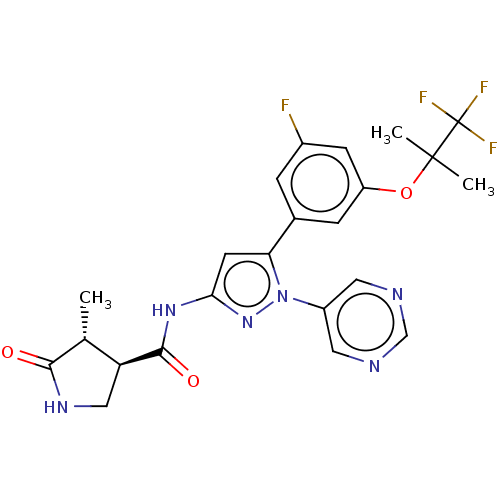 (US10988462, Example 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493898 (US10988462, Example 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493927 (US10988462, Example 38) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493896 (US10988462, Example 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493924 (US10988462, Example 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493909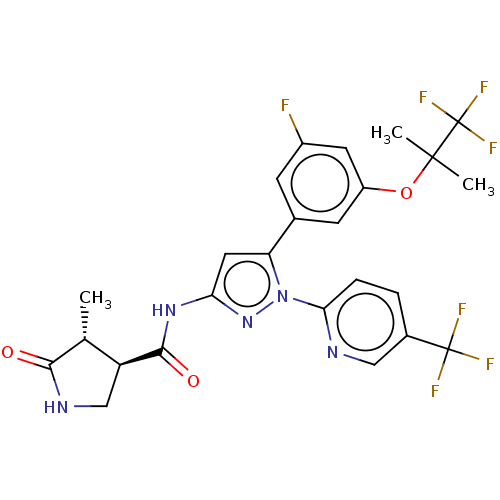 (US10988462, Example 24) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493918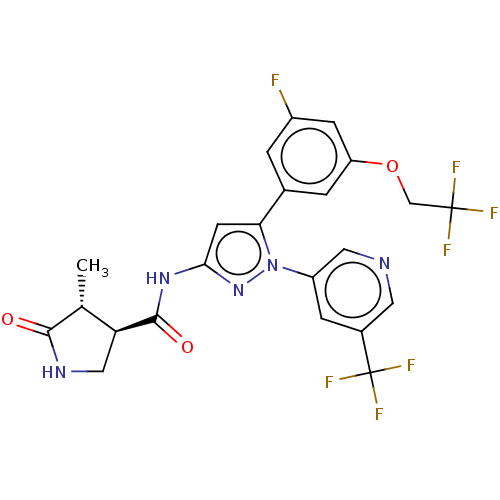 (US10988462, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493910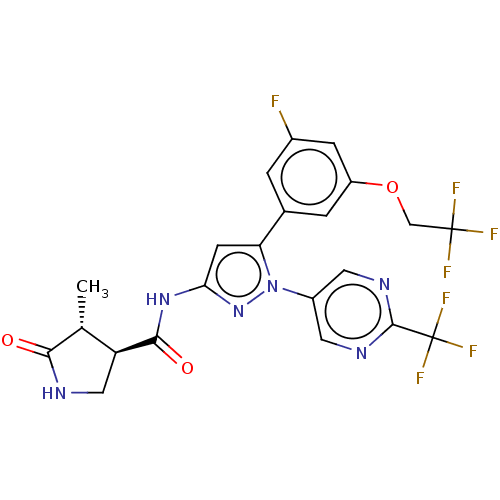 (US10988462, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium/glucose cotransporter 1 (Homo sapiens (Human)) | BDBM493921 (US10988462, Example 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Tobacco Inc. US Patent | Assay Description Human SGLT1-stably-expressing cell lines were seeded at 5×104 cells/well on BioCoat™ Poly-D-Lysine 96 well plate with Lid (Becton, Dickinson and Comp... | US Patent US10988462 (2021) BindingDB Entry DOI: 10.7270/Q2TH8QV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 564 total ) | Next | Last >> |