

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413761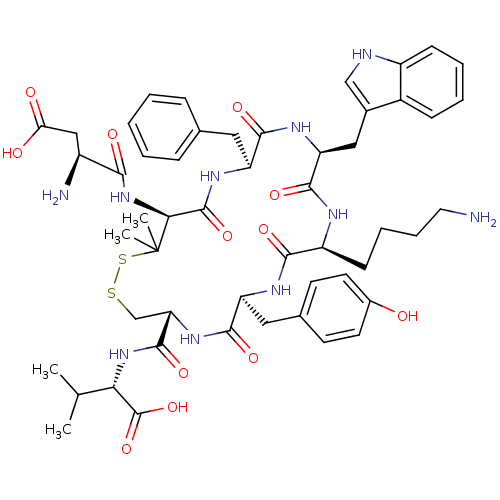 (CHEMBL390094) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413760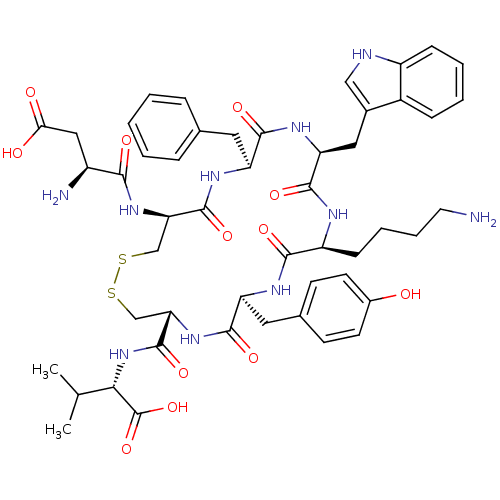 (CHEMBL426020) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413764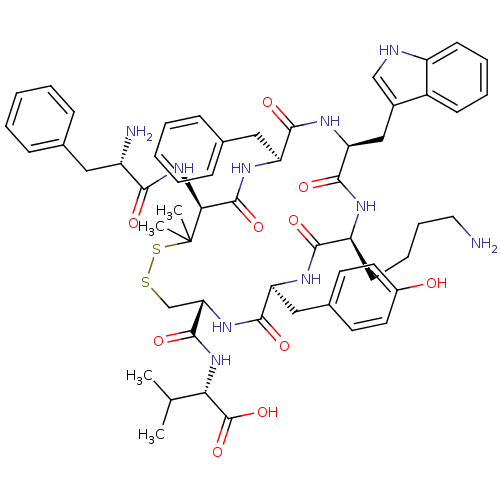 (CHEMBL504097) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50378580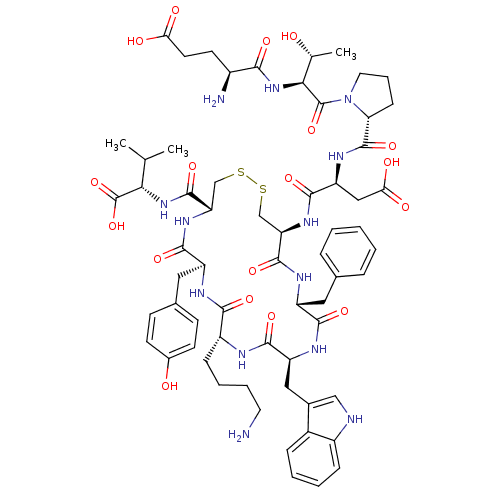 (CHEMBL437430) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413762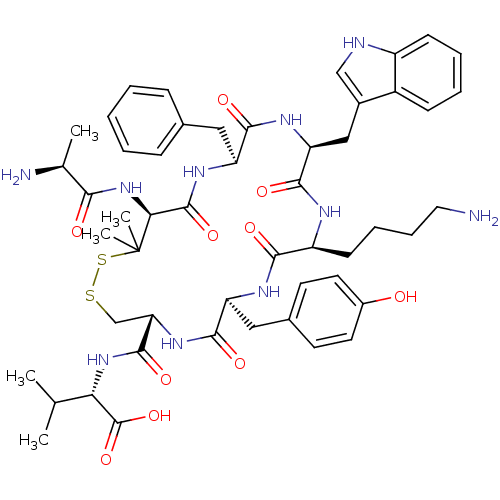 (CHEMBL509604) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413766 (CHEMBL510618) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.891 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50555050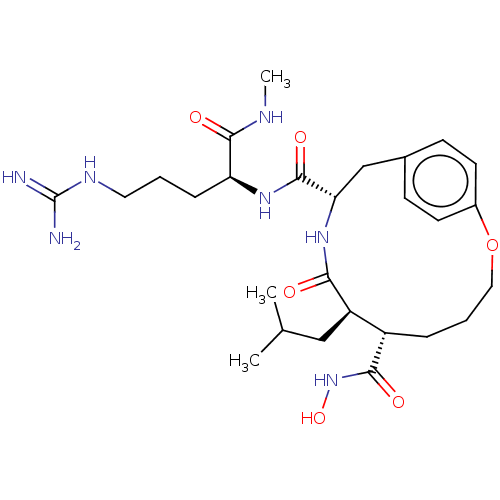 (CHEMBL4764575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP12 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50555052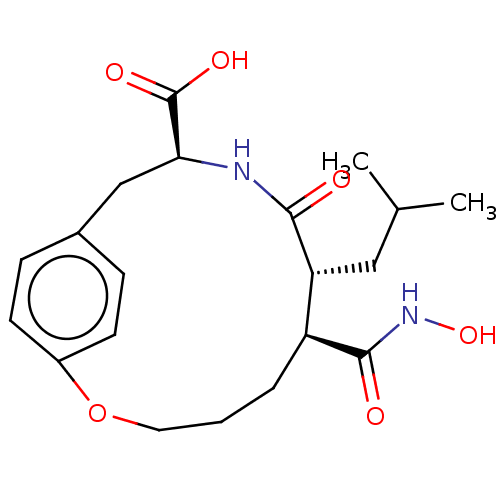 (CHEMBL4751015) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP12 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50555051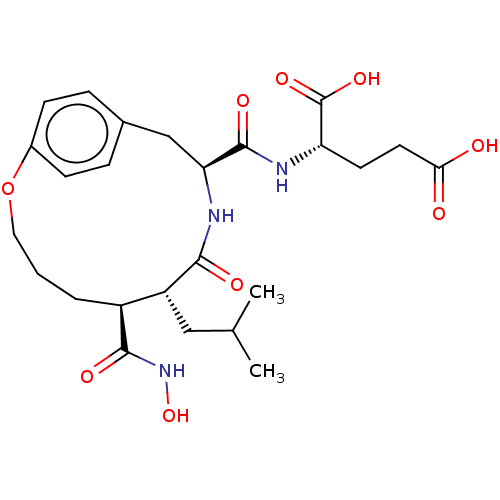 (CHEMBL4739996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP12 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50432208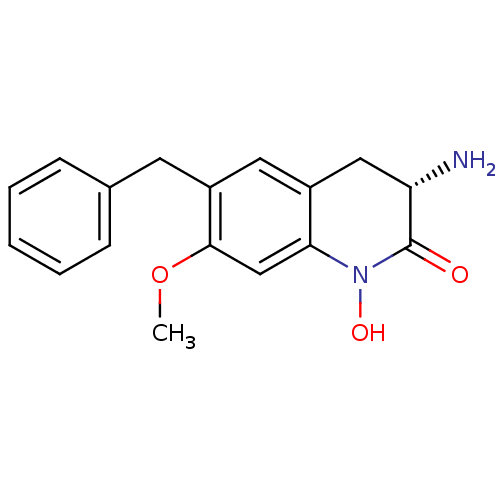 (CHEMBL2347110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413763 (CHEMBL448403) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426340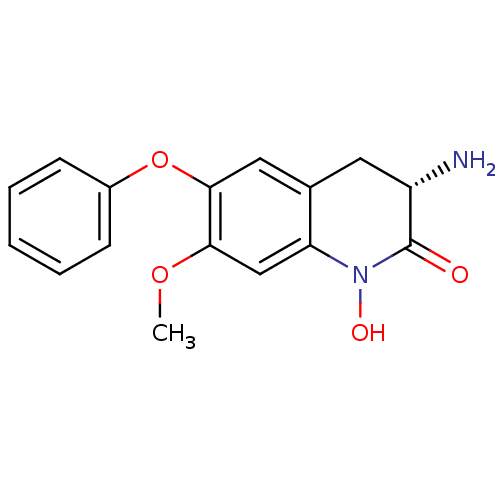 (CHEMBL2321943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107730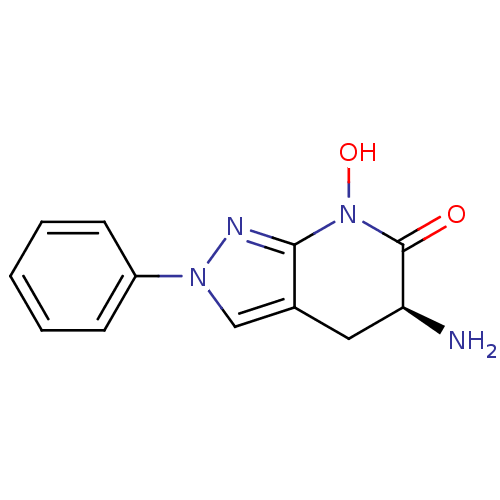 (CHEMBL2347108 | US8933095, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413777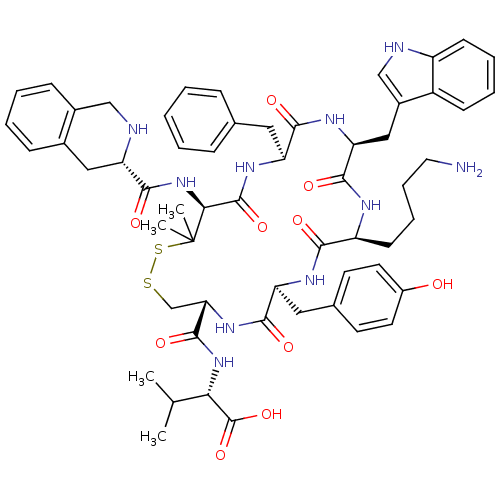 (CHEMBL524855) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413769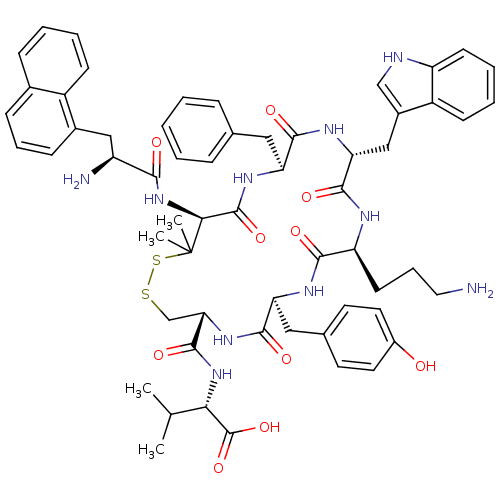 (CHEMBL507406) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50465935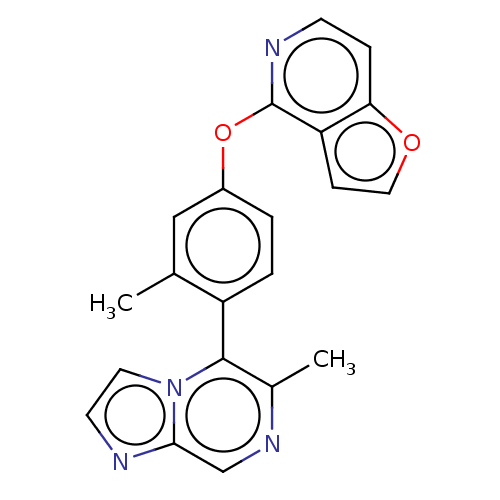 (CHEMBL4277264) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at dopamine D5 receptor (unknown origin) | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50554340 (CHEMBL4788866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged human AMCase expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50411333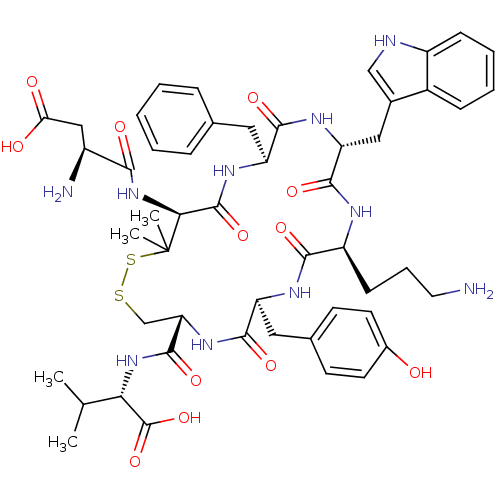 (URANTIDE) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50554340 (CHEMBL4788866) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full-length C-terminal his-tagged mouse AMCase expressed in expressed in CHO-K1 cells assessed as reduction in chitinolytic activity us... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01179 BindingDB Entry DOI: 10.7270/Q2B85CS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50555050 (CHEMBL4764575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP2 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413770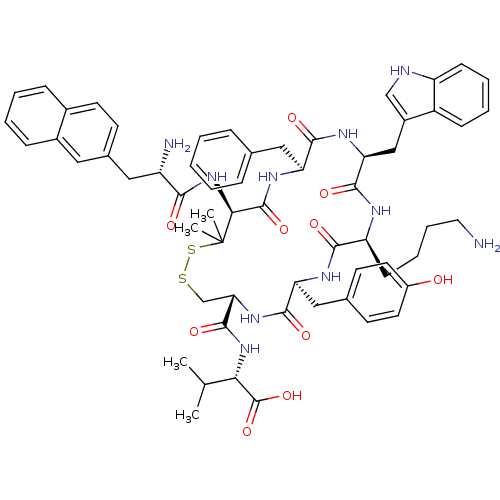 (CHEMBL452403) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465945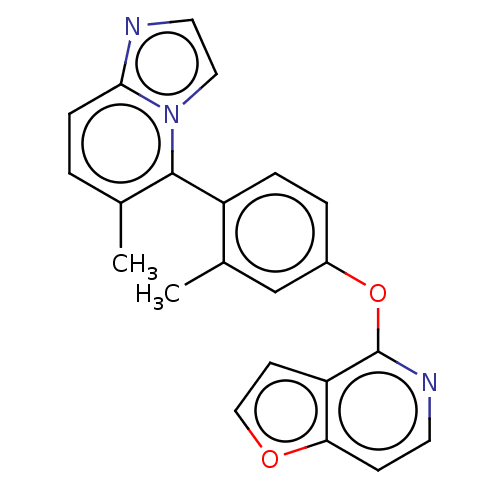 (CHEMBL4279267) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197827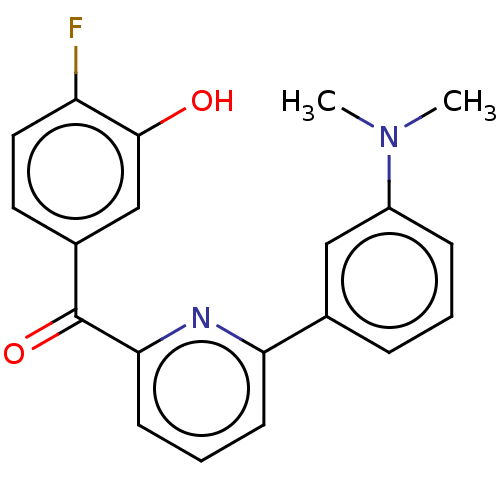 (CHEMBL3894507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197835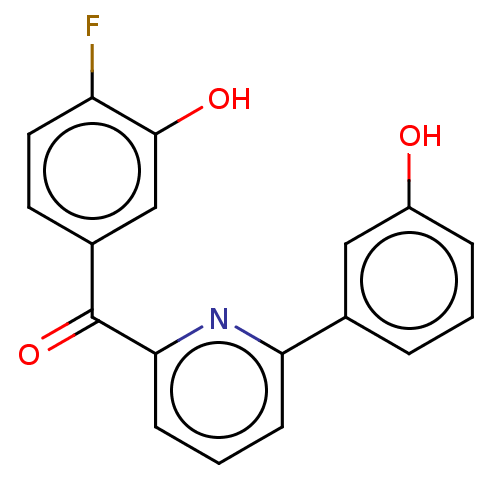 (CHEMBL3965905) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50189884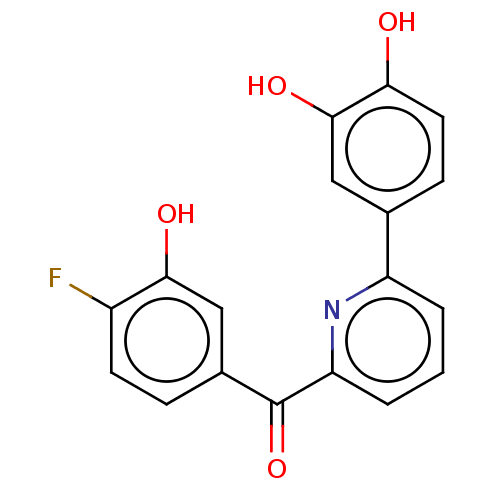 (CHEMBL3827618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50426341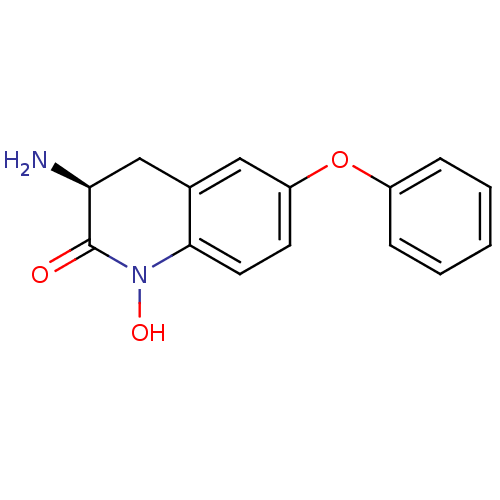 (CHEMBL2321944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50555048 (CHEMBL4793938) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP12 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386310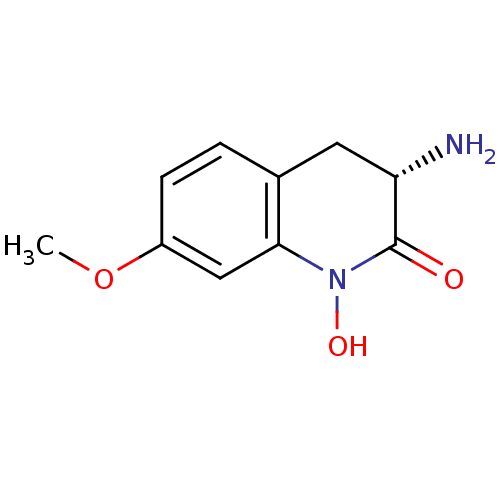 (CHEMBL2049092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465944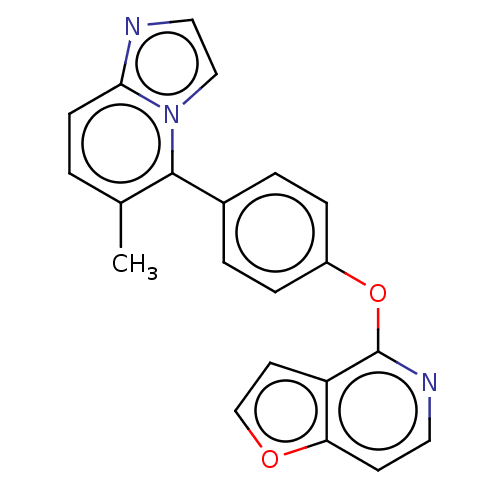 (CHEMBL4286177) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50555047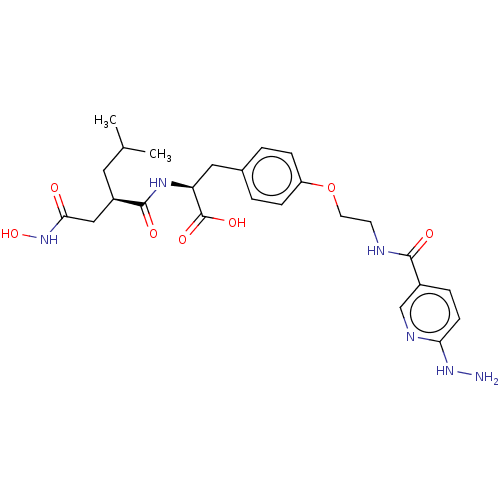 (CHEMBL4794902) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP12 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197841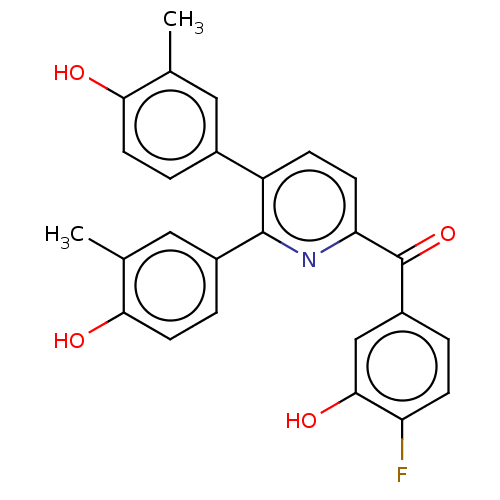 (CHEMBL3932068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197843 (CHEMBL3941236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413775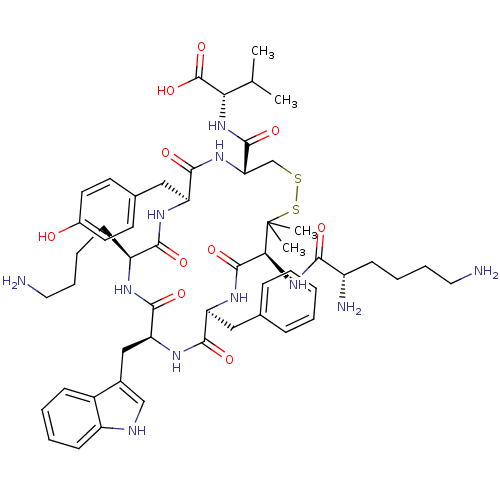 (CHEMBL501794) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413774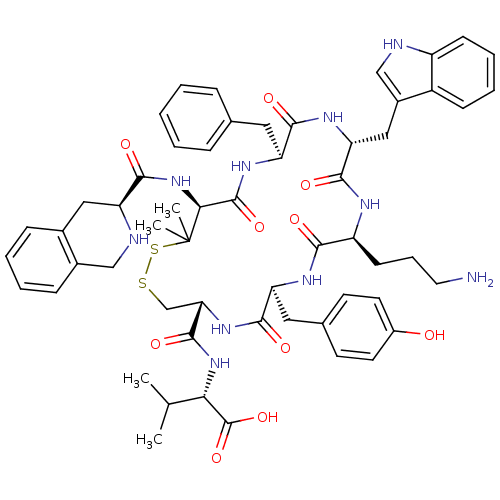 (CHEMBL509009) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413767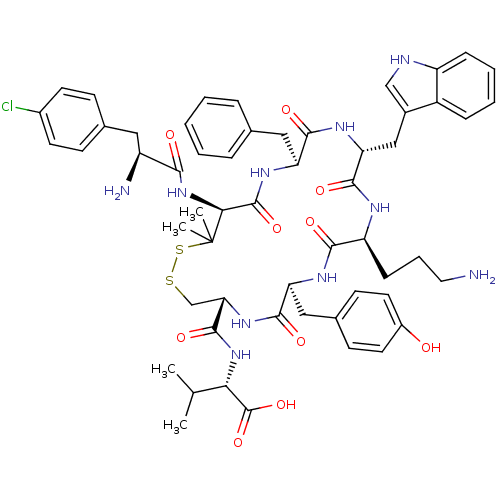 (CHEMBL504723) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197832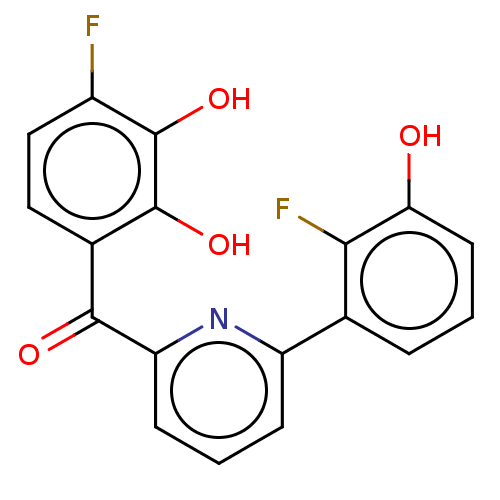 (CHEMBL3913249) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413771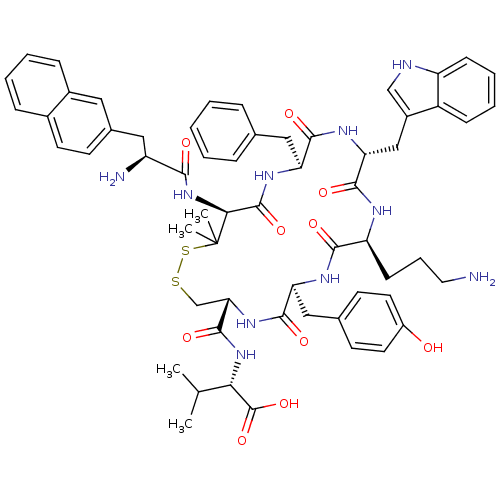 (CHEMBL509042) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM50555049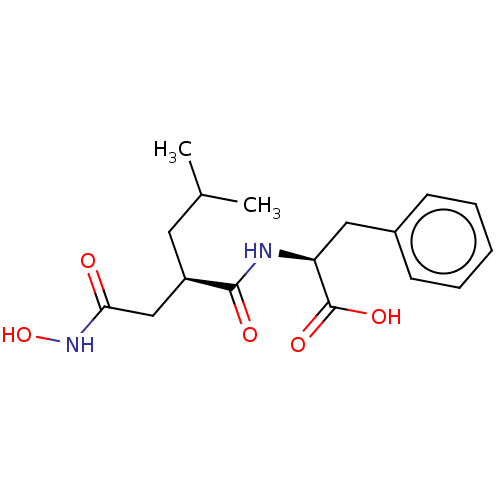 (CHEMBL4747528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP12 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM107747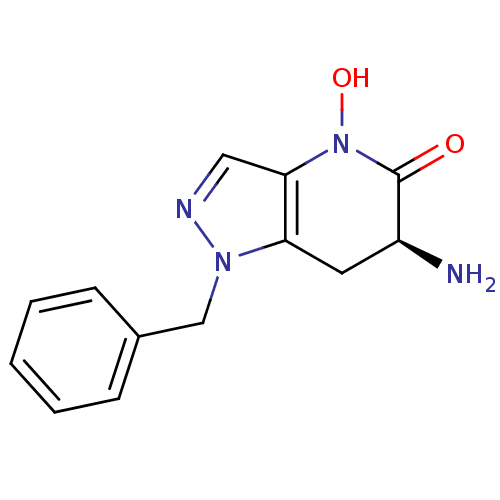 (CHEMBL2347115 | US8933095, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197831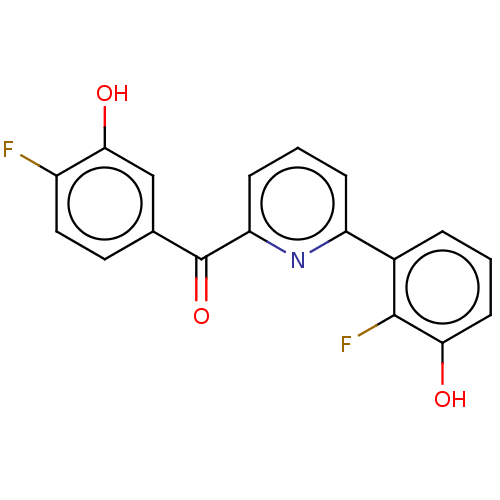 (CHEMBL3949996) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413772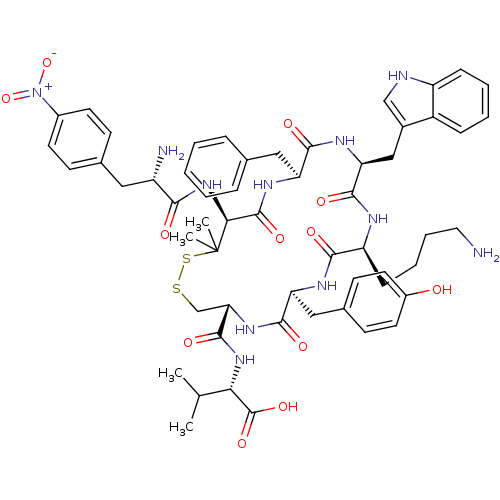 (CHEMBL508811) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292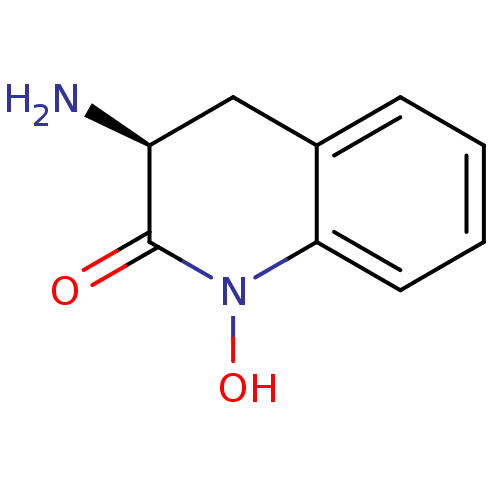 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human KAT2 using L-kynurenine as substrate after 15 to 20 hrs by UV-visible spectra analysis | Bioorg Med Chem Lett 23: 1961-6 (2013) Article DOI: 10.1016/j.bmcl.2013.02.039 BindingDB Entry DOI: 10.7270/Q2N87C48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kynurenine/alpha-aminoadipate aminotransferase, mitochondrial (Homo sapiens (Human)) | BDBM50386292 (CHEMBL2047851) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Irreversible inhibition of human KAT2 using L-kynurenine as substrate measured every 5 mins over 16 hrs by SpectraMax plate reader analysis | ACS Med Chem Lett 4: 37-40 (2013) Article DOI: 10.1021/ml300237v BindingDB Entry DOI: 10.7270/Q2KH0PNV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197865 (CHEMBL3938843) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50555050 (CHEMBL4764575) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP13 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50555051 (CHEMBL4739996) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human recombinant MMP7 using Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 substrate by spectrophotometry | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01514 BindingDB Entry DOI: 10.7270/Q2PC361P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM50413773 (CHEMBL500949) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Displacement of [125I]urotensin-2 from human UT2 receptor expressed in CHO-K1 cells by liquid scintillation counting | J Med Chem 52: 3927-40 (2009) Article DOI: 10.1021/jm900148c BindingDB Entry DOI: 10.7270/Q27W6DFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase 14 (Homo sapiens (Human)) | BDBM50197837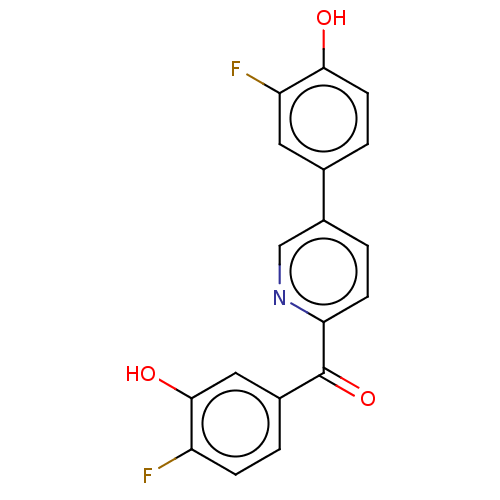 (CHEMBL3911402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg Curated by ChEMBL | Assay Description Inhibition of N-terminal 6His-tagged human human HSD17B14 expressed in Escherichia coli BL21 (DE3) pLysS using E2 substrate and NAD+ incubated for 2 ... | J Med Chem 59: 10719-10737 (2016) Article DOI: 10.1021/acs.jmedchem.6b01436 BindingDB Entry DOI: 10.7270/Q2KH0Q9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1297 total ) | Next | Last >> |