 Found 716 hits with Last Name = 'salunke' and Initial = 'd'
Found 716 hits with Last Name = 'salunke' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406601

(CHEMBL5276854)Show SMILES CC(C)c1nc(c(C)c(-c2ccc(F)cc2)c1CCP(O)([O-])CC(=O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C27H30FNO5P/c1-17(2)26-23(13-14-35(33,34)16-22(30)15-24(31)32)25(19-9-11-21(28)12-10-19)18(3)27(29-26)20-7-5-4-6-8-20/h4-12,17,33,35H,13-16H2,1-3H3,(H,31,32)/q-1/p-1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative log concentration of antagonist was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406600
(CHEMBL5267159)Show SMILES CC(C)c1nc(c(C)c(-c2ccc(F)cc2)c1CCP(O)([O-])=CC(=O)CC([O-])=O)-c1ccccc1 Show InChI InChI=1S/C27H29FNO5P/c1-17(2)26-23(13-14-35(33,34)16-22(30)15-24(31)32)25(19-9-11-21(28)12-10-19)18(3)27(29-26)20-7-5-4-6-8-20/h4-12,16-17H,13-15H2,1-3H3,(H3,30,31,32,33,34)/p-2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative log concentration of antagonist was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608747

(CHEMBL5271653) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608741

(CHEMBL5284391) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608748
(CHEMBL5287590) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Toll-like receptor 2
(Homo sapiens (Human)) | BDBM50406599

(CHEMBL5276410)Show SMILES Cc1c(nc(C2CC2)c(C=CP(O)([O-])=CC(=O)CC([O-])=O)c1-c1ccc(F)cc1)-c1ccccc1 |w:10.11| Show InChI InChI=1S/C27H25FNO5P/c1-17-25(18-9-11-21(28)12-10-18)23(13-14-35(33,34)16-22(30)15-24(31)32)27(20-7-8-20)29-26(17)19-5-3-2-4-6-19/h2-6,9-14,16,20H,7-8,15H2,1H3,(H3,30,31,32,33,34)/p-2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Negative log concentration of antagonistic compound was determined on 5-hydroxytryptamine 2B receptor of Rat stomach fundus |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608744
(CHEMBL270735) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608743
(CHEMBL5280544) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608742
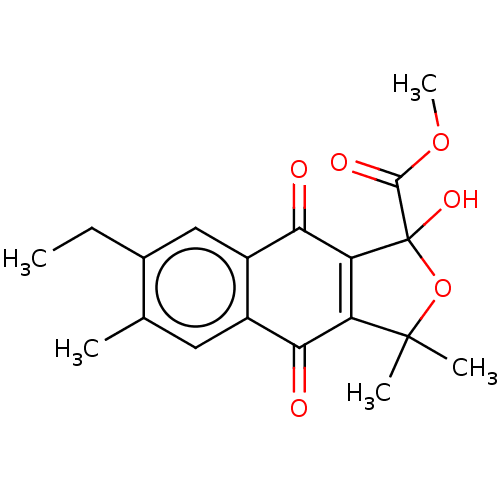
(CHEMBL5276994) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50183456
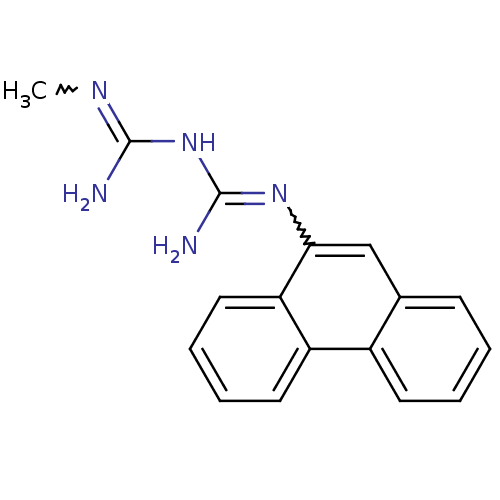
(CHEMBL425403 | N-methyl-N'-9-phenanthrylimidodicar...)Show SMILES CN=C(N)NC(N)=Nc1cc2ccccc2c2ccccc12 |w:7.7,1.0| Show InChI InChI=1S/C17H17N5/c1-20-16(18)22-17(19)21-15-10-11-6-2-3-7-12(11)13-8-4-5-9-14(13)15/h2-10H,1H3,(H5,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50184688
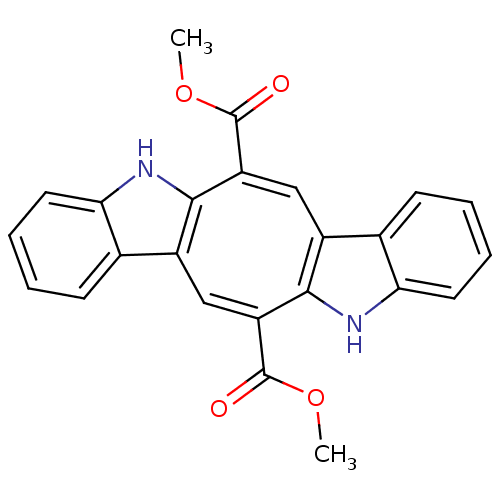
(CHEMBL377236 | Caulerpin)Show SMILES COC(=O)C1=C/c2c([nH]c3ccccc23)\C(=C/c2c\1[nH]c1ccccc21)C(=O)OC |c:17,t:4| Show InChI InChI=1S/C24H18N2O4/c1-29-23(27)17-11-15-13-7-3-6-10-20(13)26-22(15)18(24(28)30-2)12-16-14-8-4-5-9-19(14)25-21(16)17/h3-12,25-26H,1-2H3/b15-11-,16-12-,17-11+,18-12+,21-17-,22-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421815

(CHEMBL5276478)Show InChI InChI=1S/C12H15N5O/c1-18-10-5-7(2-3-9(10)13)4-8-6-16-12(15)17-11(8)14/h2-3,5-6H,4,13H2,1H3,(H4,14,15,16,17) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421812
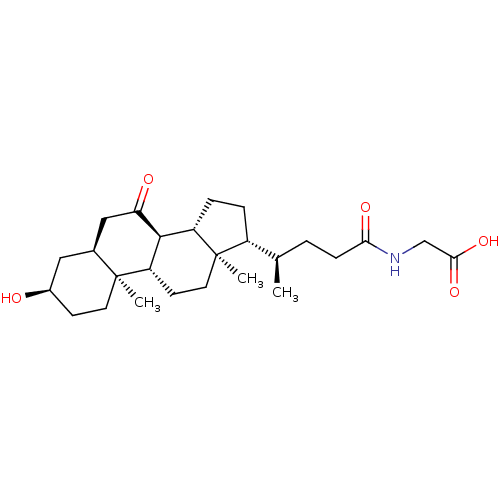
(CHEMBL5279244)Show InChI InChI=1S/C16H22N4O/c1-3-5-12-7-10(6-11(4-2)14(12)21)8-13-9-19-16(18)20-15(13)17/h6-7,9,21H,3-5,8H2,1-2H3,(H4,17,18,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 4.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421811
(CHEMBL5287944)Show InChI InChI=1S/C17H24N4O3/c1-4-22-13-8-11(7-12-10-20-17(19)21-16(12)18)9-14(23-5-2)15(13)24-6-3/h8-10H,4-7H2,1-3H3,(H4,18,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 5.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421811

(CHEMBL5287944)Show InChI InChI=1S/C17H24N4O3/c1-4-22-13-8-11(7-12-10-20-17(19)21-16(12)18)9-14(23-5-2)15(13)24-6-3/h8-10H,4-7H2,1-3H3,(H4,18,19,20,21) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 6.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608746
(CHEMBL5275669) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421815

(CHEMBL5276478)Show InChI InChI=1S/C12H15N5O/c1-18-10-5-7(2-3-9(10)13)4-8-6-16-12(15)17-11(8)14/h2-3,5-6H,4,13H2,1H3,(H4,14,15,16,17) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 8.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421813
(CHEMBL5272039)Show InChI InChI=1S/C11H11Cl2N5/c12-7-2-5(3-8(13)9(7)14)1-6-4-17-11(16)18-10(6)15/h2-4H,1,14H2,(H4,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 9.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421814

(CHEMBL5287717)Show InChI InChI=1S/C12H12I2N4O/c1-19-10-8(13)3-6(4-9(10)14)2-7-5-17-12(16)18-11(7)15/h3-5H,2H2,1H3,(H4,15,16,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421814

(CHEMBL5287717)Show InChI InChI=1S/C12H12I2N4O/c1-19-10-8(13)3-6(4-9(10)14)2-7-5-17-12(16)18-11(7)15/h3-5H,2H2,1H3,(H4,15,16,17,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421813

(CHEMBL5272039)Show InChI InChI=1S/C11H11Cl2N5/c12-7-2-5(3-8(13)9(7)14)1-6-4-17-11(16)18-10(6)15/h2-4H,1,14H2,(H4,15,16,17,18) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ileal sodium/bile acid cotransporter
(Homo sapiens (Human)) | BDBM50421812

(CHEMBL5279244)Show InChI InChI=1S/C16H22N4O/c1-3-5-12-7-10(6-11(4-2)14(12)21)8-13-9-19-16(18)20-15(13)17/h6-7,9,21H,3-5,8H2,1-2H3,(H4,17,18,19,20) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50428658
(MITOMYCIN | Mitomycin C | Mitosol | Mitozytrex | M...)Show SMILES CO[C@]12[C@H]3N[C@H]3CN1C1=C([C@H]2COC(N)=O)C(=O)C(N)=C(C)C1=O |r,c:10,t:22| Show InChI InChI=1S/C15H18N4O5/c1-5-9(16)12(21)8-6(4-24-14(17)22)15(23-2)13-7(18-13)3-19(15)10(8)11(5)20/h6-7,13,18H,3-4,16H2,1-2H3,(H2,17,22)/t6-,7+,13+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608745
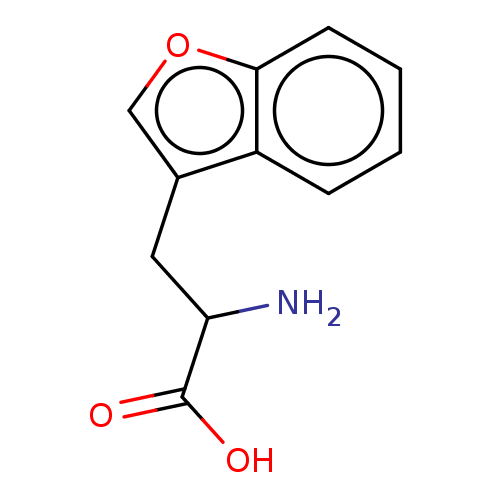
(CHEMBL5272397) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM34233

(2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...)Show InChI InChI=1S/C13H9NOSe/c15-13-11-8-4-5-9-12(11)16-14(13)10-6-2-1-3-7-10/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 9.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24813
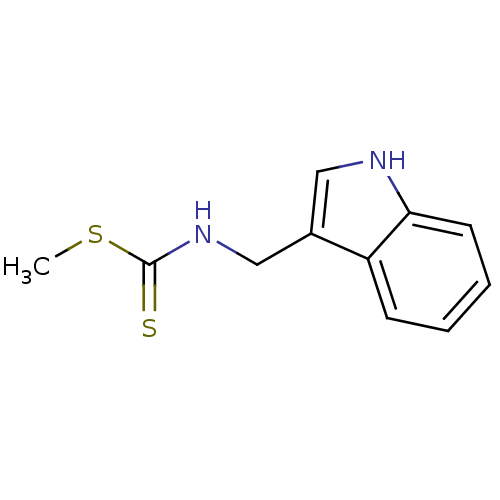
(Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...)Show InChI InChI=1S/C11H12N2S2/c1-15-11(14)13-7-8-6-12-10-5-3-2-4-9(8)10/h2-6,12H,7H2,1H3,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 9.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50562506
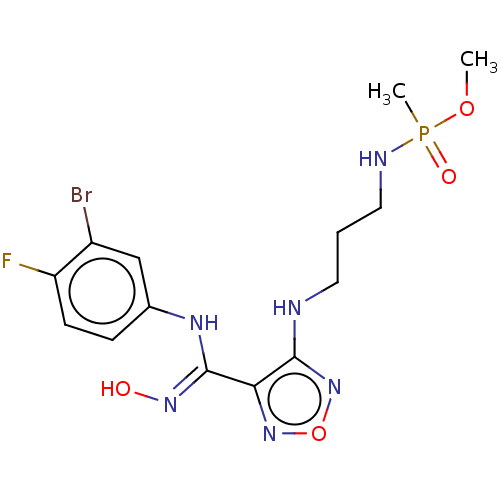
(CHEMBL4791168)Show SMILES COP(C)(=O)NCCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393129
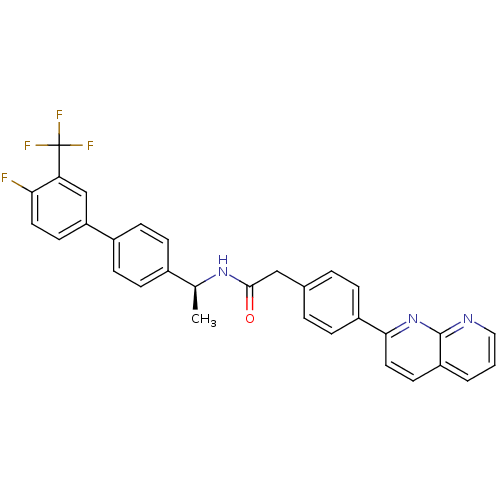
(CHEMBL2153461)Show SMILES C[C@H](NC(=O)Cc1ccc(cc1)-c1ccc2cccnc2n1)c1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H23F4N3O/c1-19(21-8-10-22(11-9-21)25-12-14-27(32)26(18-25)31(33,34)35)37-29(39)17-20-4-6-23(7-5-20)28-15-13-24-3-2-16-36-30(24)38-28/h2-16,18-19H,17H2,1H3,(H,37,39)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Rattus norvegicus) | BDBM50393161
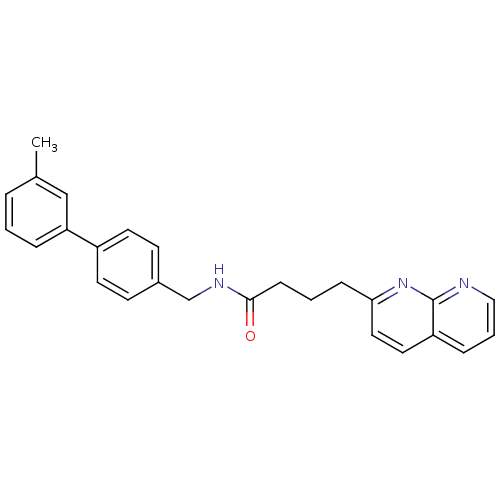
(CHEMBL2153439)Show SMILES Cc1cccc(c1)-c1ccc(CNC(=O)CCCc2ccc3cccnc3n2)cc1 Show InChI InChI=1S/C26H25N3O/c1-19-5-2-6-23(17-19)21-12-10-20(11-13-21)18-28-25(30)9-3-8-24-15-14-22-7-4-16-27-26(22)29-24/h2,4-7,10-17H,3,8-9,18H2,1H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at rat GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-induc... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50590774

(CHEMBL5203175) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393123
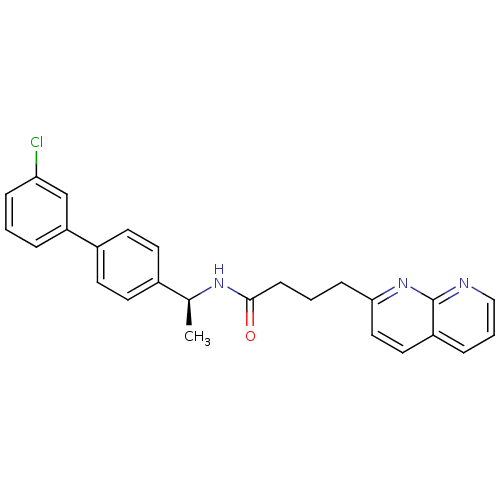
(CHEMBL2153446)Show SMILES C[C@H](NC(=O)CCCc1ccc2cccnc2n1)c1ccc(cc1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C26H24ClN3O/c1-18(19-10-12-20(13-11-19)22-5-2-7-23(27)17-22)29-25(31)9-3-8-24-15-14-21-6-4-16-28-26(21)30-24/h2,4-7,10-18H,3,8-9H2,1H3,(H,29,31)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393133
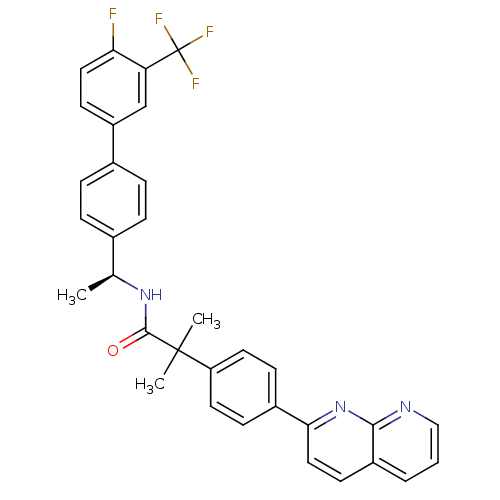
(CHEMBL2153465)Show SMILES C[C@H](NC(=O)C(C)(C)c1ccc(cc1)-c1ccc2cccnc2n1)c1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C33H27F4N3O/c1-20(21-6-8-22(9-7-21)25-12-16-28(34)27(19-25)33(35,36)37)39-31(41)32(2,3)26-14-10-23(11-15-26)29-17-13-24-5-4-18-38-30(24)40-29/h4-20H,1-3H3,(H,39,41)/t20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393122
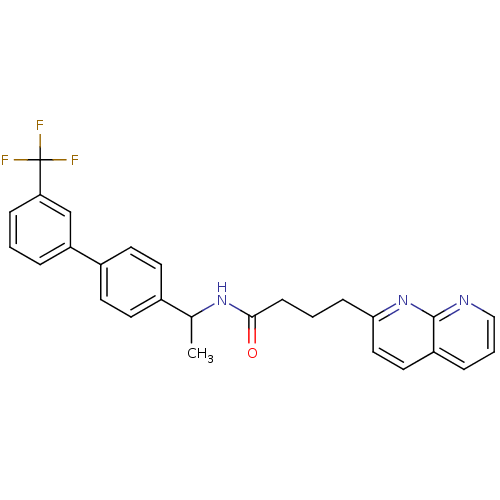
(CHEMBL2153445)Show SMILES CC(NC(=O)CCCc1ccc2cccnc2n1)c1ccc(cc1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C27H24F3N3O/c1-18(19-10-12-20(13-11-19)22-5-2-7-23(17-22)27(28,29)30)32-25(34)9-3-8-24-15-14-21-6-4-16-31-26(21)33-24/h2,4-7,10-18H,3,8-9H2,1H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393136
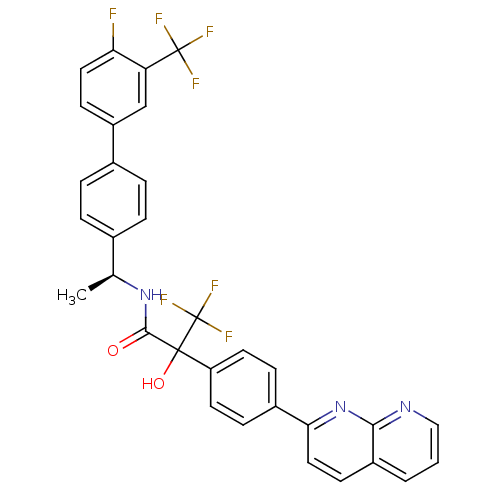
(CHEMBL2153468)Show SMILES C[C@H](NC(=O)C(O)(c1ccc(cc1)-c1ccc2cccnc2n1)C(F)(F)F)c1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H22F7N3O2/c1-18(19-4-6-20(7-5-19)23-10-14-26(33)25(17-23)31(34,35)36)41-29(43)30(44,32(37,38)39)24-12-8-21(9-13-24)27-15-11-22-3-2-16-40-28(22)42-27/h2-18,44H,1H3,(H,41,43)/t18-,30?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393130
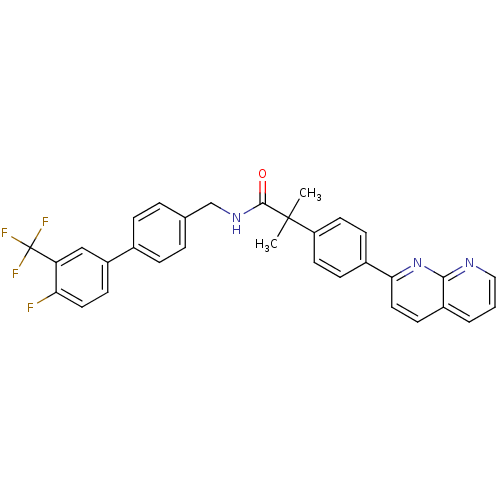
(CHEMBL2153462)Show SMILES CC(C)(C(=O)NCc1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F)c1ccc(cc1)-c1ccc2cccnc2n1 Show InChI InChI=1S/C32H25F4N3O/c1-31(2,25-13-9-22(10-14-25)28-16-12-23-4-3-17-37-29(23)39-28)30(40)38-19-20-5-7-21(8-6-20)24-11-15-27(33)26(18-24)32(34,35)36/h3-18H,19H2,1-2H3,(H,38,40) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393135
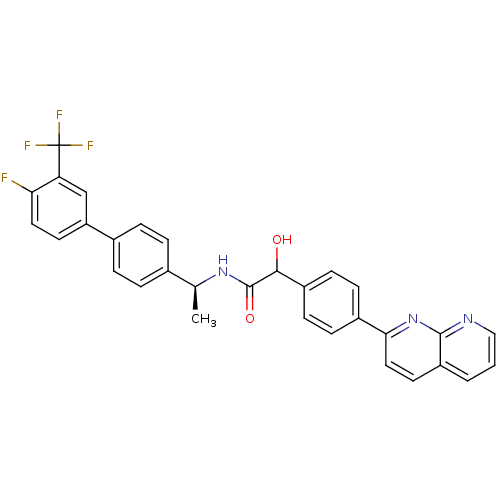
(CHEMBL2153467)Show SMILES C[C@H](NC(=O)C(O)c1ccc(cc1)-c1ccc2cccnc2n1)c1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H23F4N3O2/c1-18(19-4-6-20(7-5-19)24-12-14-26(32)25(17-24)31(33,34)35)37-30(40)28(39)22-10-8-21(9-11-22)27-15-13-23-3-2-16-36-29(23)38-27/h2-18,28,39H,1H3,(H,37,40)/t18-,28?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393134
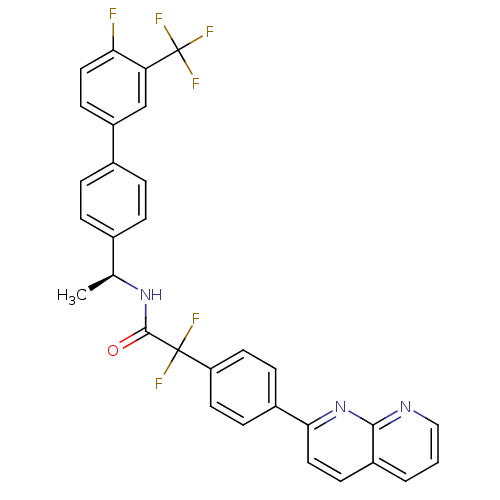
(CHEMBL2153466)Show SMILES C[C@H](NC(=O)C(F)(F)c1ccc(cc1)-c1ccc2cccnc2n1)c1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C31H21F6N3O/c1-18(19-4-6-20(7-5-19)23-10-14-26(32)25(17-23)31(35,36)37)39-29(41)30(33,34)24-12-8-21(9-13-24)27-15-11-22-3-2-16-38-28(22)40-27/h2-18H,1H3,(H,39,41)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
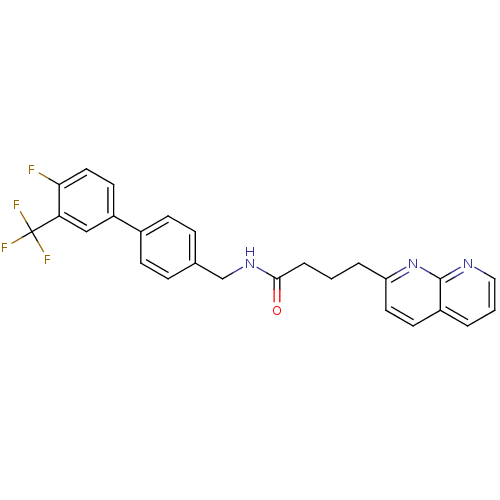
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393118
(CHEMBL2153441)Show SMILES Fc1ccc(cc1C(F)(F)F)-c1ccc(CNC(=O)CCCc2ccc3cccnc3n2)cc1 Show InChI InChI=1S/C26H21F4N3O/c27-23-13-11-20(15-22(23)26(28,29)30)18-8-6-17(7-9-18)16-32-24(34)5-1-4-21-12-10-19-3-2-14-31-25(19)33-21/h2-3,6-15H,1,4-5,16H2,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
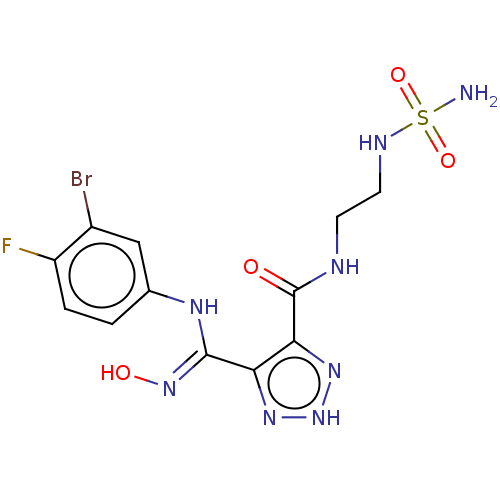
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608773
(CHEMBL5265978) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50608773

(CHEMBL5265978) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393161

(CHEMBL2153439)Show SMILES Cc1cccc(c1)-c1ccc(CNC(=O)CCCc2ccc3cccnc3n2)cc1 Show InChI InChI=1S/C26H25N3O/c1-19-5-2-6-23(17-19)21-12-10-20(11-13-21)18-28-25(30)9-3-8-24-15-14-22-7-4-16-27-26(22)29-24/h2,4-7,10-17H,3,8-9,18H2,1H3,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
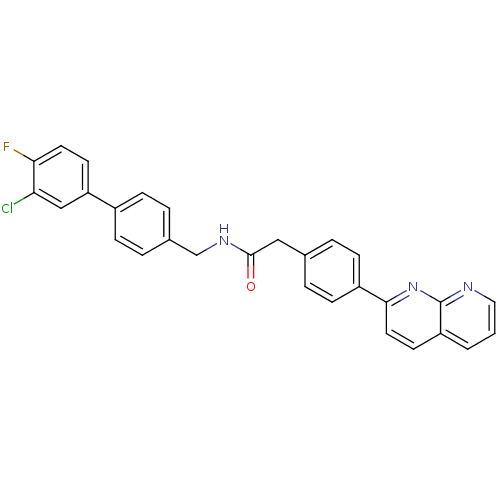
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393127
(CHEMBL2153459)Show SMILES Fc1ccc(cc1Cl)-c1ccc(CNC(=O)Cc2ccc(cc2)-c2ccc3cccnc3n2)cc1 Show InChI InChI=1S/C29H21ClFN3O/c30-25-17-24(11-13-26(25)31)21-7-5-20(6-8-21)18-33-28(35)16-19-3-9-22(10-4-19)27-14-12-23-2-1-15-32-29(23)34-27/h1-15,17H,16,18H2,(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
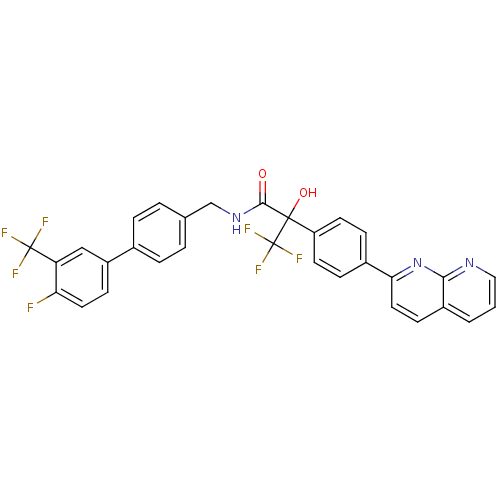
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393131
(CHEMBL2153463)Show SMILES OC(C(=O)NCc1ccc(cc1)-c1ccc(F)c(c1)C(F)(F)F)(c1ccc(cc1)-c1ccc2cccnc2n1)C(F)(F)F Show InChI InChI=1S/C31H20F7N3O2/c32-25-13-9-22(16-24(25)30(33,34)35)19-5-3-18(4-6-19)17-40-28(42)29(43,31(36,37)38)23-11-7-20(8-12-23)26-14-10-21-2-1-15-39-27(21)41-26/h1-16,43H,17H2,(H,40,42) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393143
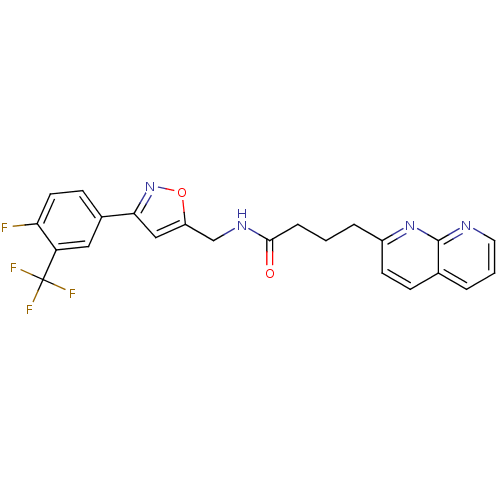
(CHEMBL2153581)Show SMILES Fc1ccc(cc1C(F)(F)F)-c1cc(CNC(=O)CCCc2ccc3cccnc3n2)on1 Show InChI InChI=1S/C23H18F4N4O2/c24-19-9-7-15(11-18(19)23(25,26)27)20-12-17(33-31-20)13-29-21(32)5-1-4-16-8-6-14-3-2-10-28-22(14)30-16/h2-3,6-12H,1,4-5,13H2,(H,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393119
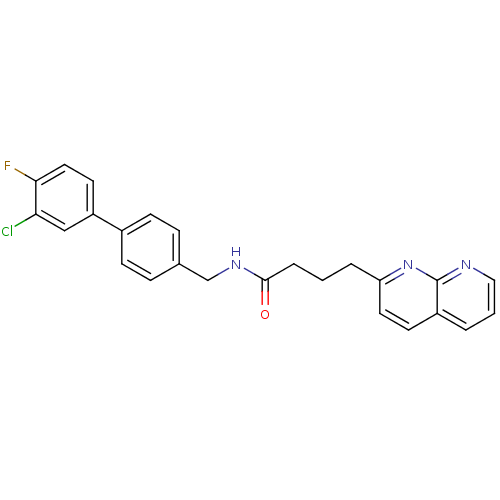
(CHEMBL2153442)Show SMILES Fc1ccc(cc1Cl)-c1ccc(CNC(=O)CCCc2ccc3cccnc3n2)cc1 Show InChI InChI=1S/C25H21ClFN3O/c26-22-15-20(11-13-23(22)27)18-8-6-17(7-9-18)16-29-24(31)5-1-4-21-12-10-19-3-2-14-28-25(19)30-21/h2-3,6-15H,1,4-5,16H2,(H,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393117
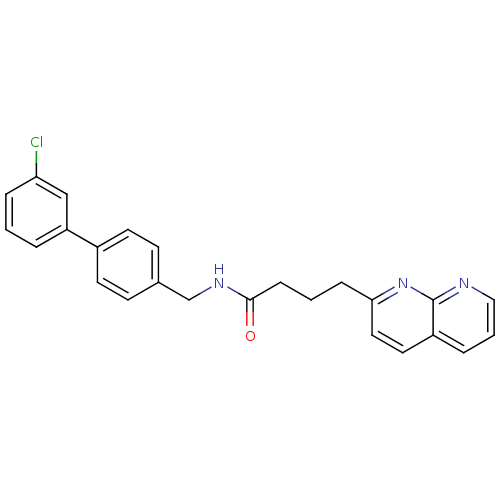
(CHEMBL2153440)Show SMILES Clc1cccc(c1)-c1ccc(CNC(=O)CCCc2ccc3cccnc3n2)cc1 Show InChI InChI=1S/C25H22ClN3O/c26-22-6-1-4-21(16-22)19-11-9-18(10-12-19)17-28-24(30)8-2-7-23-14-13-20-5-3-15-27-25(20)29-23/h1,3-6,9-16H,2,7-8,17H2,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393148

(CHEMBL2153586)Show SMILES Clc1cccc(c1)-c1ccc(cc1)-c1nnc(CCCc2ccc3cccnc3n2)o1 Show InChI InChI=1S/C25H19ClN4O/c26-21-6-1-4-20(16-21)17-9-11-19(12-10-17)25-30-29-23(31-25)8-2-7-22-14-13-18-5-3-15-27-24(18)28-22/h1,3-6,9-16H,2,7-8H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393120
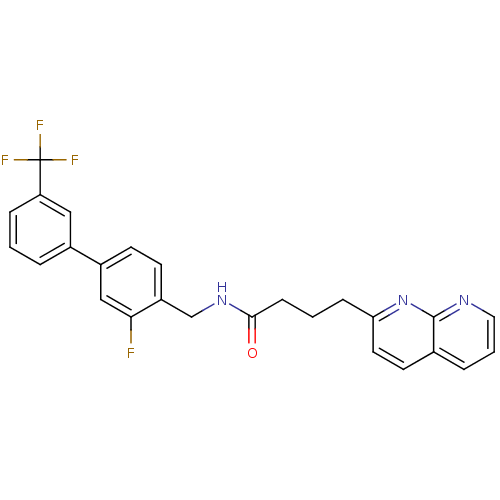
(CHEMBL2153443)Show SMILES Fc1cc(ccc1CNC(=O)CCCc1ccc2cccnc2n1)-c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C26H21F4N3O/c27-23-15-19(18-4-1-6-21(14-18)26(28,29)30)9-10-20(23)16-32-24(34)8-2-7-22-12-11-17-5-3-13-31-25(17)33-22/h1,3-6,9-15H,2,7-8,16H2,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Succinate receptor 1
(Homo sapiens (Human)) | BDBM50393137
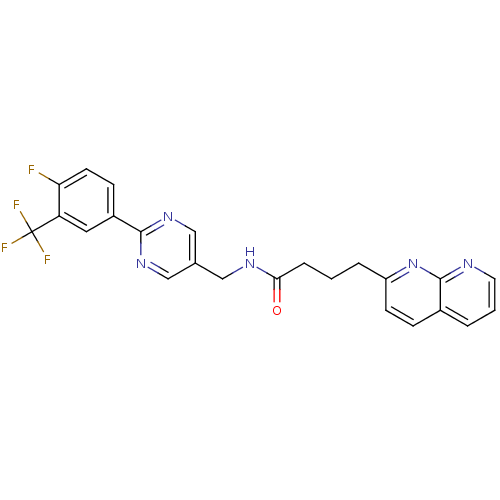
(CHEMBL2153469)Show SMILES Fc1ccc(cc1C(F)(F)F)-c1ncc(CNC(=O)CCCc2ccc3cccnc3n2)cn1 Show InChI InChI=1S/C24H19F4N5O/c25-20-9-7-17(11-19(20)24(26,27)28)22-31-13-15(14-32-22)12-30-21(34)5-1-4-18-8-6-16-3-2-10-29-23(16)33-18/h2-3,6-11,13-14H,1,4-5,12H2,(H,30,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics
Curated by ChEMBL
| Assay Description
Antagonist activity at human GPR91 receptor expressed in CHO-K1 cells co-expressing Galpha and Gqi5 G-protein assessed as inhibition of succinate-ind... |
Bioorg Med Chem Lett 21: 3596-602 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.091
BindingDB Entry DOI: 10.7270/Q2BK1DG6 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50084419
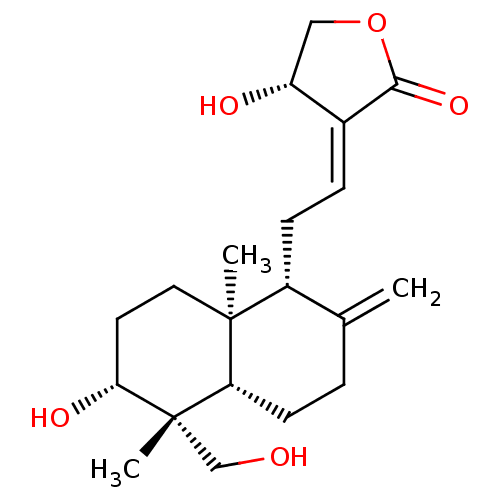
(Andrographolide | CHEBI:65408)Show SMILES [H][C@]12CCC(=C)[C@@H](C\C=C3/[C@H](O)COC3=O)[C@]1(C)CC[C@@H](O)[C@@]2(C)CO |r| Show InChI InChI=1S/C20H30O5/c1-12-4-7-16-19(2,9-8-17(23)20(16,3)11-21)14(12)6-5-13-15(22)10-25-18(13)24/h5,14-17,21-23H,1,4,6-11H2,2-3H3/b13-5+/t14-,15-,16+,17-,19+,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data