 Found 915 hits with Last Name = 'salyers' and Initial = 'k'
Found 915 hits with Last Name = 'salyers' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576
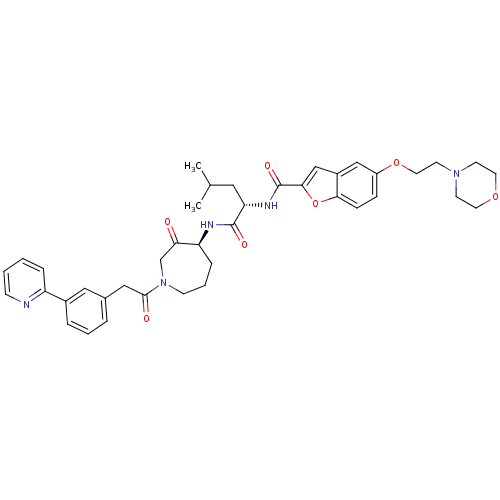
(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083761
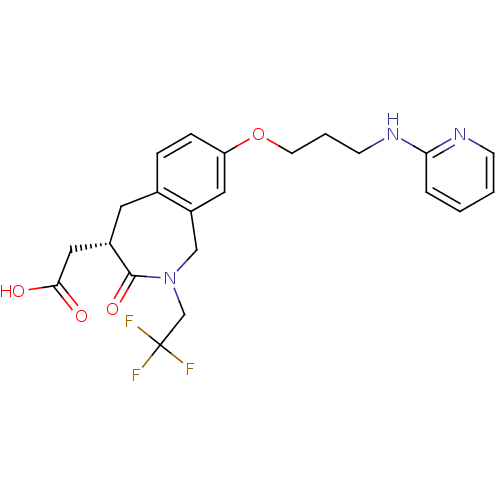
(CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083763
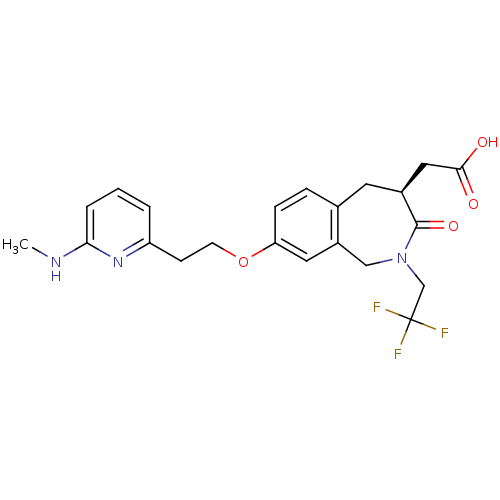
(CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...)Show SMILES CNc1cccc(CCOc2ccc3C[C@@H](CC(O)=O)C(=O)N(CC(F)(F)F)Cc3c2)n1 Show InChI InChI=1S/C22H24F3N3O4/c1-26-19-4-2-3-17(27-19)7-8-32-18-6-5-14-9-15(11-20(29)30)21(31)28(12-16(14)10-18)13-22(23,24)25/h2-6,10,15H,7-9,11-13H2,1H3,(H,26,27)(H,29,30)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083764
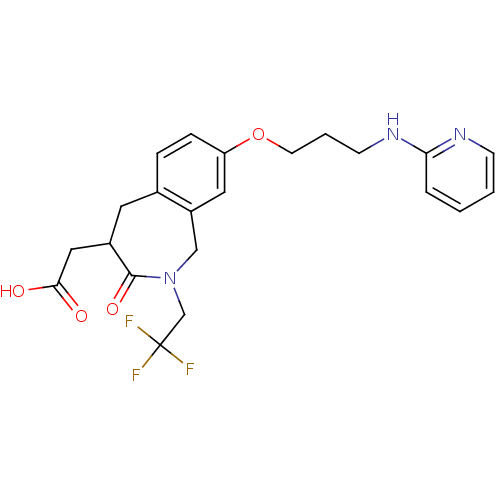
(CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...)Show SMILES OC(=O)CC1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098580
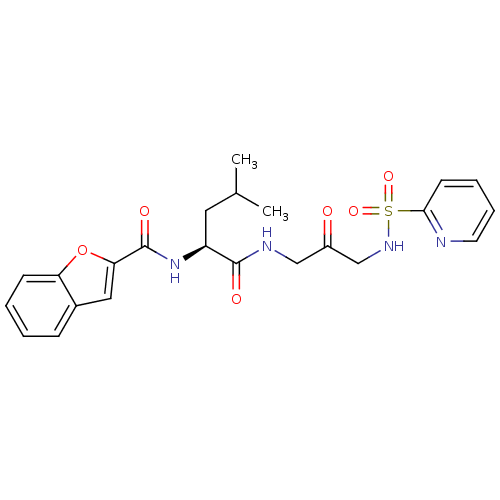
(Benzofuran-2-carboxylic acid {3-methyl-1-[2-oxo-3-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)NCC(=O)CNS(=O)(=O)c1ccccn1 Show InChI InChI=1S/C23H26N4O6S/c1-15(2)11-18(27-23(30)20-12-16-7-3-4-8-19(16)33-20)22(29)25-13-17(28)14-26-34(31,32)21-9-5-6-10-24-21/h3-10,12,15,18,26H,11,13-14H2,1-2H3,(H,25,29)(H,27,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083762
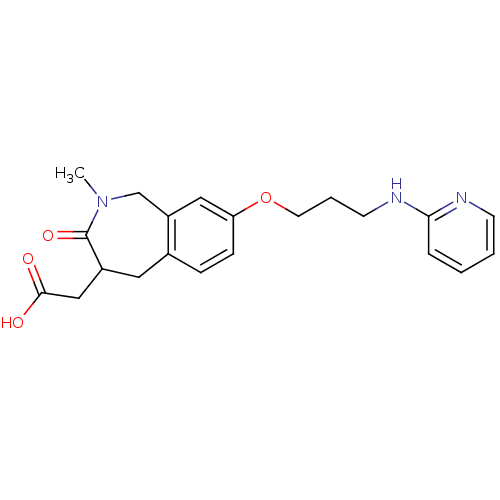
(CHEMBL314022 | {2-Methyl-3-oxo-8-[3-(pyridin-2-yla...)Show InChI InChI=1S/C21H25N3O4/c1-24-14-17-12-18(28-10-4-9-23-19-5-2-3-8-22-19)7-6-15(17)11-16(21(24)27)13-20(25)26/h2-3,5-8,12,16H,4,9-11,13-14H2,1H3,(H,22,23)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098584
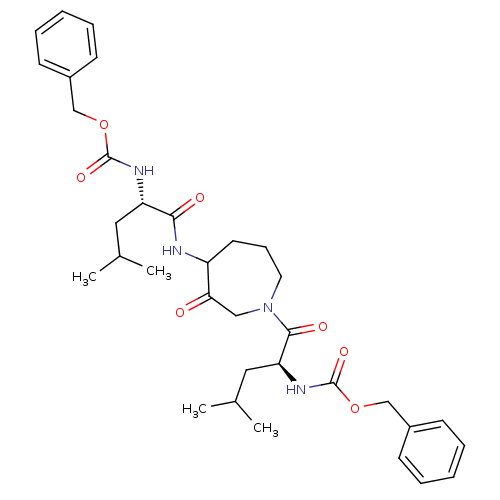
(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50059133
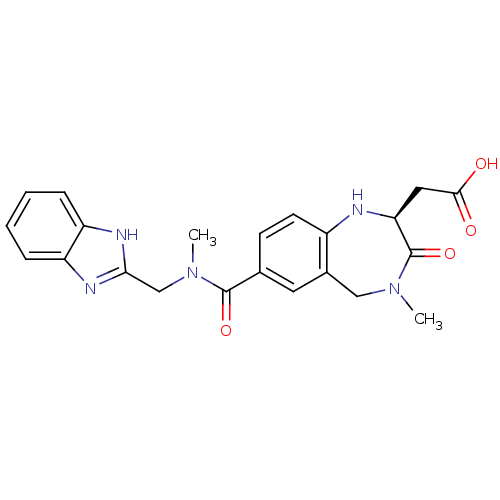
(CHEMBL50106 | SB-223245 | {(S)-7-[(1H-Benzoimidazo...)Show SMILES CN(Cc1nc2ccccc2[nH]1)C(=O)c1ccc2N[C@@H](CC(O)=O)C(=O)N(C)Cc2c1 Show InChI InChI=1S/C22H23N5O4/c1-26-11-14-9-13(7-8-15(14)23-18(22(26)31)10-20(28)29)21(30)27(2)12-19-24-16-5-3-4-6-17(16)25-19/h3-9,18,23H,10-12H2,1-2H3,(H,24,25)(H,28,29)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098579
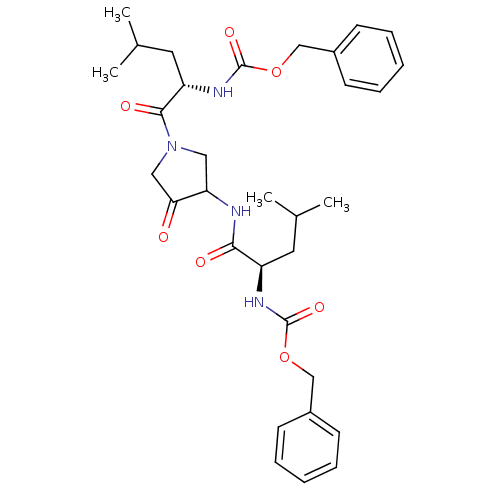
(CHEMBL29483 | {1-[3-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26+,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50126595
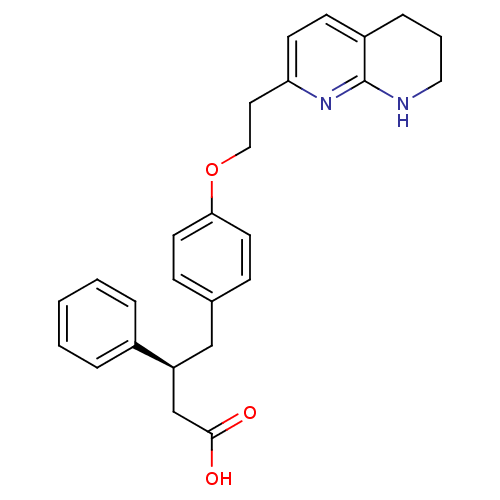
(3-Phenyl-4-{4-[2-(5,6,7,8-tetrahydro-[1,8]naphthyr...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCc2ccc3CCCNc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C26H28N2O3/c29-25(30)18-22(20-5-2-1-3-6-20)17-19-8-12-24(13-9-19)31-16-14-23-11-10-21-7-4-15-27-26(21)28-23/h1-3,5-6,8-13,22H,4,7,14-18H2,(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphav/beta 3 vitronectin receptor in HEK cells |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126595

(3-Phenyl-4-{4-[2-(5,6,7,8-tetrahydro-[1,8]naphthyr...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCc2ccc3CCCNc3n2)cc1)c1ccccc1 Show InChI InChI=1S/C26H28N2O3/c29-25(30)18-22(20-5-2-1-3-6-20)17-19-8-12-24(13-9-19)31-16-14-23-11-10-21-7-4-15-27-26(21)28-23/h1-3,5-6,8-13,22H,4,7,14-18H2,(H,27,28)(H,29,30)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta5 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098575
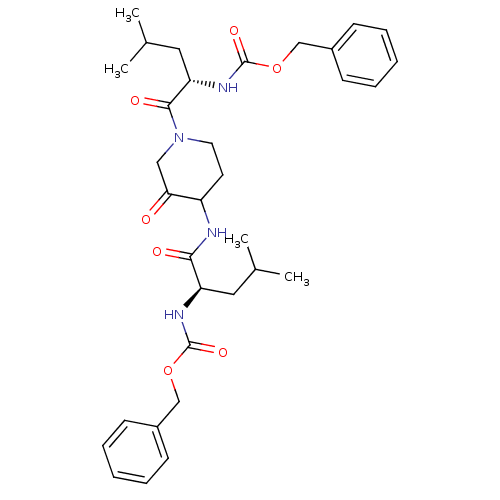
(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50078714
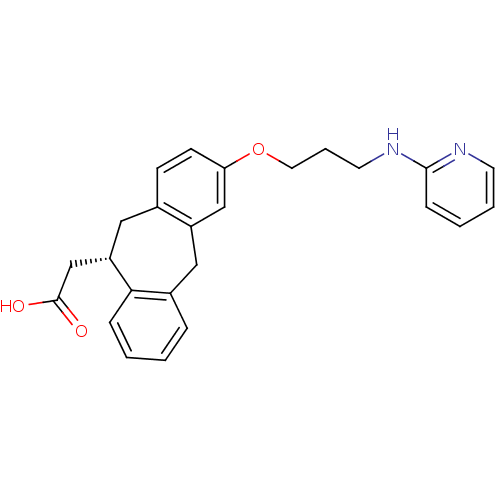
(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252353
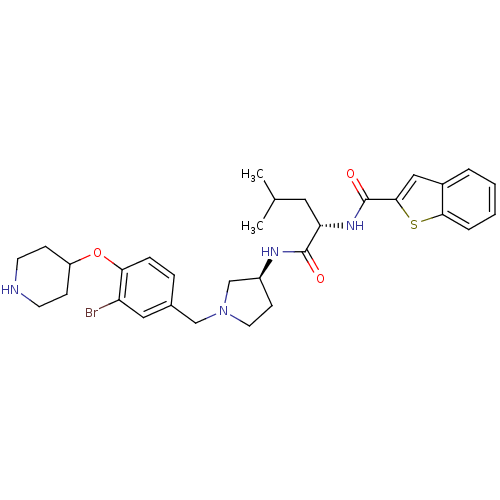
(CHEMBL479413 | N-((S)-1-((S)-1-(3-bromo-4-(piperid...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)N[C@H]1CCN(Cc2ccc(OC3CCNCC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C31H39BrN4O3S/c1-20(2)15-26(35-31(38)29-17-22-5-3-4-6-28(22)40-29)30(37)34-23-11-14-36(19-23)18-21-7-8-27(25(32)16-21)39-24-9-12-33-13-10-24/h3-8,16-17,20,23-24,26,33H,9-15,18-19H2,1-2H3,(H,34,37)(H,35,38)/t23-,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252659
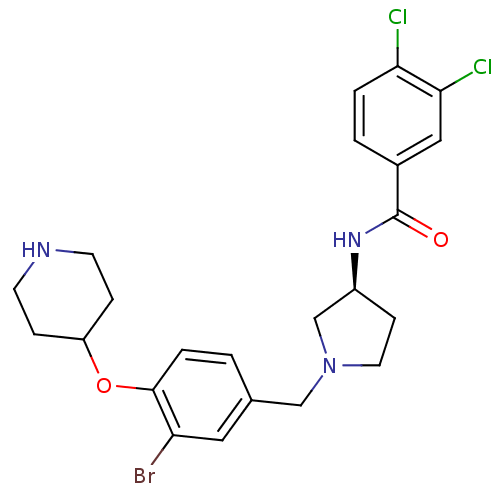
((S)-N-(1-(3-bromo-4-(piperidin-4-yloxy)benzyl)pyrr...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccc(OC3CCNCC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C23H26BrCl2N3O2/c24-19-11-15(1-4-22(19)31-18-5-8-27-9-6-18)13-29-10-7-17(14-29)28-23(30)16-2-3-20(25)21(26)12-16/h1-4,11-12,17-18,27H,5-10,13-14H2,(H,28,30)/t17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [I125]hU2 from human recombinant urotensin 2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50078714

(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaIIb-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50078714

(CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...)Show SMILES OC(=O)C[C@@H]1Cc2ccc(OCCCNc3ccccn3)cc2Cc2ccccc12 Show InChI InChI=1S/C25H26N2O3/c28-25(29)17-21-14-18-9-10-22(16-20(18)15-19-6-1-2-7-23(19)21)30-13-5-12-27-24-8-3-4-11-26-24/h1-4,6-11,16,21H,5,12-15,17H2,(H,26,27)(H,28,29)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098581

(5-(2-Morpholin-4-yl-ethoxy)-benzofuran-2-carboxyli...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Rattus norvegicus) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Rat cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252147
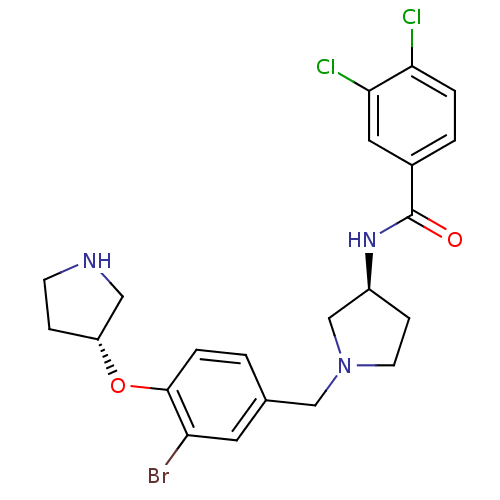
(CHEMBL482259 | N-((S)-1-((R)-3-bromo-4-((R)-pyrrol...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccc(O[C@@H]3CCNC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C22H24BrCl2N3O2/c23-18-9-14(1-4-21(18)30-17-5-7-26-11-17)12-28-8-6-16(13-28)27-22(29)15-2-3-19(24)20(25)10-15/h1-4,9-10,16-17,26H,5-8,11-13H2,(H,27,29)/t16-,17+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50126594

(3-Phenyl-4-{4-[3-(pyridin-2-ylamino)-propoxy]-phen...)Show InChI InChI=1S/C24H26N2O3/c27-24(28)18-21(20-7-2-1-3-8-20)17-19-10-12-22(13-11-19)29-16-6-15-26-23-9-4-5-14-25-23/h1-5,7-14,21H,6,15-18H2,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126597
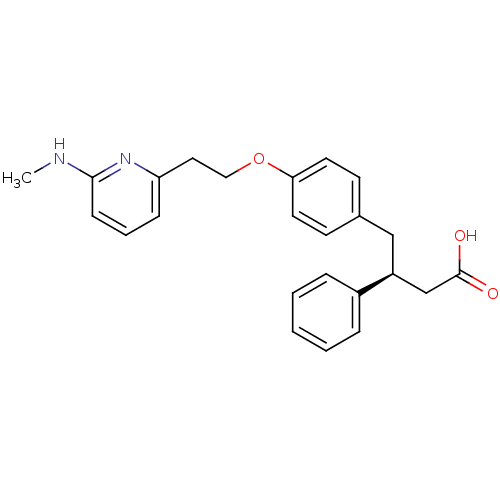
(4-{4-[2-(6-Methylamino-pyridin-2-yl)-ethoxy]-pheny...)Show SMILES CNc1cccc(CCOc2ccc(C[C@@H](CC(O)=O)c3ccccc3)cc2)n1 Show InChI InChI=1S/C24H26N2O3/c1-25-23-9-5-8-21(26-23)14-15-29-22-12-10-18(11-13-22)16-20(17-24(27)28)19-6-3-2-4-7-19/h2-13,20H,14-17H2,1H3,(H,25,26)(H,27,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta3 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19782
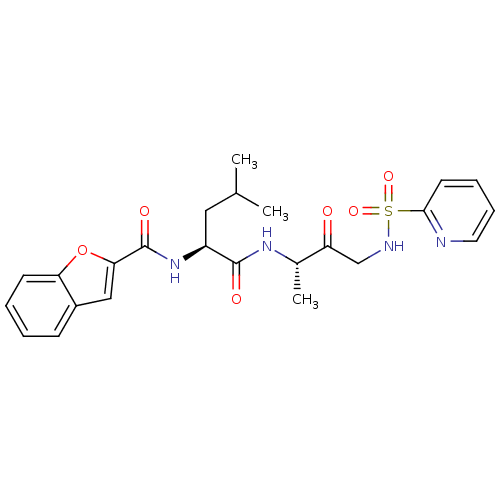
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(2...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H](C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H28N4O6S/c1-15(2)12-18(28-24(31)21-13-17-8-4-5-9-20(17)34-21)23(30)27-16(3)19(29)14-26-35(32,33)22-10-6-7-11-25-22/h4-11,13,15-16,18,26H,12,14H2,1-3H3,(H,27,30)(H,28,31)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252197
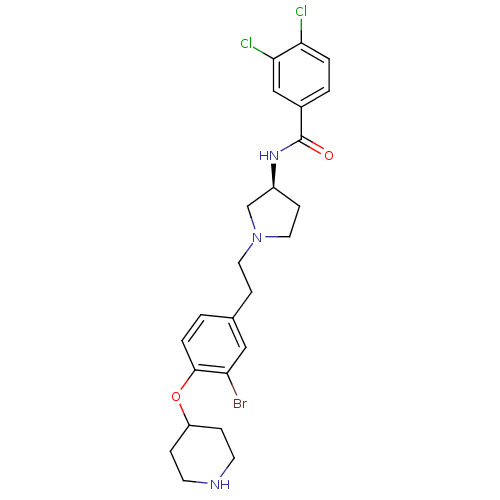
((S)-N-(1-(3-bromo-4-(piperidin-4-yloxy)phenethyl)p...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(CCc2ccc(OC3CCNCC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C24H28BrCl2N3O2/c25-20-13-16(1-4-23(20)32-19-5-9-28-10-6-19)7-11-30-12-8-18(15-30)29-24(31)17-2-3-21(26)22(27)14-17/h1-4,13-14,18-19,28H,5-12,15H2,(H,29,31)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252355

(CHEMBL505360 | N-((S)-1-((S)-1-(3-bromo-4-(piperid...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)N[C@H]1CCN(C1)S(=O)(=O)c1ccc(OC2CCNCC2)c(Br)c1 |r| Show InChI InChI=1S/C30H37BrN4O5S2/c1-19(2)15-25(34-30(37)28-16-20-5-3-4-6-27(20)41-28)29(36)33-21-11-14-35(18-21)42(38,39)23-7-8-26(24(31)17-23)40-22-9-12-32-13-10-22/h3-8,16-17,19,21-22,25,32H,9-15,18H2,1-2H3,(H,33,36)(H,34,37)/t21-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126596
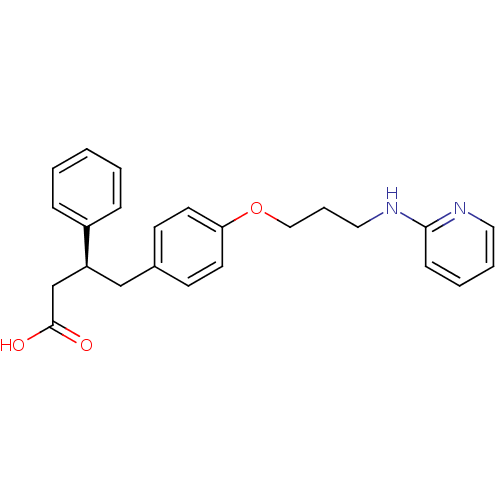
(3-Phenyl-4-{4-[3-(pyridin-2-ylamino)-propoxy]-phen...)Show SMILES OC(=O)C[C@H](Cc1ccc(OCCCNc2ccccn2)cc1)c1ccccc1 Show InChI InChI=1S/C24H26N2O3/c27-24(28)18-21(20-7-2-1-3-8-20)17-19-10-12-22(13-11-19)29-16-6-15-26-23-9-4-5-14-25-23/h1-5,7-14,21H,6,15-18H2,(H,25,26)(H,27,28)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphav/beta 3 vitronectin receptor in HEK cells |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252198

((S)-N-(1-(3-chloro-4-(piperidin-4-yloxy)phenethyl)...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(CCc2ccc(OC3CCNCC3)c(Cl)c2)C1 |r| Show InChI InChI=1S/C24H28Cl3N3O2/c25-20-3-2-17(14-21(20)26)24(31)29-18-8-12-30(15-18)11-7-16-1-4-23(22(27)13-16)32-19-5-9-28-10-6-19/h1-4,13-14,18-19,28H,5-12,15H2,(H,29,31)/t18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098578
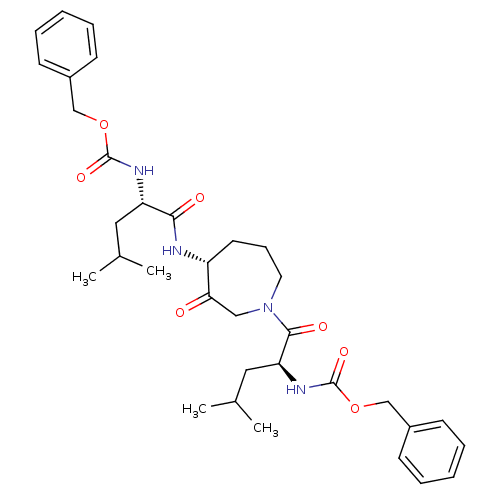
(CHEMBL286034 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098575

(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252354
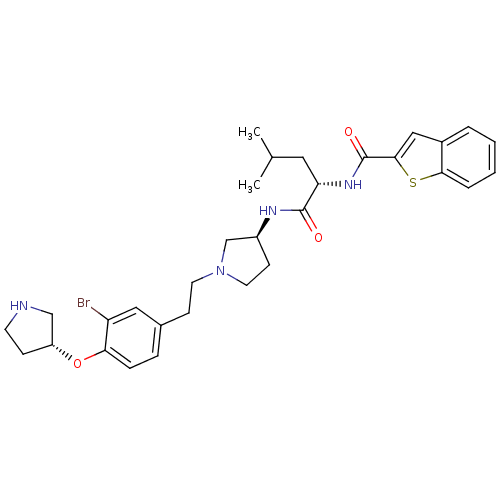
(CHEMBL517991 | N-((S)-1-((S)-1-((R)-3-bromo-4-((R)...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)N[C@H]1CCN(CCc2ccc(O[C@@H]3CCNC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C31H39BrN4O3S/c1-20(2)15-26(35-31(38)29-17-22-5-3-4-6-28(22)40-29)30(37)34-23-11-14-36(19-23)13-10-21-7-8-27(25(32)16-21)39-24-9-12-33-18-24/h3-8,16-17,20,23-24,26,33H,9-15,18-19H2,1-2H3,(H,34,37)(H,35,38)/t23-,24+,26-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50083760
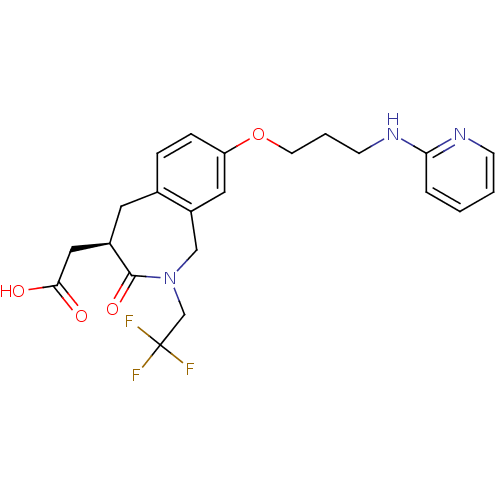
(CHEMBL86991 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...)Show SMILES OC(=O)C[C@H]1Cc2ccc(OCCCNc3ccccn3)cc2CN(CC(F)(F)F)C1=O Show InChI InChI=1S/C22H24F3N3O4/c23-22(24,25)14-28-13-17-11-18(32-9-3-8-27-19-4-1-2-7-26-19)6-5-15(17)10-16(21(28)31)12-20(29)30/h1-2,4-7,11,16H,3,8-10,12-14H2,(H,26,27)(H,29,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for Vitronectin receptor (alpha V beta 3) |
J Med Chem 43: 22-6 (2000)
BindingDB Entry DOI: 10.7270/Q290230D |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19782

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(2...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H](C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H28N4O6S/c1-15(2)12-18(28-24(31)21-13-17-8-4-5-9-20(17)34-21)23(30)27-16(3)19(29)14-26-35(32,33)22-10-6-7-11-25-22/h4-11,13,15-16,18,26H,12,14H2,1-3H3,(H,27,30)(H,28,31)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650
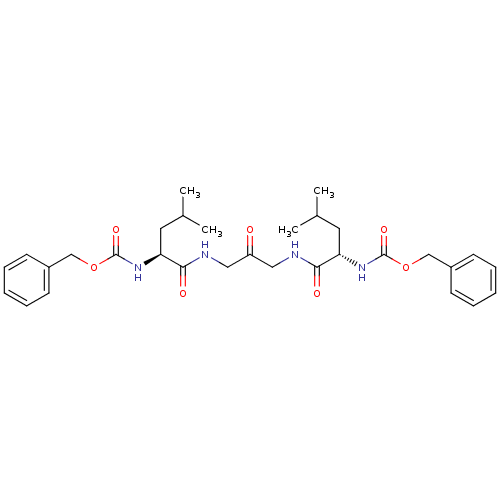
(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098584

(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098582
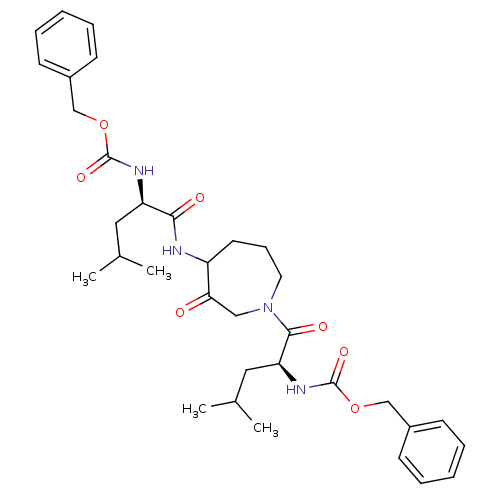
(CHEMBL31519 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50126594

(3-Phenyl-4-{4-[3-(pyridin-2-ylamino)-propoxy]-phen...)Show InChI InChI=1S/C24H26N2O3/c27-24(28)18-21(20-7-2-1-3-8-20)17-19-10-12-22(13-11-19)29-16-6-15-26-23-9-4-5-14-25-23/h1-5,7-14,21H,6,15-18H2,(H,25,26)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for alphaV-beta5 vitronectin receptor |
Bioorg Med Chem Lett 13: 1483-6 (2003)
BindingDB Entry DOI: 10.7270/Q2FN15K8 |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252239
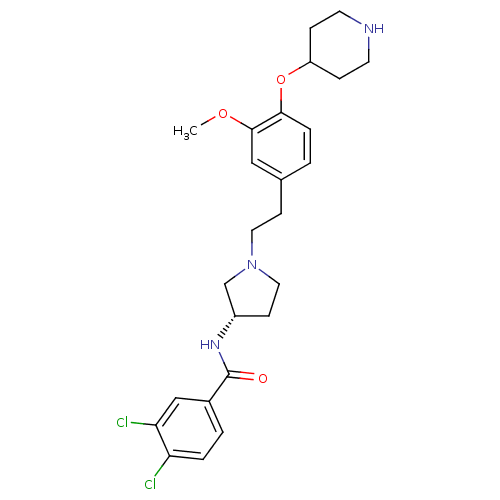
((S)-N-(1-(3-methoxy-4-(piperidin-4-yloxy)phenethyl...)Show SMILES COc1cc(CCN2CC[C@@H](C2)NC(=O)c2ccc(Cl)c(Cl)c2)ccc1OC1CCNCC1 |r| Show InChI InChI=1S/C25H31Cl2N3O3/c1-32-24-14-17(2-5-23(24)33-20-6-10-28-11-7-20)8-12-30-13-9-19(16-30)29-25(31)18-3-4-21(26)22(27)15-18/h2-5,14-15,19-20,28H,6-13,16H2,1H3,(H,29,31)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50252659

((S)-N-(1-(3-bromo-4-(piperidin-4-yloxy)benzyl)pyrr...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccc(OC3CCNCC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C23H26BrCl2N3O2/c24-19-11-15(1-4-22(19)31-18-5-8-27-9-6-18)13-29-10-7-17(14-29)28-23(30)16-2-3-20(25)21(26)12-16/h1-4,11-12,17-18,27H,5-10,13-14H2,(H,28,30)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT1A receptor |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098579

(CHEMBL29483 | {1-[3-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26+,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252096
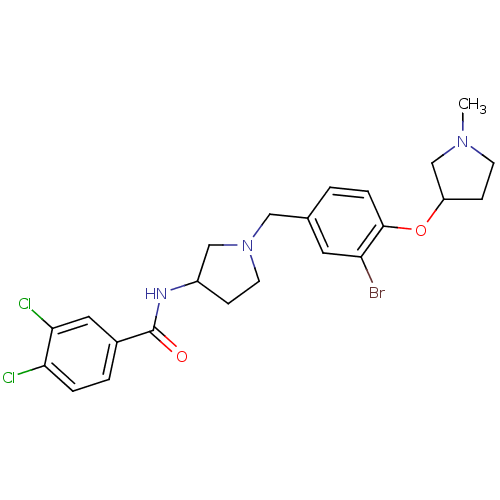
((+/-)-N-(1-(3-bromo-4-(1-methylpyrrolidin-3-yloxy)...)Show SMILES CN1CCC(C1)Oc1ccc(CN2CCC(C2)NC(=O)c2ccc(Cl)c(Cl)c2)cc1Br Show InChI InChI=1S/C23H26BrCl2N3O2/c1-28-8-7-18(14-28)31-22-5-2-15(10-19(22)24)12-29-9-6-17(13-29)27-23(30)16-3-4-20(25)21(26)11-16/h2-5,10-11,17-18H,6-9,12-14H2,1H3,(H,27,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252241
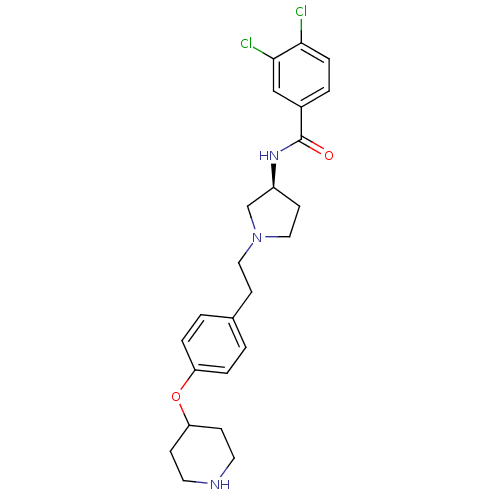
((S)-N-(1-(4-(piperidin-4-yloxy)phenethyl)pyrrolidi...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(CCc2ccc(OC3CCNCC3)cc2)C1 |r| Show InChI InChI=1S/C24H29Cl2N3O2/c25-22-6-3-18(15-23(22)26)24(30)28-19-10-14-29(16-19)13-9-17-1-4-20(5-2-17)31-21-7-11-27-12-8-21/h1-6,15,19,21,27H,7-14,16H2,(H,28,30)/t19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252148
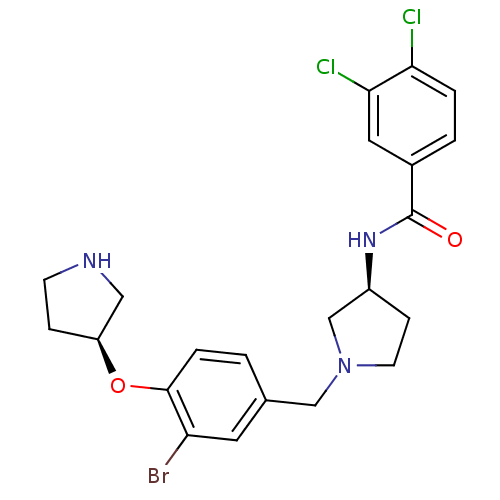
(CHEMBL516606 | N-((S)-1-((S)-3-bromo-4-((S)-pyrrol...)Show SMILES Clc1ccc(cc1Cl)C(=O)N[C@H]1CCN(Cc2ccc(O[C@H]3CCNC3)c(Br)c2)C1 |r| Show InChI InChI=1S/C22H24BrCl2N3O2/c23-18-9-14(1-4-21(18)30-17-5-7-26-11-17)12-28-8-6-16(13-28)27-22(29)15-2-3-19(24)20(25)10-15/h1-4,9-10,16-17,26H,5-8,11-13H2,(H,27,29)/t16-,17-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098584

(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Urotensin-2 receptor
(Homo sapiens (Human)) | BDBM50252095
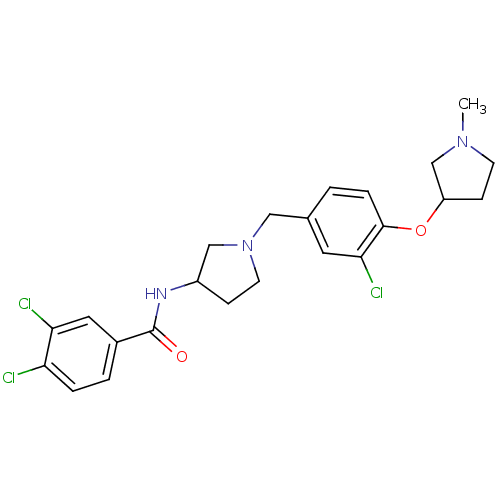
((+/-)-N-(1-(3-chloro-4-(1-methylpyrrolidin-3-yloxy...)Show SMILES CN1CCC(C1)Oc1ccc(CN2CCC(C2)NC(=O)c2ccc(Cl)c(Cl)c2)cc1Cl Show InChI InChI=1S/C23H26Cl3N3O2/c1-28-8-7-18(14-28)31-22-5-2-15(10-21(22)26)12-29-9-6-17(13-29)27-23(30)16-3-4-19(24)20(25)11-16/h2-5,10-11,17-18H,6-9,12-14H2,1H3,(H,27,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant urotensin 2 receptor expressed in HEK293 cells assessed as inhibition of urotensin 2-induced calcium mobiliz... |
Bioorg Med Chem Lett 18: 3950-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.06.019
BindingDB Entry DOI: 10.7270/Q2FT8KTR |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098585
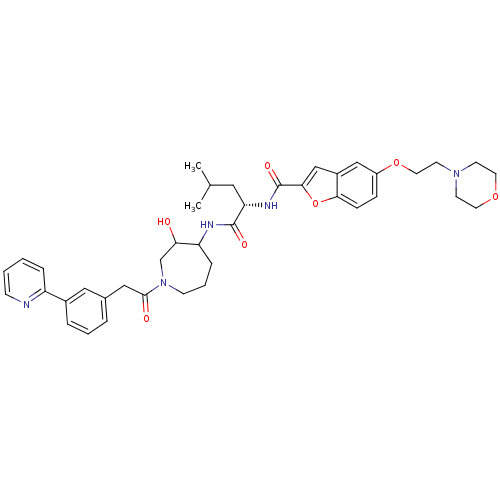
(5-(2-Morpholin-4-yl-ethoxy)-benzofuran-2-carboxyli...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)NC1CCCN(CC1O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H49N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-35,46H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33?,34-,35?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data