 Found 1352 hits with Last Name = 'sari' and Initial = 'm'
Found 1352 hits with Last Name = 'sari' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417035

(CHEMBL1257993)Show SMILES Fc1ccccc1-c1cnc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)cn1 |r,wU:13.13,wD:16.22,(15.52,-7.53,;14.76,-6.18,;13.23,-6.17,;12.47,-4.82,;13.26,-3.49,;14.8,-3.52,;15.55,-4.86,;17.09,-4.88,;17.88,-3.56,;19.42,-3.58,;20.17,-4.93,;21.71,-4.96,;22.45,-6.3,;23.99,-6.33,;24.79,-5.01,;26.33,-5.03,;27.07,-6.38,;28.2,-5.34,;29.54,-6.12,;29.22,-7.62,;30.25,-8.77,;27.69,-7.78,;30.95,-5.5,;32.19,-6.4,;33.6,-5.78,;33.76,-4.25,;32.51,-3.34,;31.11,-3.97,;26.27,-7.7,;24.74,-7.67,;19.38,-6.25,;17.85,-6.23,)| Show InChI InChI=1S/C24H24FN5O2/c25-19-6-2-1-5-18(19)20-14-29-21(15-27-20)28-13-17-8-10-24(11-9-17)16-30(23(31)32-24)22-7-3-4-12-26-22/h1-7,12,14-15,17H,8-11,13,16H2,(H,28,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417033
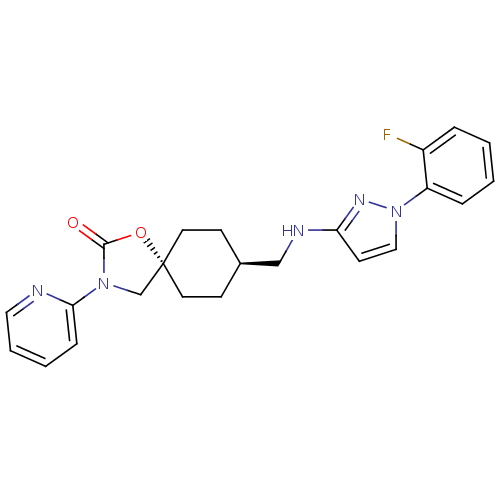
(CHEMBL1258111)Show SMILES Fc1ccccc1-n1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)n1 |r,wU:13.13,wD:16.22,(15.91,-.57,;14.51,.08,;13.26,-.81,;11.85,-.16,;11.72,1.37,;12.98,2.26,;14.38,1.6,;15.63,2.49,;15.65,4.03,;17.12,4.48,;18.01,3.23,;19.55,3.2,;20.3,1.86,;21.84,1.83,;22.63,3.15,;24.17,3.13,;24.91,1.78,;26.05,2.82,;27.39,2.04,;27.06,.54,;28.09,-.61,;25.53,.38,;28.79,2.66,;30.04,1.75,;31.44,2.38,;31.61,3.91,;30.35,4.82,;28.95,4.19,;24.12,.46,;22.59,.49,;17.08,1.99,)| Show InChI InChI=1S/C23H24FN5O2/c24-18-5-1-2-6-19(18)29-14-10-20(27-29)26-15-17-8-11-23(12-9-17)16-28(22(30)31-23)21-7-3-4-13-25-21/h1-7,10,13-14,17H,8-9,11-12,15-16H2,(H,26,27)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417056
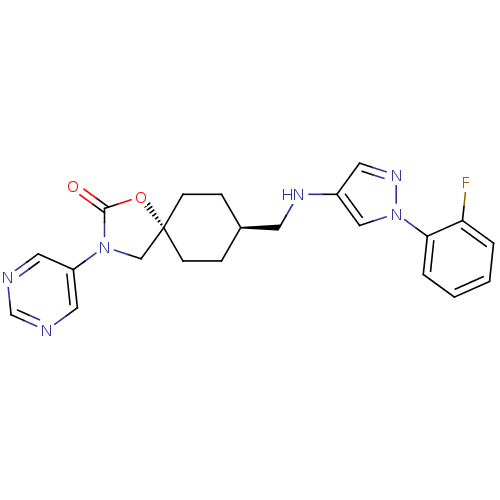
(CHEMBL1258225)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cncnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-5.79,-9.02,;-7.19,-8.37,;-8.44,-9.26,;-9.85,-8.61,;-9.98,-7.07,;-8.73,-6.19,;-7.33,-6.84,;-6.08,-5.96,;-4.62,-6.46,;-3.69,-5.22,;-2.15,-5.25,;-1.4,-6.59,;.14,-6.62,;.94,-5.3,;2.48,-5.31,;3.21,-6.67,;4.35,-5.63,;5.69,-6.41,;5.37,-7.91,;6.4,-9.06,;3.84,-8.08,;7.1,-5.79,;8.34,-6.69,;9.75,-6.07,;9.91,-4.54,;8.66,-3.63,;7.26,-4.26,;2.42,-7.99,;.89,-7.96,;-4.58,-3.96,;-6.05,-4.42,)| Show InChI InChI=1S/C22H23FN6O2/c23-19-3-1-2-4-20(19)29-13-17(10-27-29)26-9-16-5-7-22(8-6-16)14-28(21(30)31-22)18-11-24-15-25-12-18/h1-4,10-13,15-16,26H,5-9,14H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21397

(8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...)Show SMILES Fc1ccc(cc1)C(=O)CCCN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C23H26FN3O2/c24-19-10-8-18(9-11-19)21(28)7-4-14-26-15-12-23(13-16-26)22(29)25-17-27(23)20-5-2-1-3-6-20/h1-3,5-6,8-11H,4,7,12-17H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417045

(CHEMBL1258341)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cnccn3)CC2)cn1 |r,wU:12.12,wD:15.21,(-6.54,-17.19,;-7.94,-16.54,;-9.19,-17.43,;-10.59,-16.78,;-10.73,-15.24,;-9.48,-14.36,;-8.08,-15.01,;-6.82,-14.13,;-5.37,-14.63,;-4.44,-13.39,;-2.9,-13.41,;-2.15,-14.76,;-.61,-14.79,;.19,-13.47,;1.73,-13.48,;2.46,-14.84,;3.6,-13.8,;4.94,-14.58,;4.62,-16.08,;5.64,-17.23,;3.09,-16.24,;6.35,-13.96,;7.58,-14.86,;8.99,-14.24,;9.15,-12.71,;7.91,-11.8,;6.5,-12.43,;1.67,-16.16,;.14,-16.13,;-5.33,-12.13,;-6.8,-12.59,)| Show InChI InChI=1S/C22H23FN6O2/c23-18-3-1-2-4-19(18)29-14-17(12-27-29)26-11-16-5-7-22(8-6-16)15-28(21(30)31-22)20-13-24-9-10-25-20/h1-4,9-10,12-14,16,26H,5-8,11,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417036

(CHEMBL1257992)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nn1 |r,wU:13.13,wD:16.22,(-7.93,-14.34,;-8.69,-13,;-10.22,-12.98,;-10.98,-11.63,;-10.19,-10.31,;-8.65,-10.33,;-7.9,-11.68,;-6.36,-11.69,;-5.6,-13.04,;-4.07,-13.06,;-3.28,-11.75,;-1.74,-11.77,;-1,-13.12,;.54,-13.14,;1.34,-11.82,;2.88,-11.84,;3.62,-13.19,;4.75,-12.16,;6.09,-12.93,;5.77,-14.44,;6.8,-15.58,;4.24,-14.6,;7.5,-12.31,;8.74,-13.22,;10.15,-12.59,;10.31,-11.06,;9.06,-10.15,;7.66,-10.78,;2.82,-14.51,;1.29,-14.48,;-4.03,-10.4,;-5.57,-10.37,)| Show InChI InChI=1S/C24H24FN5O2/c25-19-6-2-1-5-18(19)20-8-9-21(29-28-20)27-15-17-10-12-24(13-11-17)16-30(23(31)32-24)22-7-3-4-14-26-22/h1-9,14,17H,10-13,15-16H2,(H,27,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417051

(CHEMBL1258787)Show SMILES Cc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:13.13,wD:16.22,(17.45,-6.84,;16.7,-5.5,;15.16,-5.48,;14.41,-4.13,;15.2,-2.81,;16.74,-2.83,;17.48,-4.18,;19.03,-4.2,;19.78,-5.55,;21.32,-5.57,;22.1,-4.25,;23.64,-4.27,;24.39,-5.62,;25.93,-5.64,;26.72,-4.32,;28.26,-4.34,;29,-5.69,;30.14,-4.66,;31.48,-5.43,;31.16,-6.94,;32.19,-8.08,;29.62,-7.1,;32.88,-4.81,;34.13,-5.72,;35.54,-5.1,;35.7,-3.56,;34.44,-2.66,;33.04,-3.29,;28.21,-7.01,;26.68,-6.98,;21.36,-2.9,;19.82,-2.87,)| Show InChI InChI=1S/C24H26N6O2/c1-17-5-2-3-6-19(17)20-8-9-21(28-27-20)25-15-18-10-12-24(13-11-18)16-30(23(31)32-24)22-7-4-14-26-29-22/h2-9,14,18H,10-13,15-16H2,1H3,(H,25,28)/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417032

(CHEMBL1258110)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1nc(cs1)-c1ccccn1)CC2 |r,wU:14.16,wD:3.2,(5.76,.15,;4.73,1.3,;3.2,1.14,;2.57,2.54,;3.71,3.58,;5.05,2.8,;6.45,3.43,;7.7,2.52,;9.11,3.14,;9.27,4.67,;8.02,5.58,;6.61,4.95,;1.83,3.89,;.29,3.91,;-.5,2.59,;-2.04,2.62,;-2.79,3.96,;-4.33,3.99,;-5.25,2.75,;-6.71,3.25,;-6.69,4.79,;-5.21,5.24,;-7.96,2.37,;-7.82,.84,;-9.08,-.05,;-10.48,.6,;-10.62,2.14,;-9.36,3.02,;.25,1.25,;1.78,1.22,)| Show InChI InChI=1S/C22H23N5O2S/c28-21-27(19-6-2-4-12-24-19)15-22(29-21)9-7-16(8-10-22)13-25-20-26-18(14-30-20)17-5-1-3-11-23-17/h1-6,11-12,14,16H,7-10,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417042

(CHEMBL1257636)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-7.01,-.66,;-8.41,-.01,;-9.65,-.9,;-11.06,-.25,;-11.21,1.29,;-9.94,2.17,;-8.54,1.52,;-7.29,2.4,;-5.83,1.9,;-4.9,3.14,;-3.36,3.12,;-2.62,1.77,;-1.08,1.74,;-.28,3.07,;1.26,3.05,;2,1.7,;3.14,2.73,;4.48,1.96,;4.16,.45,;5.18,-.7,;2.62,.29,;5.88,2.57,;7.12,1.67,;8.53,2.29,;8.69,3.82,;7.44,4.73,;6.04,4.1,;1.21,.37,;-.33,.41,;-5.79,4.4,;-7.27,3.94,)| Show InChI InChI=1S/C23H24FN5O2/c24-20-5-1-2-6-21(20)29-15-18(13-27-29)26-12-17-7-9-23(10-8-17)16-28(22(30)31-23)19-4-3-11-25-14-19/h1-6,11,13-15,17,26H,7-10,12,16H2/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782
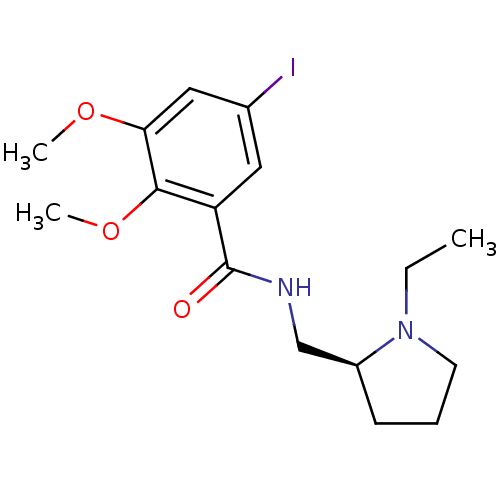
(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782

(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417050

(CHEMBL1258674)Show SMILES Fc1cc(F)cc(c1)-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:14.14,wD:17.23,(-9.07,-1.62,;-8.32,-2.96,;-9.11,-4.29,;-8.36,-5.64,;-9.14,-6.96,;-6.82,-5.65,;-6.04,-4.34,;-6.78,-2.99,;-4.49,-4.35,;-3.74,-5.7,;-2.2,-5.72,;-1.42,-4.4,;.12,-4.43,;.87,-5.77,;2.41,-5.8,;3.2,-4.48,;4.74,-4.5,;5.48,-5.85,;6.62,-4.81,;7.96,-5.59,;7.64,-7.09,;8.67,-8.24,;6.1,-7.25,;9.36,-4.97,;10.61,-5.88,;12.02,-5.25,;12.18,-3.72,;10.92,-2.81,;9.52,-3.44,;4.69,-7.17,;3.16,-7.14,;-2.16,-3.06,;-3.7,-3.03,)| Show InChI InChI=1S/C23H22F2N6O2/c24-17-10-16(11-18(25)12-17)19-3-4-20(29-28-19)26-13-15-5-7-23(8-6-15)14-31(22(32)33-23)21-2-1-9-27-30-21/h1-4,9-12,15H,5-8,13-14H2,(H,26,29)/t15-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417046

(CHEMBL1258453)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)cn1 |r,wU:12.12,wD:15.21,(16.1,-16.72,;14.7,-16.07,;13.45,-16.97,;12.04,-16.31,;11.91,-14.78,;13.16,-13.9,;14.56,-14.55,;15.81,-13.67,;17.27,-14.16,;18.2,-12.92,;19.74,-12.95,;20.49,-14.3,;22.03,-14.32,;22.82,-13,;24.36,-13.02,;25.1,-14.37,;26.24,-13.33,;27.58,-14.11,;27.26,-15.62,;28.28,-16.76,;25.73,-15.78,;28.99,-13.49,;30.23,-14.4,;31.63,-13.78,;31.8,-12.24,;30.55,-11.33,;29.15,-11.97,;24.31,-15.69,;22.78,-15.66,;17.31,-11.67,;15.84,-12.13,)| Show InChI InChI=1S/C22H23FN6O2/c23-18-4-1-2-5-19(18)29-14-17(13-26-29)24-12-16-7-9-22(10-8-16)15-28(21(30)31-22)20-6-3-11-25-27-20/h1-6,11,13-14,16,24H,7-10,12,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417044
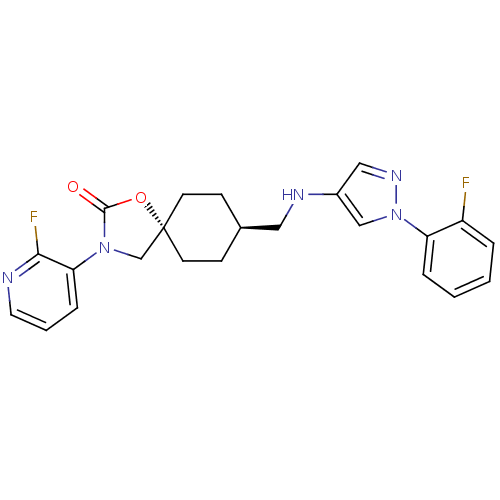
(CHEMBL1258340)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnc3F)CC2)cn1 |r,wU:12.12,wD:15.21,(17.14,-9.3,;15.74,-8.65,;14.5,-9.54,;13.09,-8.89,;12.95,-7.35,;14.21,-6.47,;15.61,-7.12,;16.86,-6.24,;18.32,-6.74,;19.25,-5.5,;20.79,-5.52,;21.54,-6.87,;23.08,-6.9,;23.87,-5.57,;25.41,-5.59,;26.15,-6.95,;27.29,-5.92,;28.63,-6.69,;28.31,-8.19,;29.34,-9.34,;26.78,-8.35,;30.04,-6.07,;31.28,-6.97,;32.69,-6.35,;32.85,-4.82,;31.6,-3.91,;30.2,-4.54,;28.95,-3.63,;25.36,-8.27,;23.83,-8.24,;18.36,-4.24,;16.89,-4.7,)| Show InChI InChI=1S/C23H23F2N5O2/c24-18-4-1-2-5-19(18)30-14-17(13-28-30)27-12-16-7-9-23(10-8-16)15-29(22(31)32-23)20-6-3-11-26-21(20)25/h1-6,11,13-14,16,27H,7-10,12,15H2/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50008782

(CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...)Show InChI InChI=1S/C16H23IN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417041

(CHEMBL1257637)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(cn1)-c1nccs1)CC2 |r,wU:14.16,wD:3.2,(30.13,-31.33,;29.1,-30.19,;27.57,-30.35,;26.95,-28.94,;28.09,-27.91,;29.43,-28.68,;30.83,-28.06,;32.08,-28.97,;33.48,-28.34,;33.65,-26.81,;32.39,-25.9,;30.99,-26.53,;26.21,-27.59,;24.67,-27.57,;23.88,-28.89,;22.34,-28.87,;21.59,-27.52,;20.05,-27.5,;19.26,-28.81,;17.73,-28.79,;16.97,-27.44,;17.77,-26.12,;19.3,-26.15,;15.43,-27.43,;14.51,-28.66,;13.05,-28.17,;13.06,-26.63,;14.53,-26.17,;24.62,-30.23,;26.16,-30.26,)| Show InChI InChI=1S/C22H23N5O2S/c28-21-27(19-3-1-2-10-23-19)15-22(29-21)8-6-16(7-9-22)13-25-18-5-4-17(14-26-18)20-24-11-12-30-20/h1-5,10-12,14,16H,6-9,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417039
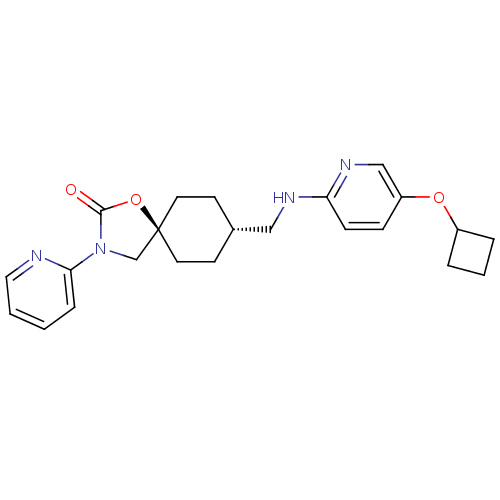
(CHEMBL1257761)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(OC3CCC3)cn1)CC2 |r,wU:14.16,wD:3.2,(29.67,-25.14,;28.64,-23.99,;27.1,-24.16,;26.48,-22.75,;27.62,-21.72,;28.96,-22.49,;30.36,-21.87,;31.61,-22.78,;33.02,-22.15,;33.18,-20.62,;31.92,-19.71,;30.52,-20.34,;25.74,-21.4,;24.2,-21.38,;23.41,-22.7,;21.87,-22.68,;21.12,-21.33,;19.58,-21.3,;18.8,-22.62,;17.26,-22.6,;16.51,-21.25,;14.96,-21.24,;14.22,-19.89,;14.64,-18.41,;13.16,-17.99,;12.74,-19.47,;17.3,-19.93,;18.84,-19.96,;24.16,-24.04,;25.69,-24.07,)| Show InChI InChI=1S/C23H28N4O3/c28-22-27(21-6-1-2-13-24-21)16-23(30-22)11-9-17(10-12-23)14-25-20-8-7-19(15-26-20)29-18-4-3-5-18/h1-2,6-8,13,15,17-18H,3-5,9-12,14,16H2,(H,25,26)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417054

(CHEMBL1258673)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nn1 |r,wU:13.13,wD:16.22,(14.72,.44,;13.96,1.78,;12.43,1.8,;11.67,3.15,;12.46,4.47,;14,4.45,;14.75,3.1,;16.29,3.08,;17.04,1.73,;18.58,1.71,;19.36,3.03,;20.9,3.01,;21.65,1.66,;23.19,1.64,;23.99,2.96,;25.53,2.94,;26.26,1.59,;27.4,2.62,;28.74,1.85,;28.42,.34,;29.45,-.8,;26.89,.18,;30.15,2.47,;31.39,1.56,;32.8,2.18,;32.96,3.72,;31.71,4.62,;30.31,3.99,;25.47,.27,;23.94,.3,;18.62,4.38,;17.08,4.41,)| Show InChI InChI=1S/C23H23FN6O2/c24-18-5-2-1-4-17(18)19-7-8-20(28-27-19)25-14-16-9-11-23(12-10-16)15-30(22(31)32-23)21-6-3-13-26-29-21/h1-8,13,16H,9-12,14-15H2,(H,25,28)/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417040
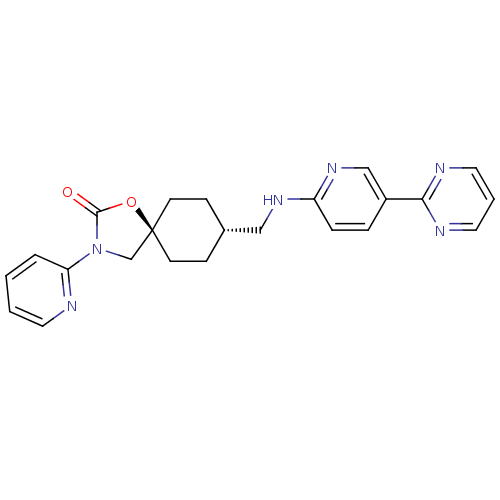
(CHEMBL1257760)Show SMILES O=C1O[C@@]2(CN1c1ccccn1)CC[C@H](CNc1ccc(cn1)-c1ncccn1)CC2 |r,wU:14.16,wD:3.2,(7.1,-28.95,;6.07,-27.81,;4.54,-27.97,;3.92,-26.56,;5.06,-25.53,;6.4,-26.3,;7.8,-25.68,;9.05,-26.59,;10.45,-25.96,;10.62,-24.43,;9.36,-23.52,;7.96,-24.15,;3.18,-25.21,;1.64,-25.19,;.85,-26.51,;-.69,-26.49,;-1.44,-25.14,;-2.98,-25.12,;-3.77,-26.43,;-5.3,-26.41,;-6.06,-25.06,;-5.26,-23.74,;-3.73,-23.77,;-7.6,-25.05,;-8.35,-23.7,;-9.89,-23.68,;-10.68,-25,;-9.92,-26.35,;-8.38,-26.37,;1.59,-27.85,;3.13,-27.88,)| Show InChI InChI=1S/C23H24N6O2/c30-22-29(20-4-1-2-11-24-20)16-23(31-22)9-7-17(8-10-23)14-27-19-6-5-18(15-28-19)21-25-12-3-13-26-21/h1-6,11-13,15,17H,7-10,14,16H2,(H,27,28)/t17-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417048
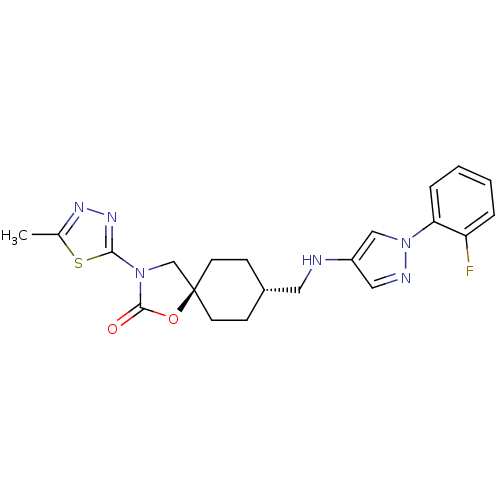
(CHEMBL1258562)Show SMILES Cc1nnc(s1)N1C[C@@]2(CC[C@H](CNc3cnn(c3)-c3ccccc3F)CC2)OC1=O |r,wU:11.12,wD:8.31,(32.21,-20.6,;30.68,-20.6,;29.77,-19.35,;28.3,-19.82,;28.3,-21.36,;29.77,-21.84,;26.9,-21.98,;25.57,-21.2,;24.42,-22.24,;23.69,-20.88,;22.15,-20.86,;21.35,-22.19,;19.81,-22.16,;19.07,-20.81,;17.53,-20.79,;16.64,-19.53,;15.17,-19.99,;15.14,-21.53,;16.6,-22.03,;13.89,-22.41,;12.49,-21.76,;11.24,-22.64,;11.37,-24.18,;12.78,-24.83,;14.02,-23.94,;15.42,-24.59,;22.1,-23.53,;23.64,-23.56,;25.05,-23.64,;26.58,-23.48,;27.61,-24.63,)| Show InChI InChI=1S/C21H23FN6O2S/c1-14-25-26-19(31-14)27-13-21(30-20(27)29)8-6-15(7-9-21)10-23-16-11-24-28(12-16)18-5-3-2-4-17(18)22/h2-5,11-12,15,23H,6-10,13H2,1H3/t15-,21- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417047

(CHEMBL1258454)Show SMILES Fc1ccccc1-n1cc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccnnc3)CC2)cn1 |r,wU:12.12,wD:15.21,(-7.1,-25.51,;-8.5,-24.86,;-9.75,-25.75,;-11.16,-25.1,;-11.3,-23.56,;-10.04,-22.68,;-8.64,-23.33,;-7.38,-22.45,;-5.93,-22.95,;-5,-21.71,;-3.45,-21.73,;-2.71,-23.08,;-1.17,-23.1,;-.37,-21.78,;1.17,-21.8,;1.9,-23.15,;3.04,-22.12,;4.38,-22.89,;4.06,-24.4,;5.09,-25.55,;2.53,-24.56,;5.79,-22.28,;7.03,-23.18,;8.44,-22.56,;8.6,-21.03,;7.35,-20.12,;5.95,-20.75,;1.11,-24.48,;-.42,-24.44,;-5.89,-20.45,;-7.36,-20.91,)| Show InChI InChI=1S/C22H23FN6O2/c23-19-3-1-2-4-20(19)29-14-17(12-27-29)24-11-16-5-8-22(9-6-16)15-28(21(30)31-22)18-7-10-25-26-13-18/h1-4,7,10,12-14,16,24H,5-6,8-9,11,15H2/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417055

(CHEMBL1258788)Show SMILES Cc1cccc(n1)-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nc1 |r,wU:13.13,wD:16.22,(-8.85,-9.35,;-8.11,-10.7,;-8.9,-12.02,;-8.14,-13.37,;-6.6,-13.38,;-5.82,-12.07,;-6.57,-10.72,;-4.28,-12.08,;-3.52,-13.43,;-1.98,-13.45,;-1.2,-12.13,;.34,-12.16,;1.09,-13.51,;2.63,-13.53,;3.42,-12.21,;4.96,-12.23,;5.7,-13.58,;6.84,-12.55,;8.18,-13.32,;7.85,-14.82,;8.88,-15.97,;6.32,-14.99,;9.58,-12.7,;10.83,-13.61,;12.23,-12.98,;12.4,-11.45,;11.14,-10.54,;9.74,-11.17,;4.91,-14.9,;3.37,-14.87,;-1.95,-10.79,;-3.48,-10.76,)| Show InChI InChI=1S/C24H26N6O2/c1-17-4-2-5-20(28-17)19-7-8-21(26-15-19)25-14-18-9-11-24(12-10-18)16-30(23(31)32-24)22-6-3-13-27-29-22/h2-8,13,15,18H,9-12,14,16H2,1H3,(H,25,26)/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417034
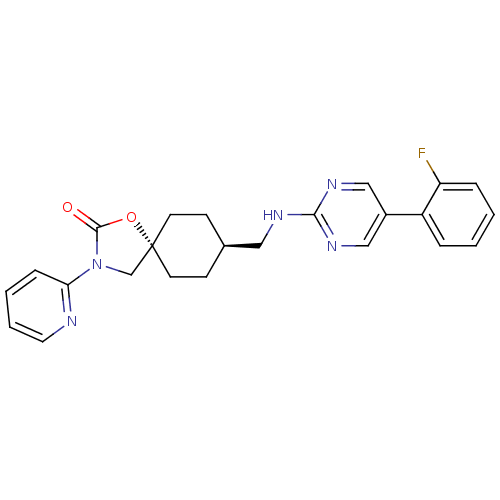
(CHEMBL1258224)Show SMILES Fc1ccccc1-c1cnc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nc1 |r,wU:13.13,wD:16.22,(-7.82,-7.93,;-8.57,-6.59,;-10.11,-6.57,;-10.86,-5.22,;-10.07,-3.9,;-8.53,-3.92,;-7.79,-5.27,;-6.24,-5.29,;-5.45,-3.96,;-3.91,-3.99,;-3.17,-5.34,;-1.63,-5.36,;-.88,-6.71,;.66,-6.73,;1.45,-5.41,;2.99,-5.43,;3.73,-6.78,;4.87,-5.75,;6.21,-6.52,;5.89,-8.03,;6.92,-9.17,;4.35,-8.19,;7.61,-5.9,;8.86,-6.81,;10.27,-6.19,;10.43,-4.65,;9.17,-3.75,;7.77,-4.37,;2.94,-8.1,;1.41,-8.07,;-3.95,-6.65,;-5.49,-6.64,)| Show InChI InChI=1S/C24H24FN5O2/c25-20-6-2-1-5-19(20)18-14-28-22(29-15-18)27-13-17-8-10-24(11-9-17)16-30(23(31)32-24)21-7-3-4-12-26-21/h1-7,12,14-15,17H,8-11,13,16H2,(H,27,28,29)/t17-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417052

(CHEMBL1258907)Show SMILES Fc1cccnc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3cccnn3)CC2)nc1 |r,wU:13.13,wD:16.22,(16.94,-15.12,;16.19,-13.77,;14.65,-13.76,;13.89,-12.41,;14.68,-11.08,;16.22,-11.11,;16.97,-12.46,;18.51,-12.47,;19.27,-13.82,;20.8,-13.84,;21.59,-12.52,;23.13,-12.55,;23.88,-13.89,;25.42,-13.92,;26.21,-12.6,;27.75,-12.62,;28.49,-13.97,;29.63,-12.93,;30.97,-13.71,;30.64,-15.21,;31.67,-16.36,;29.11,-15.37,;32.37,-13.09,;33.62,-14,;35.02,-13.37,;35.19,-11.84,;33.93,-10.93,;32.53,-11.56,;27.7,-15.29,;26.16,-15.26,;20.84,-11.18,;19.31,-11.15,)| Show InChI InChI=1S/C23H23FN6O2/c24-18-3-1-11-25-21(18)17-5-6-19(27-14-17)26-13-16-7-9-23(10-8-16)15-30(22(31)32-23)20-4-2-12-28-29-20/h1-6,11-12,14,16H,7-10,13,15H2,(H,26,27)/t16-,23- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50241107

(1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...)Show SMILES Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1 Show InChI InChI=1S/C22H24ClN5O2/c23-15-6-7-20-18(14-15)25-22(30)28(20)16-8-12-26(13-9-16)10-3-11-27-19-5-2-1-4-17(19)24-21(27)29/h1-2,4-7,14,16H,3,8-13H2,(H,24,29)(H,25,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417043
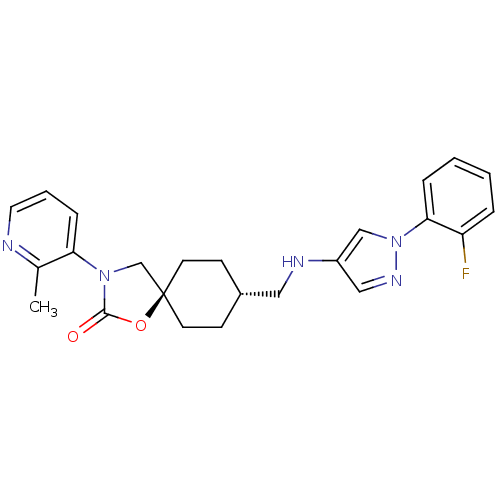
(CHEMBL1257520)Show SMILES Cc1ncccc1N1C[C@@]2(CC[C@H](CNc3cnn(c3)-c3ccccc3F)CC2)OC1=O |r,wU:12.13,wD:9.32,(27.95,3.78,;29.2,2.88,;30.61,3.51,;31.86,2.6,;31.69,1.07,;30.29,.45,;29.05,1.35,;27.64,.73,;26.3,1.5,;25.16,.47,;24.42,1.83,;22.88,1.84,;22.08,.52,;20.54,.55,;19.8,1.89,;18.26,1.92,;17.37,3.18,;15.89,2.72,;15.87,1.18,;17.33,.68,;14.61,.3,;13.22,.95,;11.96,.07,;12.1,-1.47,;13.5,-2.12,;14.75,-1.23,;16.15,-1.88,;22.84,-.82,;24.37,-.85,;25.79,-.94,;27.32,-.77,;28.34,-1.92,)| Show InChI InChI=1S/C24H26FN5O2/c1-17-21(7-4-12-26-17)29-16-24(32-23(29)31)10-8-18(9-11-24)13-27-19-14-28-30(15-19)22-6-3-2-5-20(22)25/h2-7,12,14-15,18,27H,8-11,13,16H2,1H3/t18-,24- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417038

(CHEMBL1257881)Show SMILES FC(F)(F)c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)nc1 |r,wU:10.9,wD:13.18,(-9.58,-17.43,;-8.84,-18.78,;-9.62,-20.1,;-10.38,-18.78,;-7.29,-18.8,;-6.54,-20.15,;-5,-20.16,;-4.22,-18.85,;-2.68,-18.87,;-1.93,-20.22,;-.39,-20.24,;.4,-18.92,;1.94,-18.94,;2.68,-20.29,;3.82,-19.26,;5.16,-20.03,;4.84,-21.54,;5.87,-22.68,;3.3,-21.7,;6.56,-19.41,;7.81,-20.32,;9.22,-19.7,;9.38,-18.16,;8.12,-17.26,;6.72,-17.88,;1.89,-21.61,;.36,-21.58,;-4.96,-17.5,;-6.5,-17.47,)| Show InChI InChI=1S/C20H21F3N4O2/c21-20(22,23)15-4-5-16(26-12-15)25-11-14-6-8-19(9-7-14)13-27(18(28)29-19)17-3-1-2-10-24-17/h1-5,10,12,14H,6-9,11,13H2,(H,25,26)/t14-,19- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417049

(CHEMBL1258563)Show SMILES O=C1O[C@@]2(CN1c1cccnn1)CC[C@H](CNc1ccc(cn1)-c1ncccn1)CC2 |r,wU:14.16,wD:3.2,(5.9,-.17,;4.87,.97,;3.34,.81,;2.72,2.22,;3.86,3.25,;5.2,2.48,;6.6,3.1,;7.85,2.19,;9.25,2.81,;9.42,4.35,;8.16,5.25,;6.76,4.62,;1.98,3.57,;.44,3.59,;-.35,2.27,;-1.89,2.29,;-2.64,3.64,;-4.18,3.66,;-4.96,2.34,;-6.5,2.36,;-7.26,3.71,;-6.46,5.04,;-4.92,5.01,;-8.8,3.73,;-9.55,5.08,;-11.08,5.1,;-11.88,3.78,;-11.12,2.43,;-9.58,2.41,;.4,.93,;1.93,.9,)| Show InChI InChI=1S/C22H23N7O2/c30-21-29(19-3-1-12-27-28-19)15-22(31-21)8-6-16(7-9-22)13-25-18-5-4-17(14-26-18)20-23-10-2-11-24-20/h1-5,10-12,14,16H,6-9,13,15H2,(H,25,26)/t16-,22- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417053
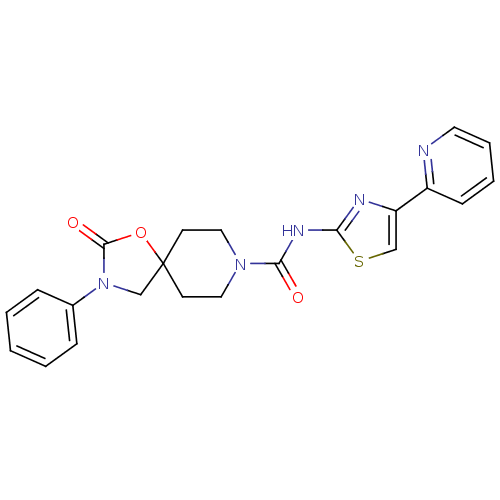
(CHEMBL1257173)Show SMILES O=C(Nc1nc(cs1)-c1ccccn1)N1CCC2(CN(C(=O)O2)c2ccccc2)CC1 Show InChI InChI=1S/C22H21N5O3S/c28-20(25-19-24-18(14-31-19)17-8-4-5-11-23-17)26-12-9-22(10-13-26)15-27(21(29)30-22)16-6-2-1-3-7-16/h1-8,11,14H,9-10,12-13,15H2,(H,24,25,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... |
Bioorg Med Chem 23: 770-8 (2015)
Article DOI: 10.1016/j.bmc.2014.12.063
BindingDB Entry DOI: 10.7270/Q2J38V8K |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50029816
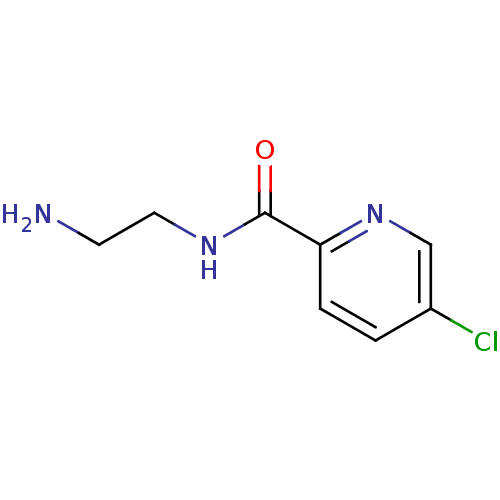
(5-Chloro-pyridine-2-carboxylic acid (2-amino-ethyl...)Show InChI InChI=1S/C8H10ClN3O/c9-6-1-2-7(12-5-6)8(13)11-4-3-10/h1-2,5H,3-4,10H2,(H,11,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... |
Bioorg Med Chem 23: 770-8 (2015)
Article DOI: 10.1016/j.bmc.2014.12.063
BindingDB Entry DOI: 10.7270/Q2J38V8K |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant microsomal MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 min... |
Bioorg Med Chem 23: 770-8 (2015)
Article DOI: 10.1016/j.bmc.2014.12.063
BindingDB Entry DOI: 10.7270/Q2J38V8K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074300
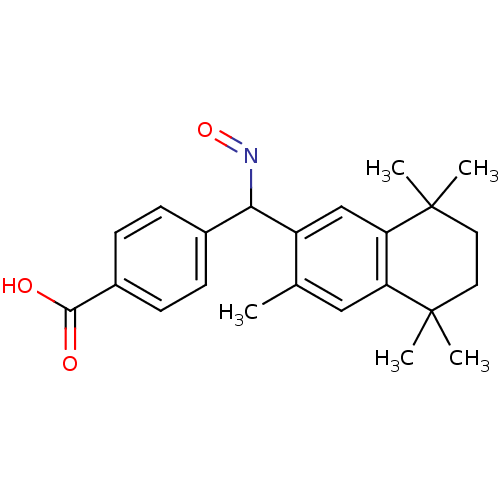
(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50074300

(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50417037
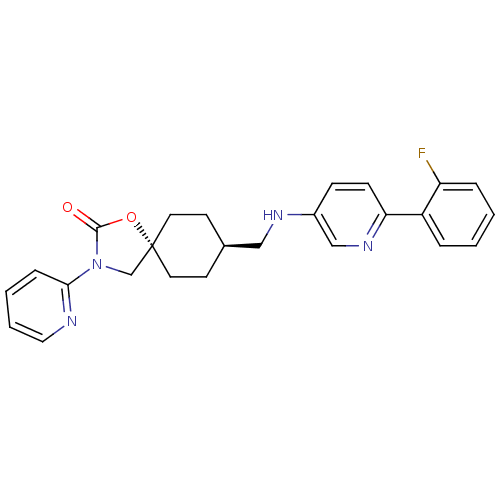
(CHEMBL1257882)Show SMILES Fc1ccccc1-c1ccc(NC[C@H]2CC[C@@]3(CN(C(=O)O3)c3ccccn3)CC2)cn1 |r,wU:13.13,wD:16.22,(16.05,-15.23,;15.29,-13.88,;13.76,-13.87,;13,-12.52,;13.79,-11.19,;15.33,-11.22,;16.08,-12.56,;17.62,-12.58,;18.37,-13.93,;19.91,-13.95,;20.69,-12.63,;22.23,-12.66,;22.98,-14,;24.52,-14.03,;25.32,-12.71,;26.86,-12.73,;27.59,-14.08,;28.73,-13.04,;30.07,-13.82,;29.75,-15.32,;30.78,-16.47,;28.22,-15.48,;31.48,-13.2,;32.72,-14.1,;34.13,-13.48,;34.29,-11.95,;33.04,-11.04,;31.64,-11.67,;26.8,-15.4,;25.27,-15.37,;19.95,-11.28,;18.41,-11.26,)| Show InChI InChI=1S/C25H25FN4O2/c26-21-6-2-1-5-20(21)22-9-8-19(16-29-22)28-15-18-10-12-25(13-11-18)17-30(24(31)32-25)23-7-3-4-14-27-23/h1-9,14,16,18,28H,10-13,15,17H2/t18-,25- | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline SpA
Curated by ChEMBL
| Assay Description
Antagonist activity at human NPY Y5 receptor |
Bioorg Med Chem Lett 20: 6103-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.041
BindingDB Entry DOI: 10.7270/Q2JM2BWB |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM19187
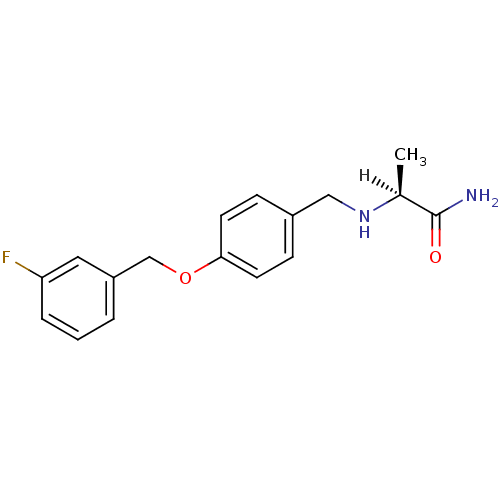
((2S)-2-[({4-[(3-fluorophenyl)methoxy]phenyl}methyl...)Show SMILES [H][C@@](C)(NCc1ccc(OCc2cccc(F)c2)cc1)C(N)=O |r| Show InChI InChI=1S/C17H19FN2O2/c1-12(17(19)21)20-10-13-5-7-16(8-6-13)22-11-14-3-2-4-15(18)9-14/h2-9,12,20H,10-11H2,1H3,(H2,19,21)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... |
Bioorg Med Chem 23: 770-8 (2015)
Article DOI: 10.1016/j.bmc.2014.12.063
BindingDB Entry DOI: 10.7270/Q2J38V8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074307
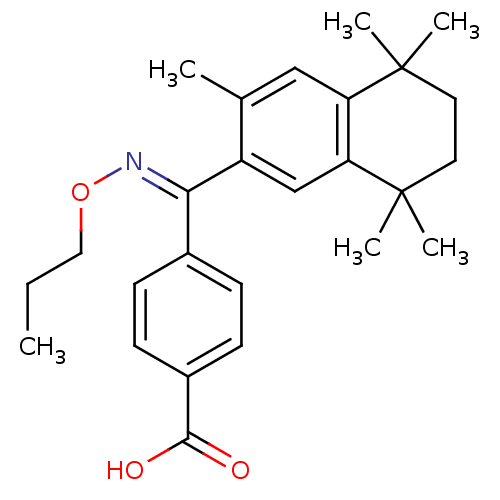
(4-{(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CCCO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H33NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h8-11,15-16H,7,12-14H2,1-6H3,(H,28,29)/b27-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074300

(4-[[(E)-Hydroxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES Cc1cc2c(cc1C(N=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H27NO3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24-27)15-6-8-16(9-7-15)21(25)26/h6-9,12-13,20H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074304
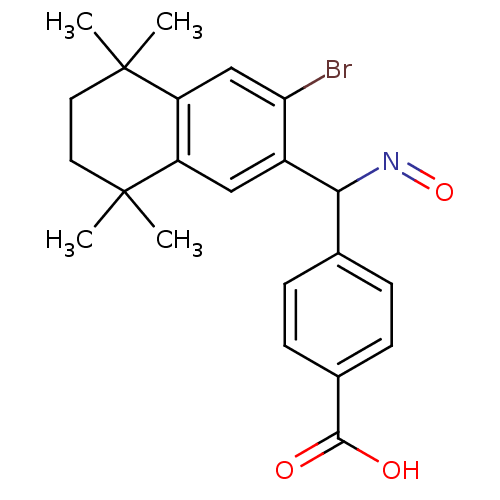
(4-{(3-Bromo-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...)Show SMILES CC1(C)CCC(C)(C)c2cc(C(N=O)c3ccc(cc3)C(O)=O)c(Br)cc12 Show InChI InChI=1S/C22H24BrNO3/c1-21(2)9-10-22(3,4)17-12-18(23)15(11-16(17)21)19(24-27)13-5-7-14(8-6-13)20(25)26/h5-8,11-12,19H,9-10H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074306
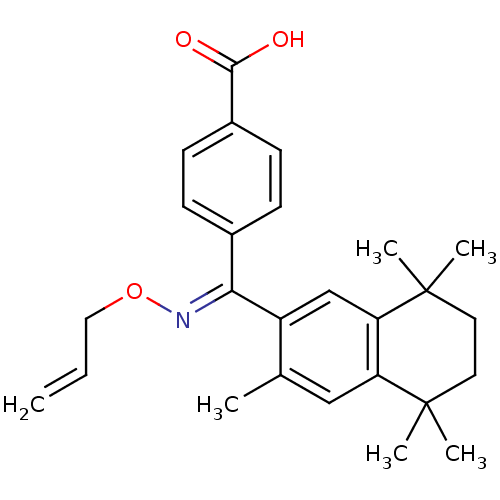
(4-[[(E)-Allyloxyimino]-(3,5,5,8,8-pentamethyl-5,6,...)Show SMILES Cc1cc2c(cc1\C(=N\OCC=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C26H31NO3/c1-7-14-30-27-23(18-8-10-19(11-9-18)24(28)29)20-16-22-21(15-17(20)2)25(3,4)12-13-26(22,5)6/h7-11,15-16H,1,12-14H2,2-6H3,(H,28,29)/b27-23+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM10989
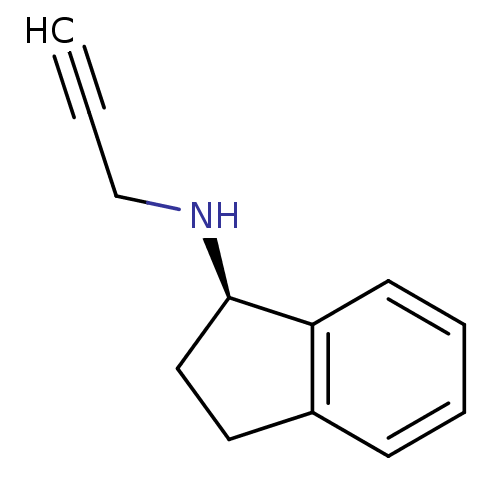
((1R)-N-(prop-2-yn-1-yl)-2,3-dihydro-1H-inden-1-ami...)Show InChI InChI=1S/C12H13N/c1-2-9-13-12-8-7-10-5-3-4-6-11(10)12/h1,3-6,12-13H,7-9H2/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dart NeuroScience, LLC
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble MAO-B expressed in Pichia pastoris incubated for 30 mins prior to substrate addition measured after 60 mins b... |
Bioorg Med Chem 23: 770-8 (2015)
Article DOI: 10.1016/j.bmc.2014.12.063
BindingDB Entry DOI: 10.7270/Q2J38V8K |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50074295
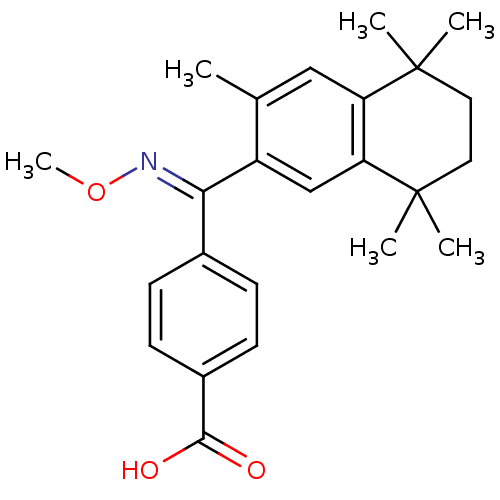
(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-gamma in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50074295

(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-beta in baculoviral sysytem, by using 5 nM [3H]-targretin in a competitiv... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50074295

(4-[[(E)-Methoxyimino]-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES CO\N=C(/c1ccc(cc1)C(O)=O)c1cc2c(cc1C)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H29NO3/c1-15-13-19-20(24(4,5)12-11-23(19,2)3)14-18(15)21(25-28-6)16-7-9-17(10-8-16)22(26)27/h7-10,13-14H,11-12H2,1-6H3,(H,26,27)/b25-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Binding affinity towards recombinantly expressed Retinoic acid receptor RXR-alpha in baculoviral sysytem, by using 5 nM [3H]-targretin in a competiti... |
J Med Chem 42: 742-50 (1999)
Article DOI: 10.1021/jm980621r
BindingDB Entry DOI: 10.7270/Q2K936Q1 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM11638

(CHEMBL26 | Compound 7 | N-[(1-ethylpyrrolidin-2-yl...)Show InChI InChI=1S/C15H23N3O4S/c1-3-18-8-4-5-11(18)10-17-15(19)13-9-12(23(16,20)21)6-7-14(13)22-2/h6-7,9,11H,3-5,8,10H2,1-2H3,(H,17,19)(H2,16,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by PDSP Ki Database
| |
Synapse 15: 169-76 (1993)
Article DOI: 10.1002/syn.890150302
BindingDB Entry DOI: 10.7270/Q2MS3R8Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data