

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106179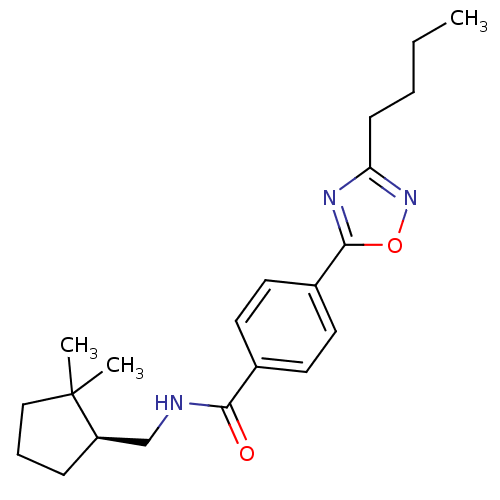 (CHEMBL125327 | Enantiomer-4-(3-Butyl-[1,2,4]oxadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106169 (CHEMBL338973 | Enantiomer-4-(3-Butyl-[1,2,4]oxadia...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106167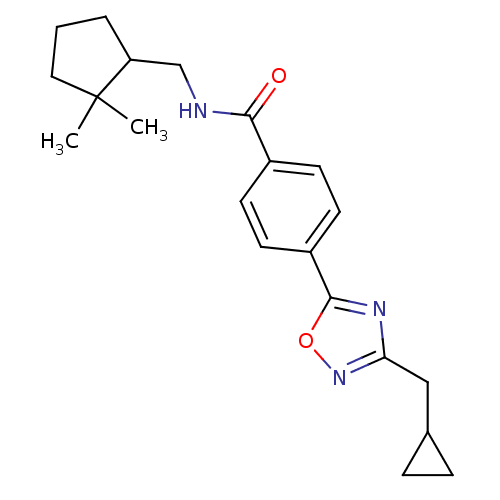 (CHEMBL125000 | Enantiomer-4-(3-Cyclopropylmethyl-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106167 (CHEMBL125000 | Enantiomer-4-(3-Cyclopropylmethyl-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from whole rat brain Nicotinic acetylcholine receptor | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106178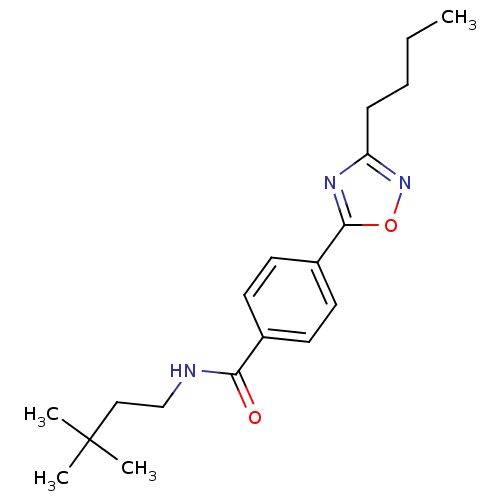 (4-(3-Butyl-[1,2,4]oxadiazol-5-yl)-N-(3,3-dimethyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106168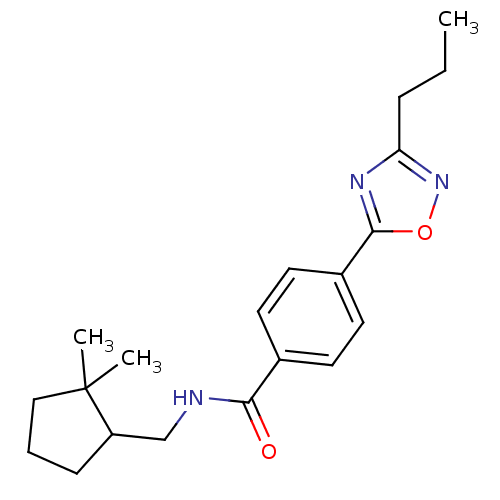 (CHEMBL124448 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from whole rat brain Nicotinic acetylcholine receptor | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106168 (CHEMBL124448 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404297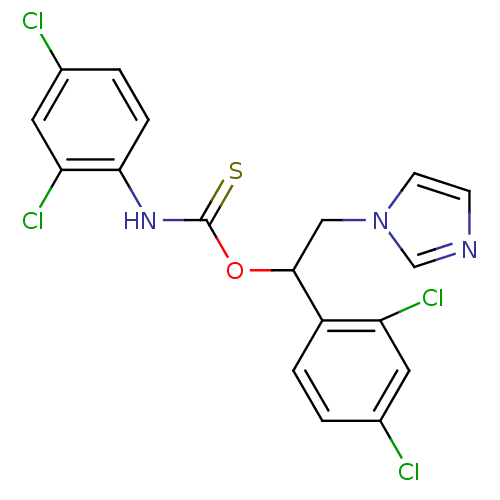 (CHEMBL269080) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50061218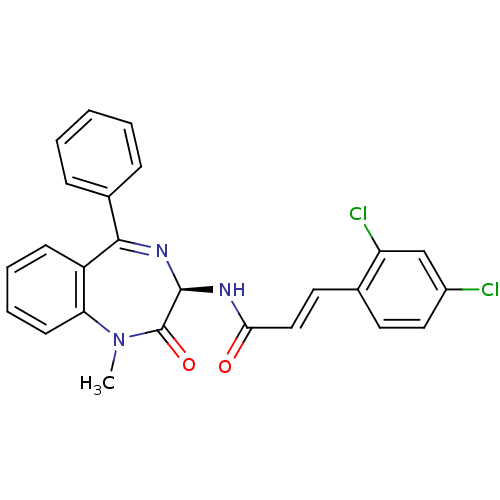 ((E)-3-(2,4-Dichloro-phenyl)-N-((R)-1-methyl-2-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404288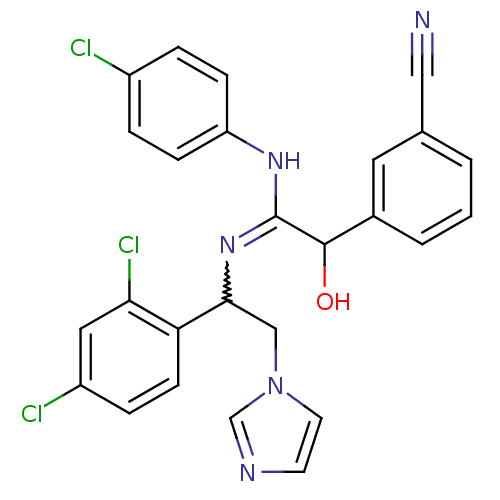 (CHEMBL269369) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0-ATP synthase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404288 (CHEMBL269369) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106176 (CHEMBL340681 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity values obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from a preparation of whole rat brain | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106176 (CHEMBL340681 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404293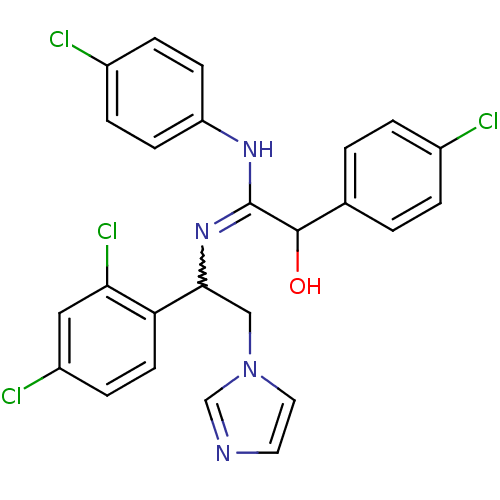 (CHEMBL8024) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106182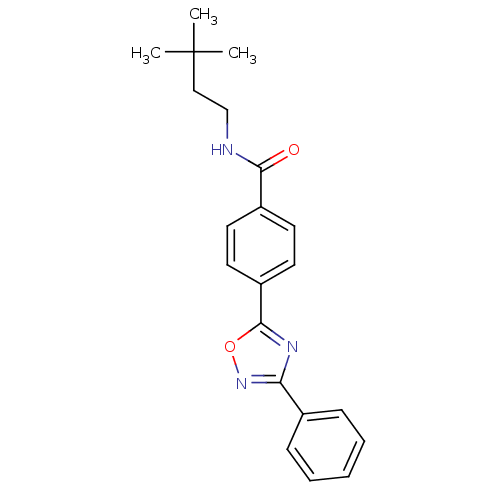 (CHEMBL123085 | N-(3,3-Dimethyl-butyl)-4-(3-phenyl-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404286 (CHEMBL7982) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0-ATP synthase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106166 (CHEMBL122341 | N-(3,3-Dimethyl-butyl)-4-hexyloxy-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106177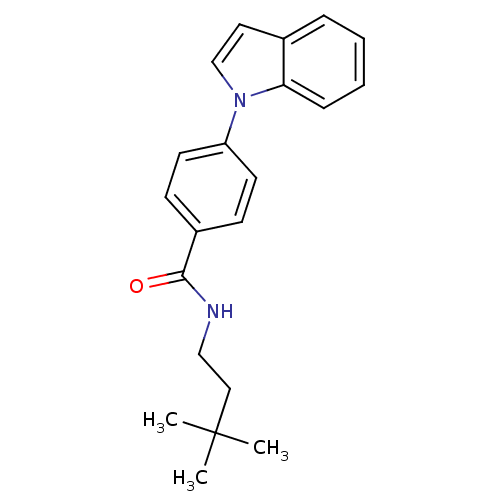 (CHEMBL431682 | N-(3,3-Dimethyl-butyl)-4-indol-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404287 (CHEMBL8116) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0-ATP synthase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287137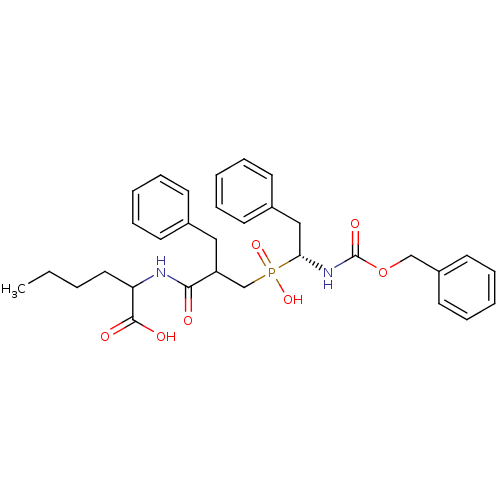 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287140 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106184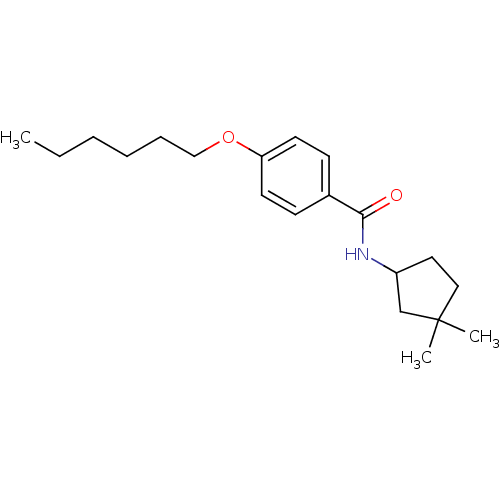 (CHEMBL121751 | N-(3,3-Dimethyl-cyclopentyl)-4-hexy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106180 (CHEMBL124595 | N-(3,3-Dimethyl-cyclohexylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50287138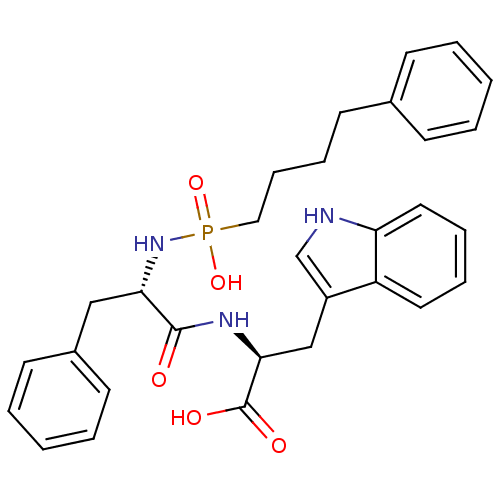 (2-[(2-{[hydroxy(4-phenyl-butyl)phosphoryl]amino}-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of endothelin-converting enzyme in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404289 (CHEMBL8376) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106181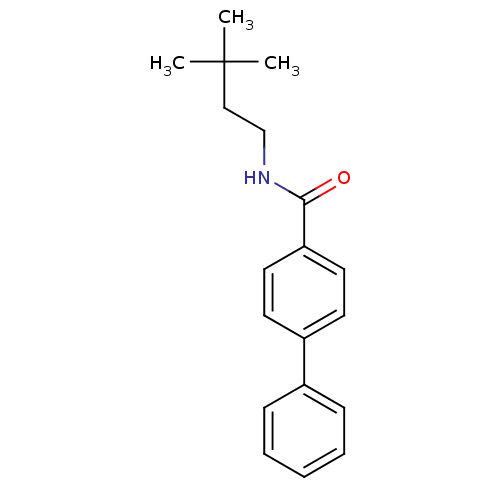 (Biphenyl-4-carboxylic acid (3,3-dimethyl-butyl)-am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106164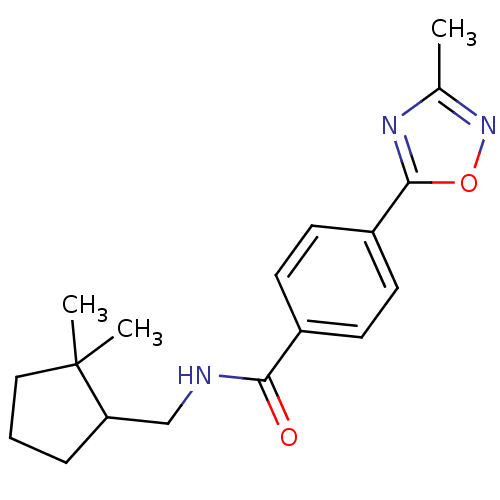 (CHEMBL123569 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from a preparation of whole rat brain | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106164 (CHEMBL123569 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404301 (CHEMBL7871) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0-ATP synthase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404284 (CHEMBL8064) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404285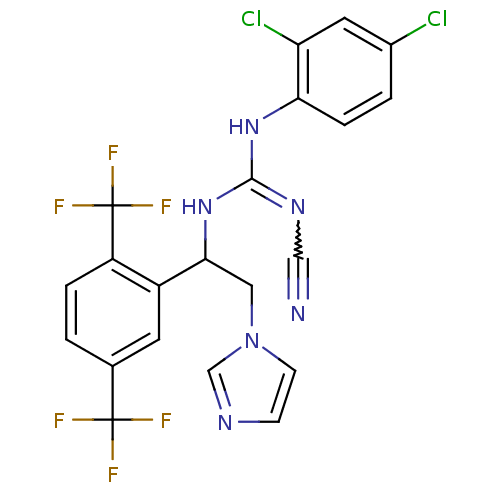 (CHEMBL8025) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106175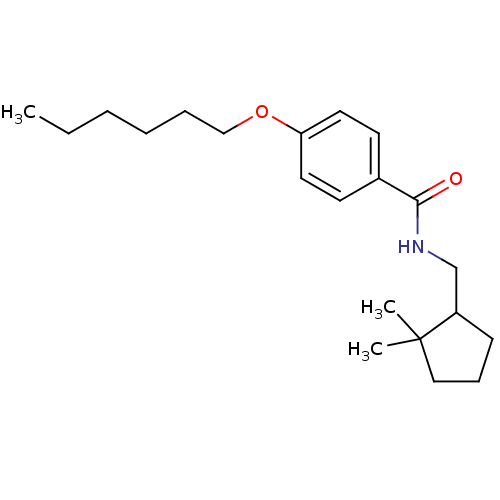 (CHEMBL121750 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106175 (CHEMBL121750 | Enantiomer-N-(2,2-Dimethyl-cyclopen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Bindind affinity value obtained by measuring the displacement of radioligand [3H]-(-)-cytisine from whole rat brain Nicotinic acetylcholine receptor | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287136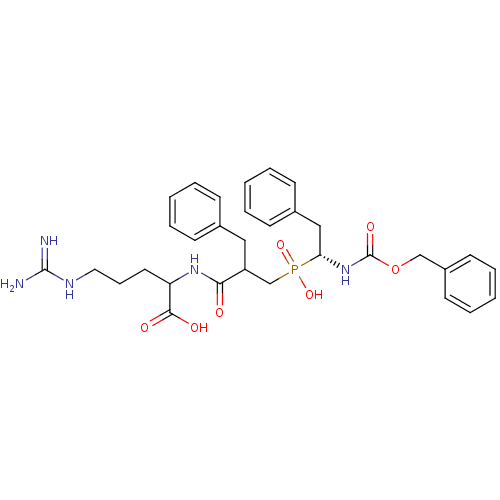 (2-{(R)-2-Benzyl-3-[(1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287134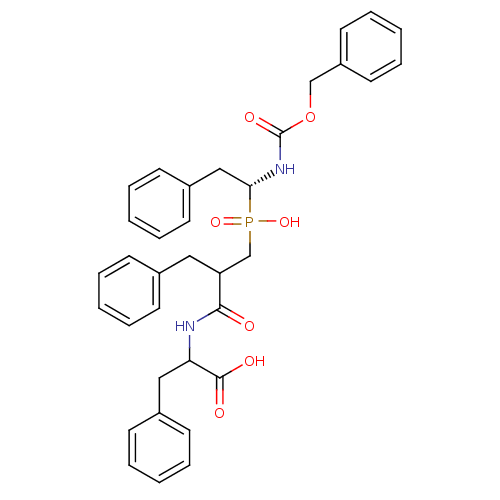 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287129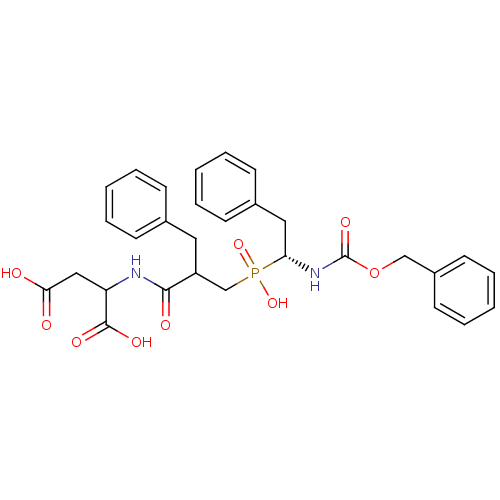 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of endothelin-converting enzyme in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287147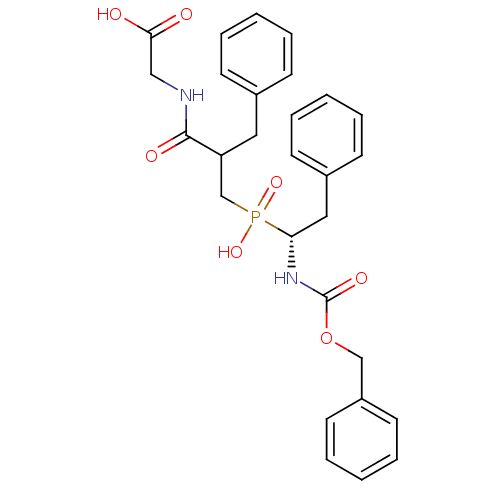 (CHEMBL282158 | {2-Benzyl-3-[((R)-1-benzyloxycarbon...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50287130 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of endothelin-converting enzyme in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50287130 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287150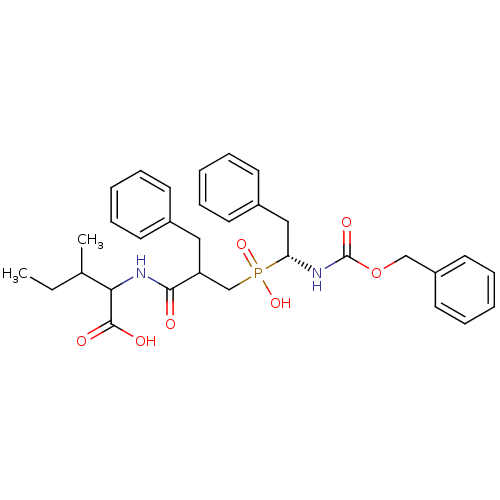 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287130 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287130 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily KQT member 1 (Cavia porcellus) | BDBM50106174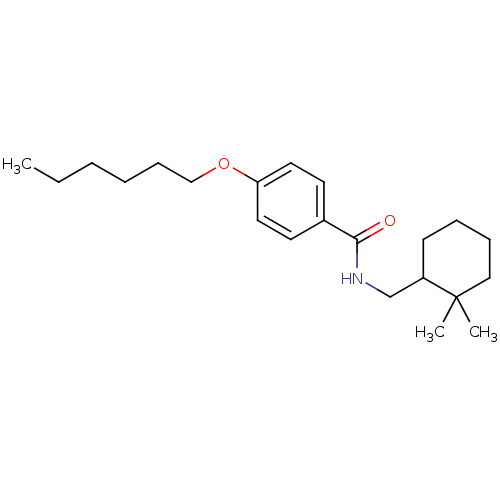 (CHEMBL332297 | N-(2,2-Dimethyl-cyclohexylmethyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Molar concentration required to inhibit 50% of the activating delayed-rectifier K+ current in isolated guinea pig ventricularmyocytes | J Med Chem 44: 3764-7 (2001) BindingDB Entry DOI: 10.7270/Q2G1604C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404290 (CHEMBL8473) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404300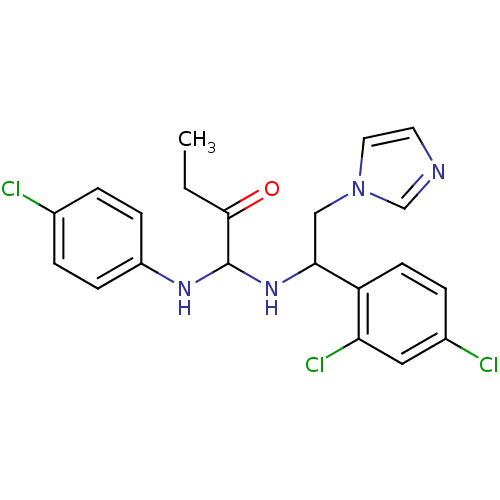 (CHEMBL7894) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287139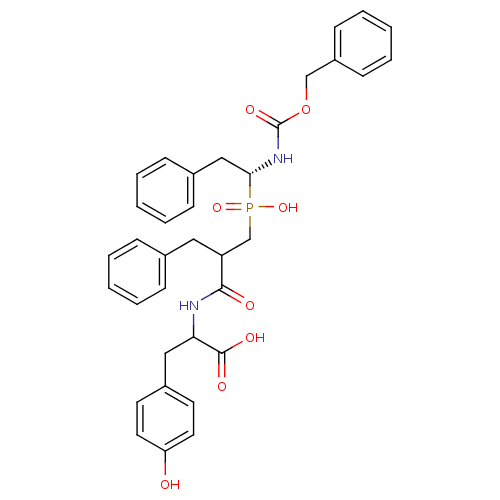 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287139 (2-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50287141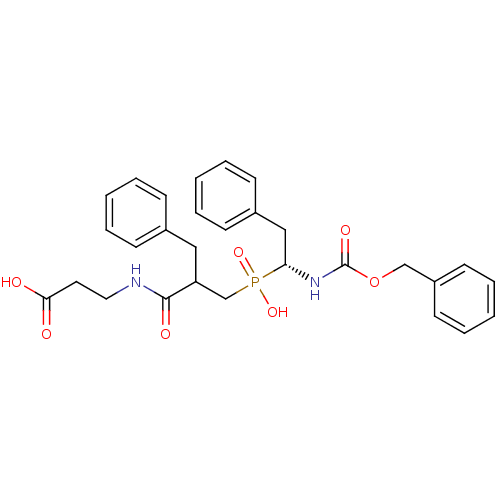 (3-{2-Benzyl-3-[((R)-1-benzyloxycarbonylamino-2-phe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human umbilical vein endothelial cells | Bioorg Med Chem Lett 6: 1323-1326 (1996) Article DOI: 10.1016/0960-894X(96)00227-2 BindingDB Entry DOI: 10.7270/Q2Q81DKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial (Bos taurus) | BDBM50404299 (CHEMBL8199) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against bovine mitochondrial F1F0-ATP synthase | Bioorg Med Chem Lett 14: 1027-30 (2004) Article DOI: 10.1016/j.bmcl.2003.11.077 BindingDB Entry DOI: 10.7270/Q2VX0HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 131 total ) | Next | Last >> |