 Found 429 hits with Last Name = 'schreier' and Initial = 'jd'
Found 429 hits with Last Name = 'schreier' and Initial = 'jd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
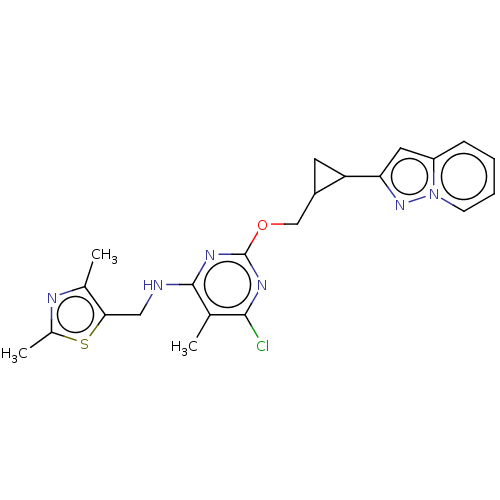
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147136
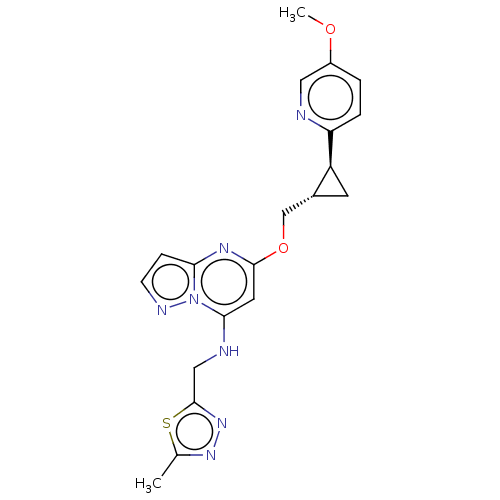
(US8957077, M-3)Show SMILES COc1ccc(nc1)[C@H]1C[C@@H]1COc1cc(NCc2nnc(C)s2)n2nccc2n1 |r| Show InChI InChI=1S/C20H21N7O2S/c1-12-25-26-20(30-12)10-22-18-8-19(24-17-5-6-23-27(17)18)29-11-13-7-15(13)16-4-3-14(28-2)9-21-16/h3-6,8-9,13,15,22H,7,10-11H2,1-2H3/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
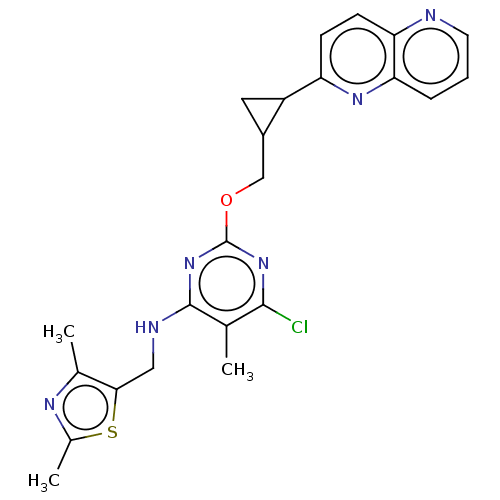
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823
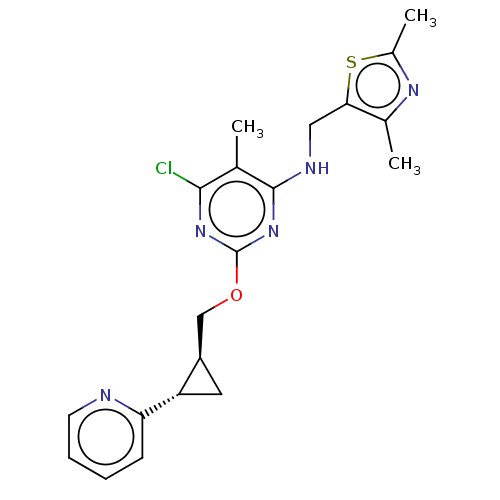
(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823

(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104700
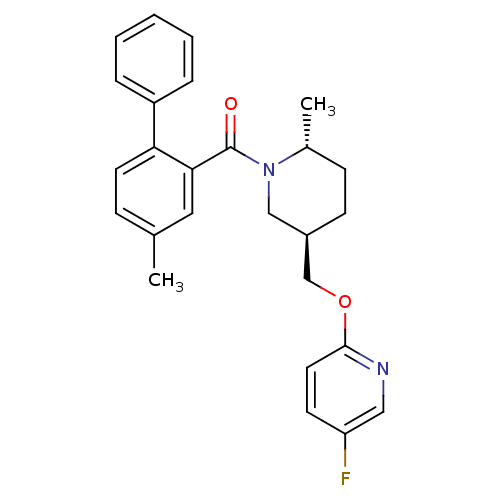
(US8569311, 1-10)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H27FN2O2/c1-18-8-12-23(21-6-4-3-5-7-21)24(14-18)26(30)29-16-20(10-9-19(29)2)17-31-25-13-11-22(27)15-28-25/h3-8,11-15,19-20H,9-10,16-17H2,1-2H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147143
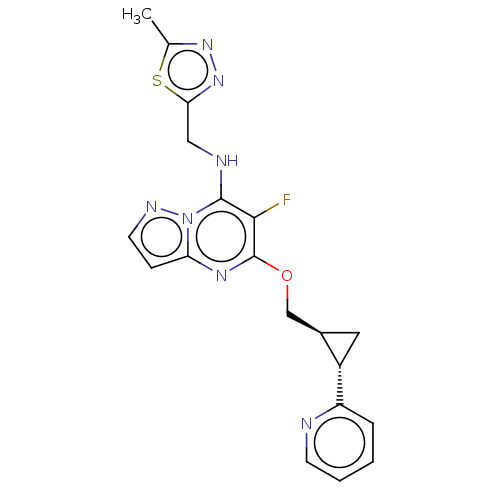
(US8957077, 1-18)Show SMILES Cc1nnc(CNc2c(F)c(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)s1 |r| Show InChI InChI=1S/C19H18FN7OS/c1-11-25-26-16(29-11)9-22-18-17(20)19(24-15-5-7-23-27(15)18)28-10-12-8-13(12)14-4-2-3-6-21-14/h2-7,12-13,22H,8-10H2,1H3/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147138
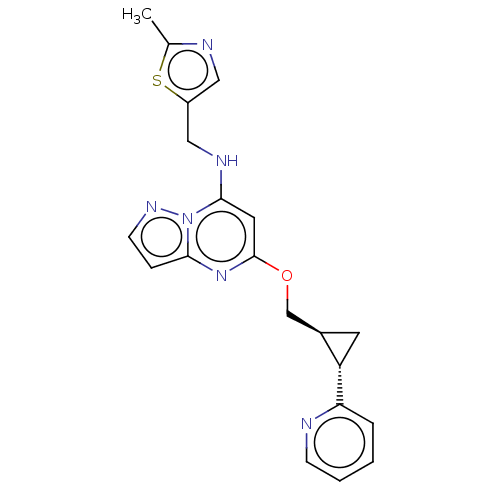
(US8957077, 1-4)Show SMILES Cc1ncc(CNc2cc(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)s1 |r| Show InChI InChI=1S/C20H20N6OS/c1-13-22-10-15(28-13)11-23-19-9-20(25-18-5-7-24-26(18)19)27-12-14-8-16(14)17-4-2-3-6-21-17/h2-7,9-10,14,16,23H,8,11-12H2,1H3/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120778
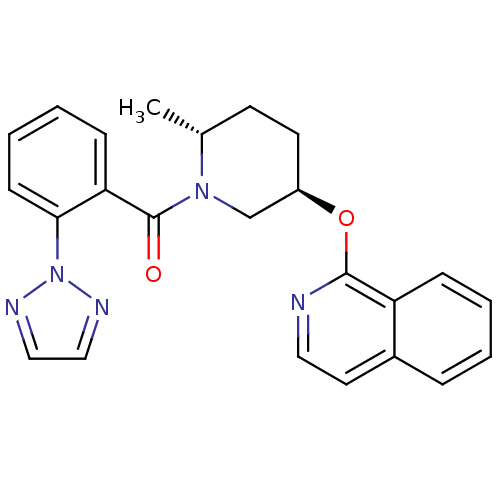
(US8710076, F-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1nccc2ccccc12 |r| Show InChI InChI=1S/C24H23N5O2/c1-17-10-11-19(31-23-20-7-3-2-6-18(20)12-13-25-23)16-28(17)24(30)21-8-4-5-9-22(21)29-26-14-15-27-29/h2-9,12-15,17,19H,10-11,16H2,1H3/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... |
US Patent US8710076 (2014)
BindingDB Entry DOI: 10.7270/Q2QN65DD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147142
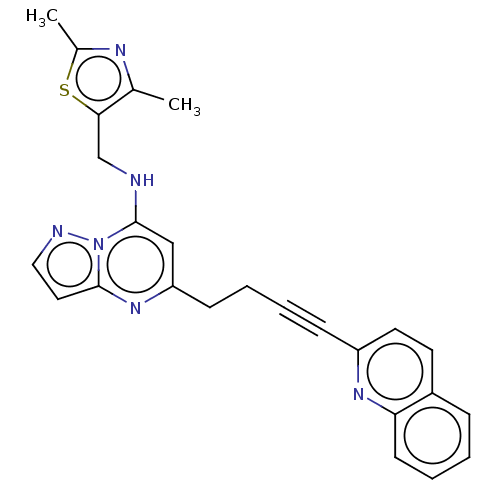
(US8957077, 1-15)Show SMILES Cc1nc(C)c(CNc2cc(CCC#Cc3ccc4ccccc4n3)nc3ccnn23)s1 Show InChI InChI=1S/C25H22N6S/c1-17-23(32-18(2)28-17)16-26-25-15-21(30-24-13-14-27-31(24)25)9-5-4-8-20-12-11-19-7-3-6-10-22(19)29-20/h3,6-7,10-15,26H,5,9,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318697
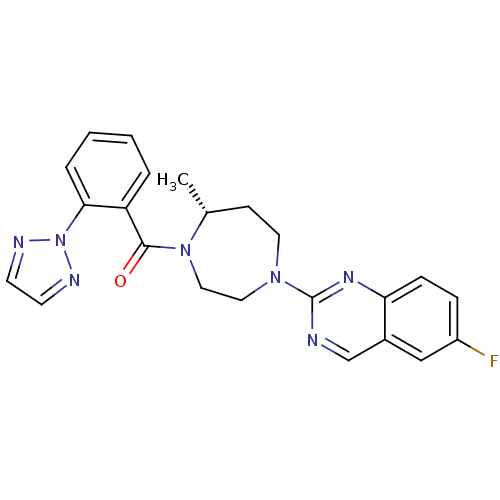
(6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C23H22FN7O/c1-16-8-11-29(23-25-15-17-14-18(24)6-7-20(17)28-23)12-13-30(16)22(32)19-4-2-3-5-21(19)31-26-9-10-27-31/h2-7,9-10,14-16H,8,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147146
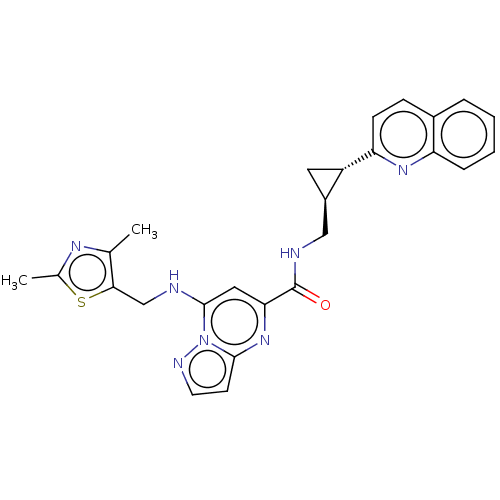
(US8957077, 1-23)Show SMILES Cc1nc(C)c(CNc2cc(nc3ccnn23)C(=O)NC[C@H]2C[C@@H]2c2ccc3ccccc3n2)s1 |r| Show InChI InChI=1S/C26H25N7OS/c1-15-23(35-16(2)30-15)14-27-25-12-22(32-24-9-10-29-33(24)25)26(34)28-13-18-11-19(18)21-8-7-17-5-3-4-6-20(17)31-21/h3-10,12,18-19,27H,11,13-14H2,1-2H3,(H,28,34)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50314676
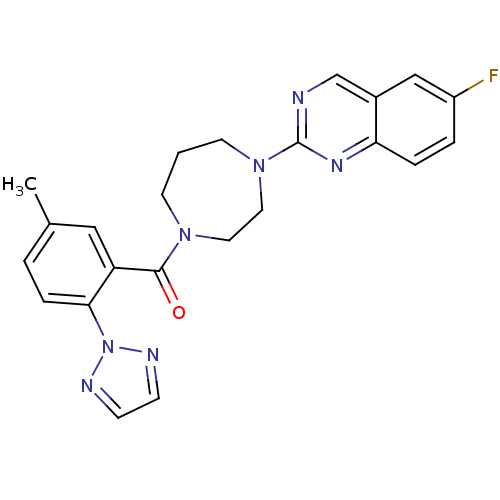
((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCN(CC1)c1ncc2cc(F)ccc2n1)-n1nccn1 Show InChI InChI=1S/C23H22FN7O/c1-16-3-6-21(31-26-7-8-27-31)19(13-16)22(32)29-9-2-10-30(12-11-29)23-25-15-17-14-18(24)4-5-20(17)28-23/h3-8,13-15H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2 receptor by radioligand displacement assay |
Bioorg Med Chem Lett 20: 2311-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.138
BindingDB Entry DOI: 10.7270/Q2MK6D26 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147134
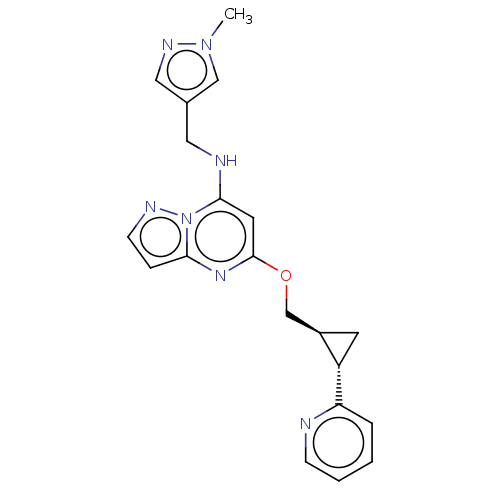
(US8957077, J-5)Show SMILES Cn1cc(CNc2cc(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)cn1 |r| Show InChI InChI=1S/C20H21N7O/c1-26-12-14(11-24-26)10-22-19-9-20(25-18-5-7-23-27(18)19)28-13-15-8-16(15)17-4-2-3-6-21-17/h2-7,9,11-12,15-16,22H,8,10,13H2,1H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104689
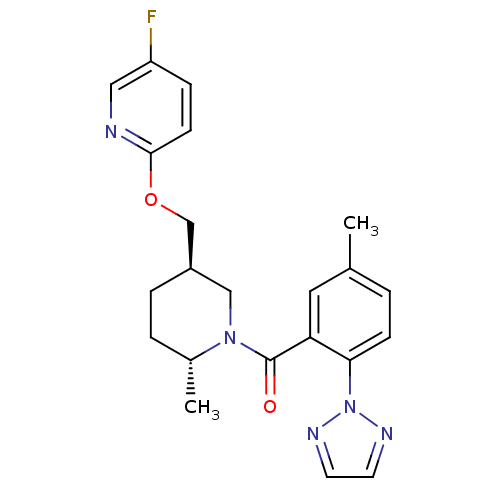
(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147135
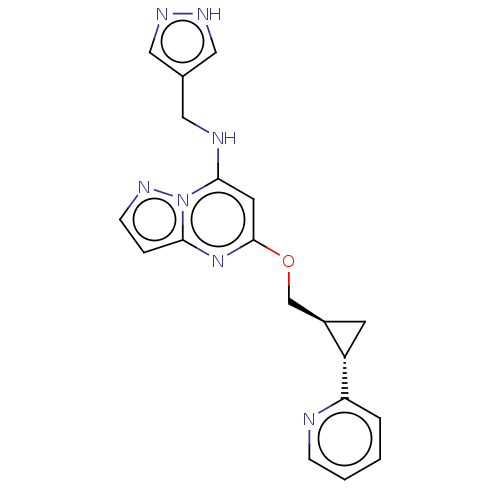
(US8957077, L-4)Show SMILES C(Nc1cc(OC[C@H]2C[C@@H]2c2ccccn2)nc2ccnn12)c1cn[nH]c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104697
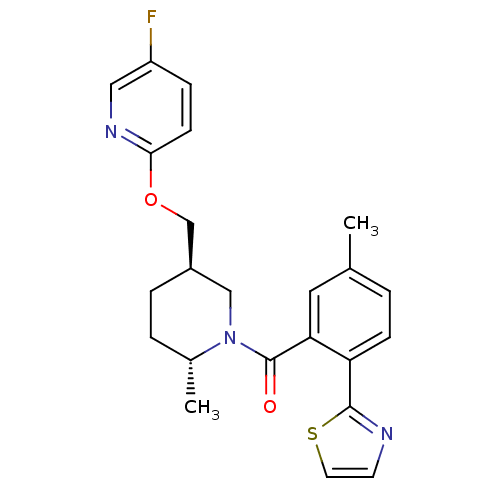
(US8569311, 1-1)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1nccs1 |r| Show InChI InChI=1S/C23H24FN3O2S/c1-15-3-7-19(22-25-9-10-30-22)20(11-15)23(28)27-13-17(5-4-16(27)2)14-29-21-8-6-18(24)12-26-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318698

(6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1cnc2cc(F)c(F)cc2n1 |r| Show InChI InChI=1S/C23H21F2N7O/c1-15-6-9-30(22-14-26-19-12-17(24)18(25)13-20(19)29-22)10-11-31(15)23(33)16-4-2-3-5-21(16)32-27-7-8-28-32/h2-5,7-8,12-15H,6,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318699
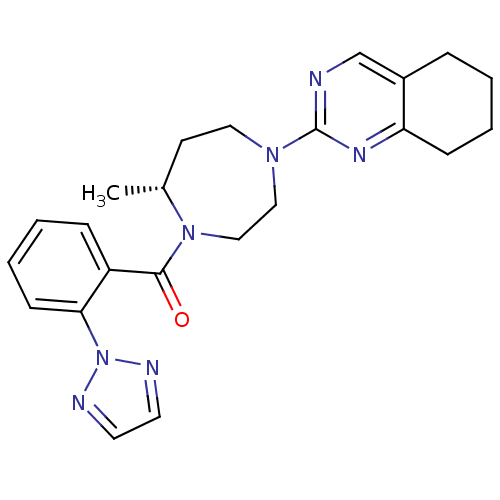
(2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2CCCCc2n1 |r| Show InChI InChI=1S/C23H27N7O/c1-17-10-13-28(23-24-16-18-6-2-4-8-20(18)27-23)14-15-29(17)22(31)19-7-3-5-9-21(19)30-25-11-12-26-30/h3,5,7,9,11-12,16-17H,2,4,6,8,10,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028040
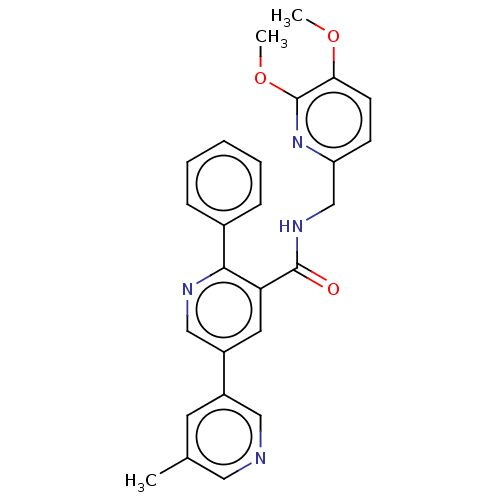
(CHEMBL3338846)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2ccccc2)-c2cncc(C)c2)nc1OC Show InChI InChI=1S/C26H24N4O3/c1-17-11-19(14-27-13-17)20-12-22(24(28-15-20)18-7-5-4-6-8-18)25(31)29-16-21-9-10-23(32-2)26(30-21)33-3/h4-15H,16H2,1-3H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 24: 4884-90 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.041
BindingDB Entry DOI: 10.7270/Q2GX4D4J |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50028045
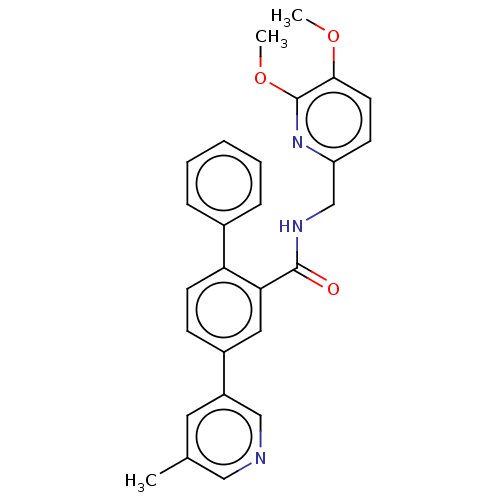
(CHEMBL3338852)Show SMILES COc1ccc(CNC(=O)c2cc(ccc2-c2ccccc2)-c2cncc(C)c2)nc1OC Show InChI InChI=1S/C27H25N3O3/c1-18-13-21(16-28-15-18)20-9-11-23(19-7-5-4-6-8-19)24(14-20)26(31)29-17-22-10-12-25(32-2)27(30-22)33-3/h4-16H,17H2,1-3H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 24: 4884-90 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.041
BindingDB Entry DOI: 10.7270/Q2GX4D4J |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104692
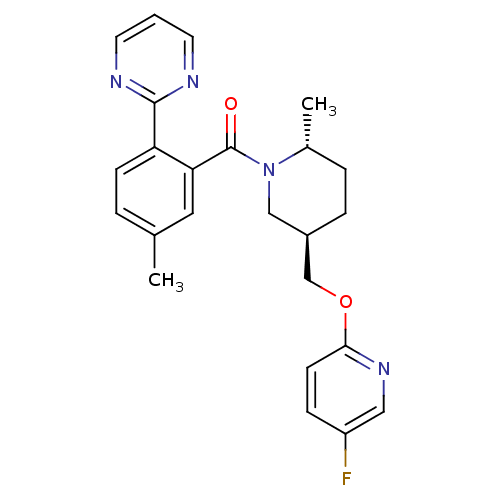
(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147134

(US8957077, J-5)Show SMILES Cn1cc(CNc2cc(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)cn1 |r| Show InChI InChI=1S/C20H21N7O/c1-26-12-14(11-24-26)10-22-19-9-20(25-18-5-7-23-27(18)19)28-13-15-8-16(15)17-4-2-3-6-21-17/h2-7,9,11-12,15-16,22H,8,10,13H2,1H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104701
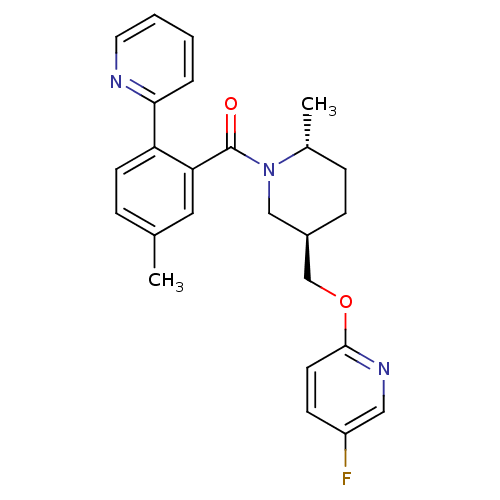
(US8569311, 1-15)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ccccn1 |r| Show InChI InChI=1S/C25H26FN3O2/c1-17-6-10-21(23-5-3-4-12-27-23)22(13-17)25(30)29-15-19(8-7-18(29)2)16-31-24-11-9-20(26)14-28-24/h3-6,9-14,18-19H,7-8,15-16H2,1-2H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318695
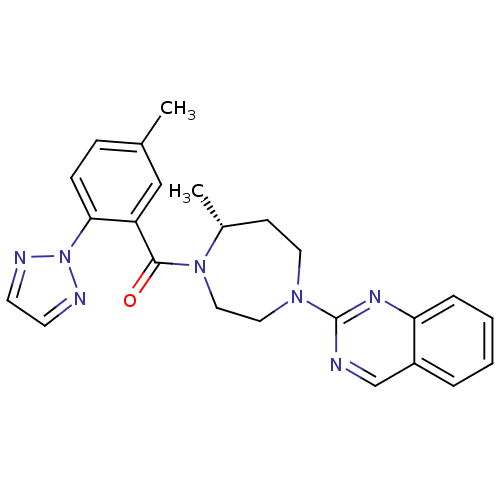
(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147140
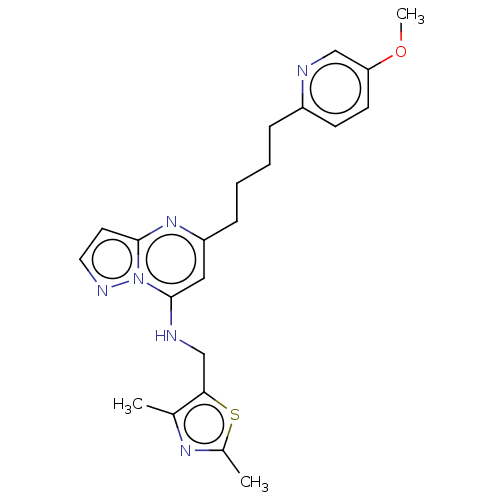
(US8957077, 1-10)Show SMILES COc1ccc(CCCCc2cc(NCc3sc(C)nc3C)n3nccc3n2)nc1 Show InChI InChI=1S/C22H26N6OS/c1-15-20(30-16(2)26-15)14-24-22-12-18(27-21-10-11-25-28(21)22)7-5-4-6-17-8-9-19(29-3)13-23-17/h8-13,24H,4-7,14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
In a typical experiment the PDE10 inhibitory activity of the compounds of the present invention was determined in accordance with the following exper... |
US Patent US8957077 (2015)
BindingDB Entry DOI: 10.7270/Q29G5KHW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104698

(US8569311, 1-4)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1ccccc1-n1nccn1 |r| Show InChI InChI=1S/C21H22FN5O2/c1-15-6-7-16(14-29-20-9-8-17(22)12-23-20)13-26(15)21(28)18-4-2-3-5-19(18)27-24-10-11-25-27/h2-5,8-12,15-16H,6-7,13-14H2,1H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318695

(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318695

(2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-triazol-2...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C24H25N7O/c1-17-7-8-22(31-26-10-11-27-31)20(15-17)23(32)30-14-13-29(12-9-18(30)2)24-25-16-19-5-3-4-6-21(19)28-24/h3-8,10-11,15-16,18H,9,12-14H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50314681
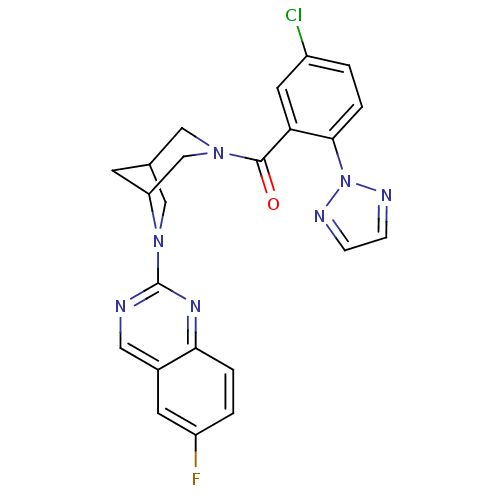
((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(6-fl...)Show SMILES Fc1ccc2nc(ncc2c1)N1CC2CC1CN(C2)C(=O)c1cc(Cl)ccc1-n1nccn1 Show InChI InChI=1S/C23H19ClFN7O/c24-16-1-4-21(32-27-5-6-28-32)19(9-16)22(33)30-11-14-7-18(13-30)31(12-14)23-26-10-15-8-17(25)2-3-20(15)29-23/h1-6,8-10,14,18H,7,11-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1 receptor by radioligand displacement assay |
Bioorg Med Chem Lett 20: 2311-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.138
BindingDB Entry DOI: 10.7270/Q2MK6D26 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500537
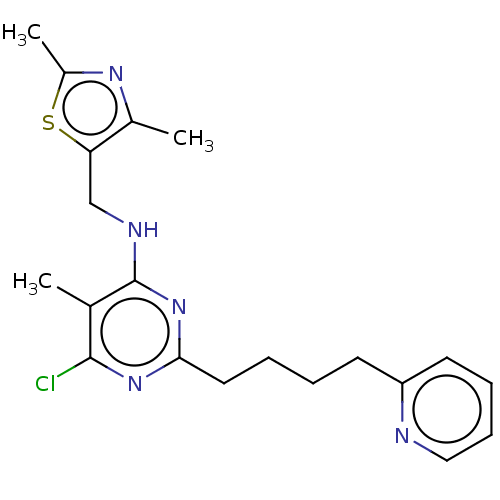
(CHEMBL3746162)Show InChI InChI=1S/C20H24ClN5S/c1-13-19(21)25-18(10-5-4-8-16-9-6-7-11-22-16)26-20(13)23-12-17-14(2)24-15(3)27-17/h6-7,9,11H,4-5,8,10,12H2,1-3H3,(H,23,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50321509
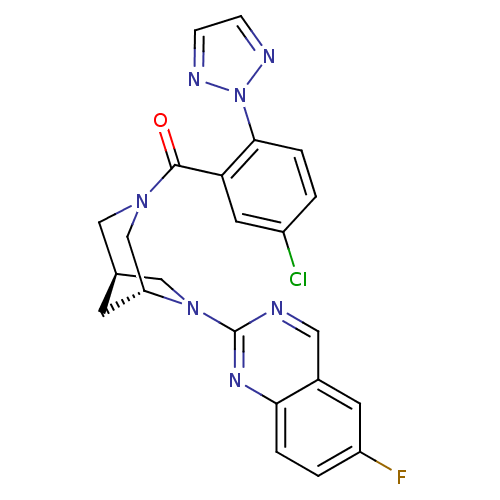
((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)((1R,5R)...)Show SMILES Fc1ccc2nc(ncc2c1)N1C[C@H]2C[C@@H]1CN(C2)C(=O)c1cc(Cl)ccc1-n1nccn1 |r| Show InChI InChI=1S/C23H19ClFN7O/c24-16-1-4-21(32-27-5-6-28-32)19(9-16)22(33)30-11-14-7-18(13-30)31(12-14)23-26-10-15-8-17(25)2-3-20(15)29-23/h1-6,8-10,14,18H,7,11-13H2/t14-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50321510
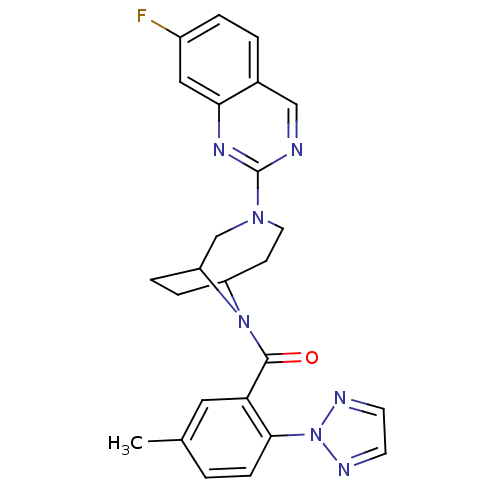
((3-(7-fluoroquinazolin-2-yl)-3,9-di azabicyclo[4.2...)Show SMILES Cc1ccc(c(c1)C(=O)N1C2CCC1CN(CC2)c1ncc2ccc(F)cc2n1)-n1nccn1 Show InChI InChI=1S/C25H24FN7O/c1-16-2-7-23(33-28-9-10-29-33)21(12-16)24(34)32-19-5-6-20(32)15-31(11-8-19)25-27-14-17-3-4-18(26)13-22(17)30-25/h2-4,7,9-10,12-14,19-20H,5-6,8,11,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R |
Bioorg Med Chem Lett 20: 4201-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.047
BindingDB Entry DOI: 10.7270/Q25Q4X3F |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM98538
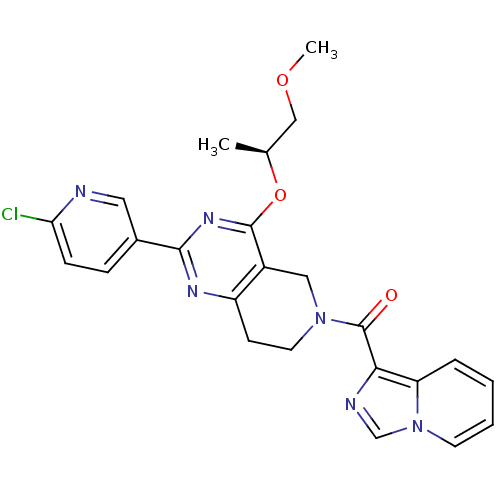
(US8492392, T-1)Show SMILES COC[C@H](C)Oc1nc(nc2CCN(Cc12)C(=O)c1ncn2ccccc12)-c1ccc(Cl)nc1 |r| Show InChI InChI=1S/C24H23ClN6O3/c1-15(13-33-2)34-23-17-12-30(24(32)21-19-5-3-4-9-31(19)14-27-21)10-8-18(17)28-22(29-23)16-6-7-20(25)26-11-16/h3-7,9,11,14-15H,8,10,12-13H2,1-2H3/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.430 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Fluorescence polarization assay: The compounds of the following examples had activity in reference assays by exhibiting the ability to inhibit the hy... |
US Patent US8492392 (2013)
BindingDB Entry DOI: 10.7270/Q2SN07M8 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126830

(US8785467, 1-39)Show SMILES Cc1c(Cl)nc(OC[C@H]2C[C@@H]2c2ccccn2)nc1NCc1cnn(C)c1 |r| Show InChI InChI=1S/C19H21ClN6O/c1-12-17(20)24-19(25-18(12)22-8-13-9-23-26(2)10-13)27-11-14-7-15(14)16-5-3-4-6-21-16/h3-6,9-10,14-15H,7-8,11H2,1-2H3,(H,22,24,25)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318696

(2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2ccccc2n1 |r| Show InChI InChI=1S/C23H23N7O/c1-17-10-13-28(23-24-16-18-6-2-4-8-20(18)27-23)14-15-29(17)22(31)19-7-3-5-9-21(19)30-25-11-12-26-30/h2-9,11-12,16-17H,10,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104703
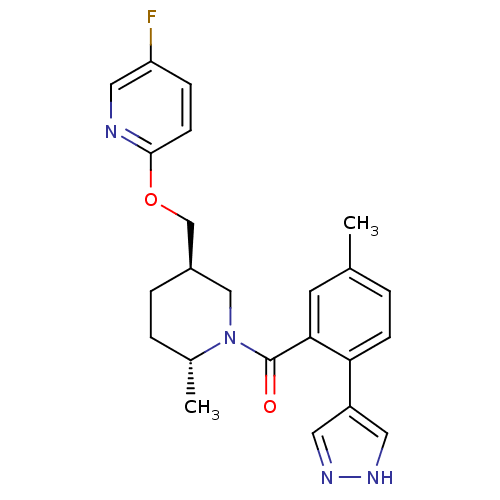
(US8569311, 1-21)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1cn[nH]c1 |r| Show InChI InChI=1S/C23H25FN4O2/c1-15-3-7-20(18-10-26-27-11-18)21(9-15)23(29)28-13-17(5-4-16(28)2)14-30-22-8-6-19(24)12-25-22/h3,6-12,16-17H,4-5,13-14H2,1-2H3,(H,26,27)/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104699

(US8569311, 1-8)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(F)ccc1-n1nccn1 |r| Show InChI InChI=1S/C21H21F2N5O2/c1-14-2-3-15(13-30-20-7-5-17(23)11-24-20)12-27(14)21(29)18-10-16(22)4-6-19(18)28-25-8-9-26-28/h4-11,14-15H,2-3,12-13H2,1H3/t14-,15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM106968

(US8592457, 1-5)Show SMILES COc1ccc(CNC(=O)c2cc(ncc2-c2ccccc2)-c2cncc(C)c2)nc1OC Show InChI InChI=1S/C26H24N4O3/c1-17-11-19(14-27-13-17)23-12-21(22(16-28-23)18-7-5-4-6-8-18)25(31)29-15-20-9-10-24(32-2)26(30-20)33-3/h4-14,16H,15H2,1-3H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of (S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-([3H]-1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 24: 4884-90 (2014)
Article DOI: 10.1016/j.bmcl.2014.08.041
BindingDB Entry DOI: 10.7270/Q2GX4D4J |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104702

(US8569311, 1-19)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(F)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C23H22F2N4O2/c1-15-3-4-16(14-31-21-8-6-18(25)12-28-21)13-29(15)23(30)20-11-17(24)5-7-19(20)22-26-9-2-10-27-22/h2,5-12,15-16H,3-4,13-14H2,1H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104690
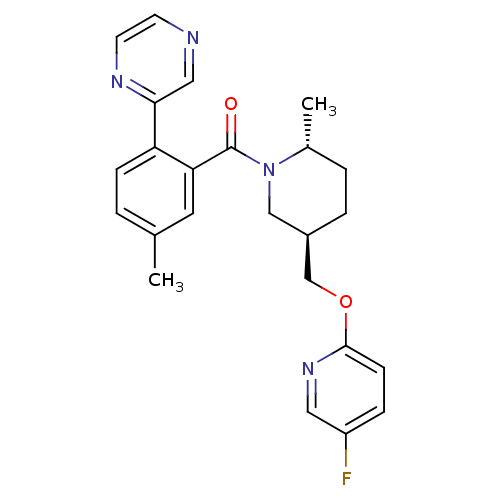
(US8569311, B-3)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1cnccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-3-7-20(22-13-26-9-10-27-22)21(11-16)24(30)29-14-18(5-4-17(29)2)15-31-23-8-6-19(25)12-28-23/h3,6-13,17-18H,4-5,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(biphenyl-2-yl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX1R expressed in C... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50318701

(CHEMBL1083659 | MK-4305 | [(7R)-4-(5-Chloro-1,3-be...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1nc2cc(Cl)ccc2o1 |r| Show InChI InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-cyclobutyl-5-methyl-N-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)ethyl)-2-(2H-1,2,3-triazol-2-yl)benzamide from human OX1R expre... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120777
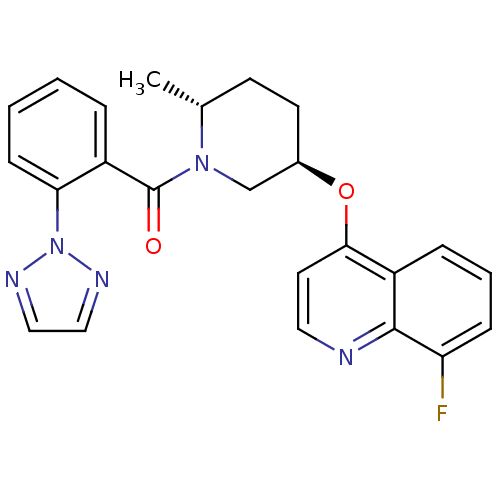
(US8710076, E-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1ccnc2c(F)cccc12 |r| Show InChI InChI=1S/C24H22FN5O2/c1-16-9-10-17(32-22-11-12-26-23-19(22)6-4-7-20(23)25)15-29(16)24(31)18-5-2-3-8-21(18)30-27-13-14-28-30/h2-8,11-14,16-17H,9-10,15H2,1H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... |
US Patent US8710076 (2014)
BindingDB Entry DOI: 10.7270/Q2QN65DD |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120779
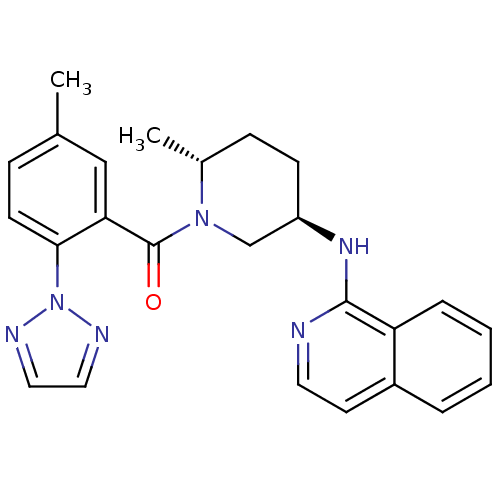
(US8710076, H-4)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1cc(C)ccc1-n1nccn1)Nc1nccc2ccccc12 |r| Show InChI InChI=1S/C25H26N6O/c1-17-7-10-23(31-27-13-14-28-31)22(15-17)25(32)30-16-20(9-8-18(30)2)29-24-21-6-4-3-5-19(21)11-12-26-24/h3-7,10-15,18,20H,8-9,16H2,1-2H3,(H,26,29)/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... |
US Patent US8710076 (2014)
BindingDB Entry DOI: 10.7270/Q2QN65DD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data