

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Translocator protein (Rattus norvegicus (rat)) | BDBM50052309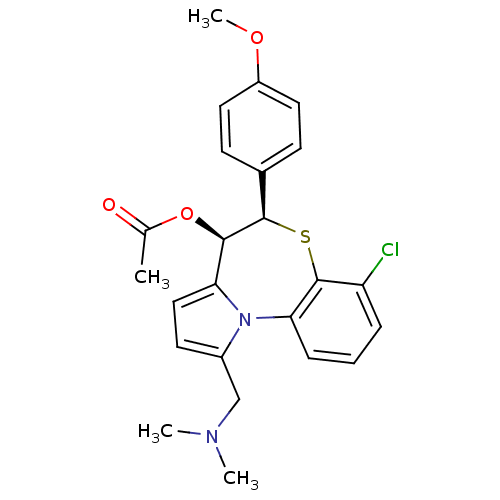 (Acetic acid (4R,5R)-7-chloro-1-dimethylaminomethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50052310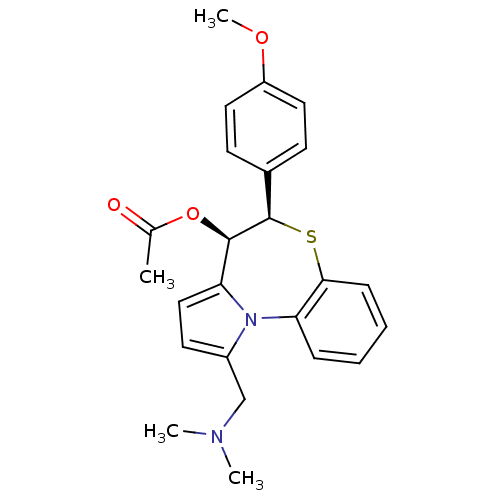 (Acetic acid (4R,5R)-1-dimethylaminomethyl-5-(4-met...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50052314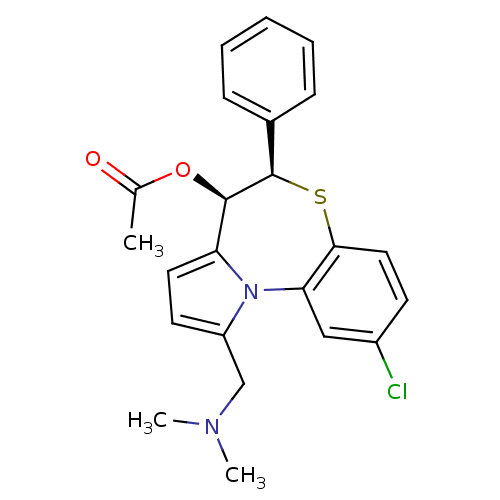 (Acetic acid (4R,5R)-9-chloro-1-dimethylaminomethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 569 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Inhibition of [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM81939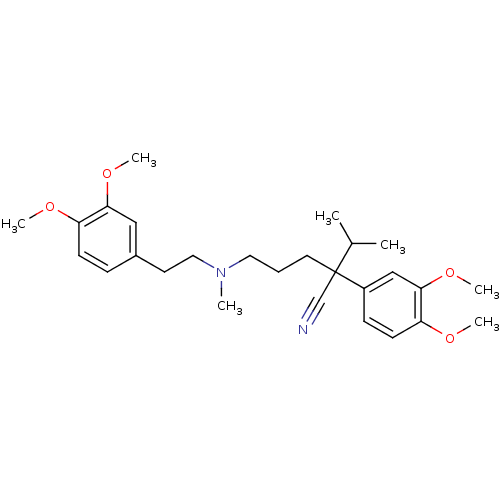 (CAS_52-53-9 | NSC_62969 | VERAPAMIL) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032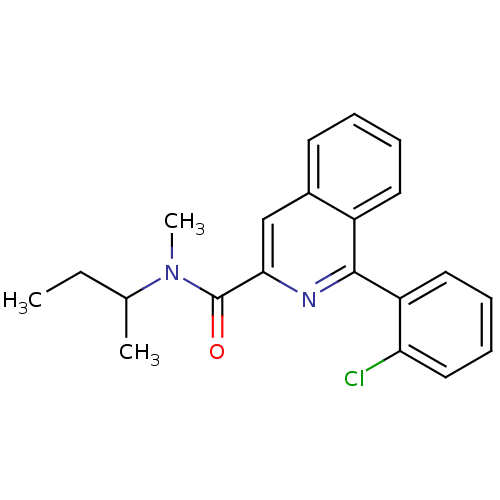 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041505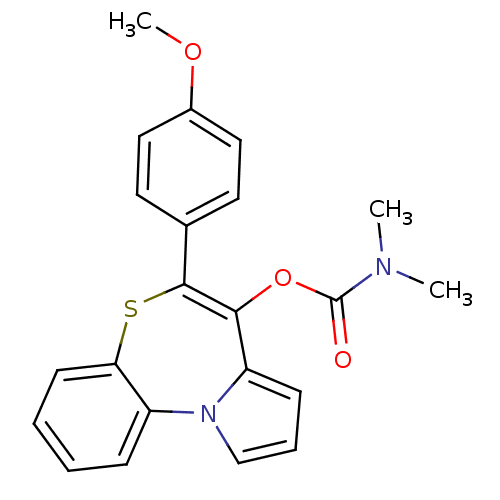 (CHEMBL33096 | Dimethyl-carbamic acid 5-(4-methoxy-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50052311 (Acetic acid (4R,5R)-7-chloro-1-dimethylaminomethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221211 (CHEMBL97077) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430948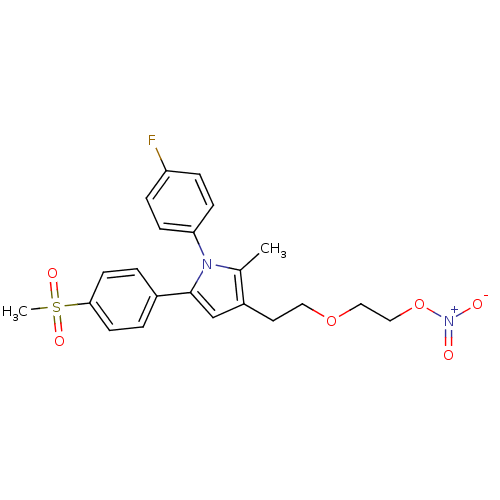 (CHEMBL2337402) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430957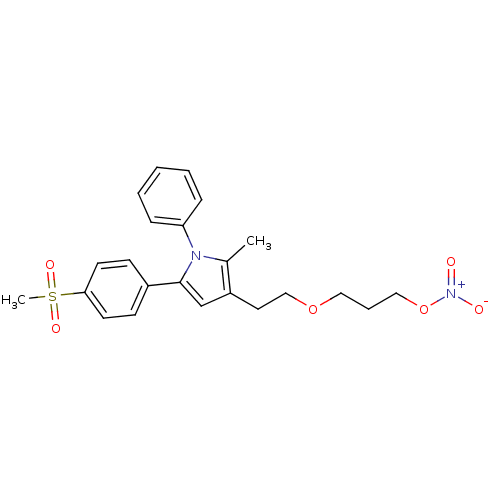 (CHEMBL2337404) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50029417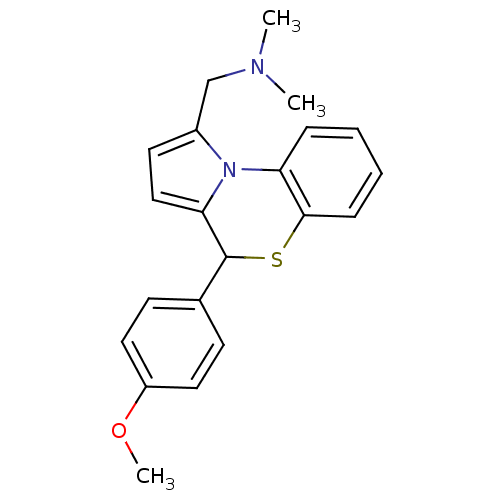 (CHEMBL277363 | [4-(4-Methoxy-phenyl)-4H-benzo[b]py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430960 (CHEMBL2337400) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50135598 ((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 17.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against translocase-I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50135599 ((4S,5S,6S)-6-{(R)-Carbamoyl-[(2S,3S,4R,5R)-5-(2,4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 17.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against translocase-I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041506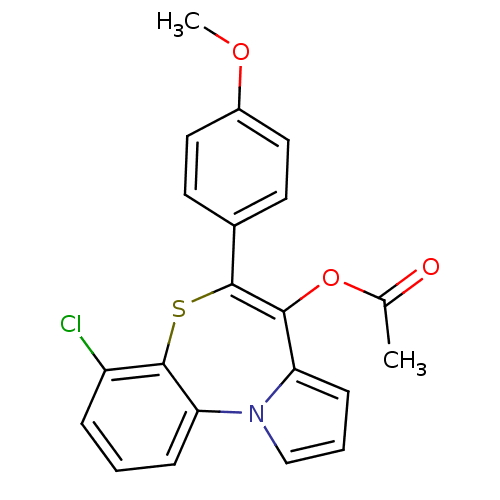 (Acetic acid 7-chloro-5-(4-methoxy-phenyl)-6-thia-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041493 (Acetic acid 5-phenyl-6-thia-10b-aza-benzo[e]azulen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041504 (Butyric acid 5-phenyl-6-thia-10b-aza-benzo[e]azule...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430956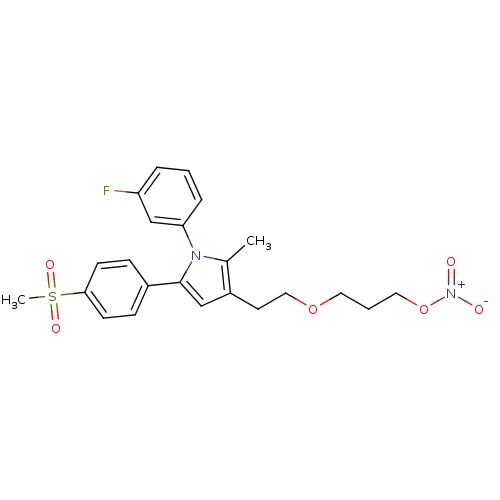 (CHEMBL2337405) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041513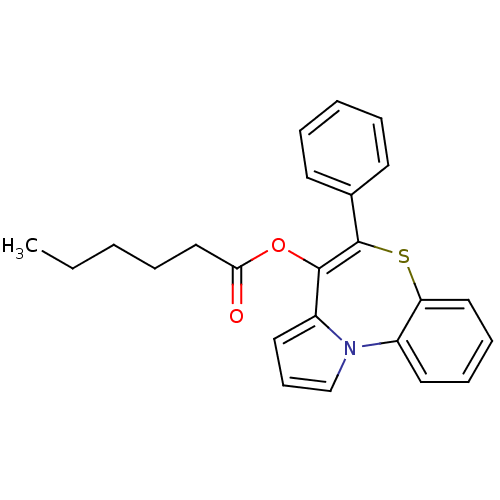 (CHEMBL416214 | Hexanoic acid 5-phenyl-6-thia-10b-a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430959 (CHEMBL2337401) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430954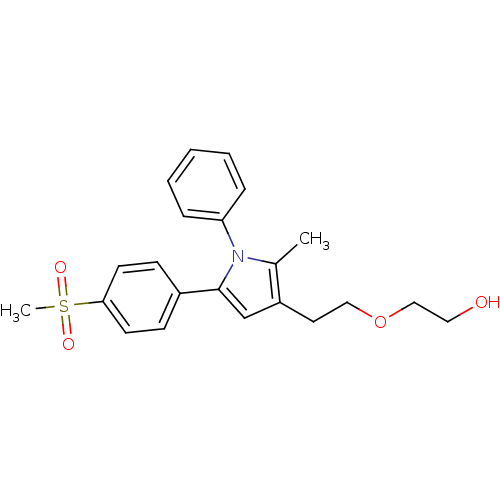 (CHEMBL2337407 | US9162979, 36-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041496 (Butyric acid 5-(4-methoxy-phenyl)-6-thia-10b-aza-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041502 (Acetic acid 5-(4-methoxy-phenyl)-6-thia-10b-aza-be...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221487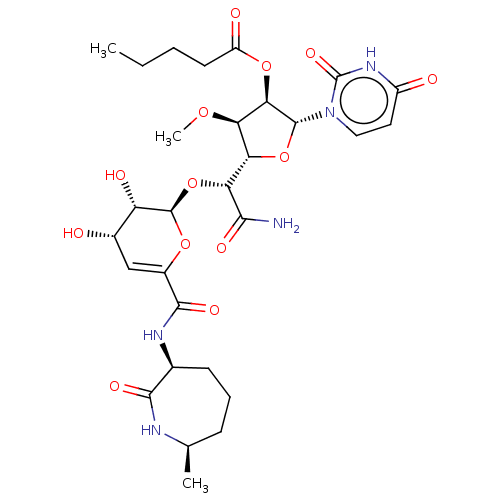 (CHEMBL94502) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM81939 (CAS_52-53-9 | NSC_62969 | VERAPAMIL) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430953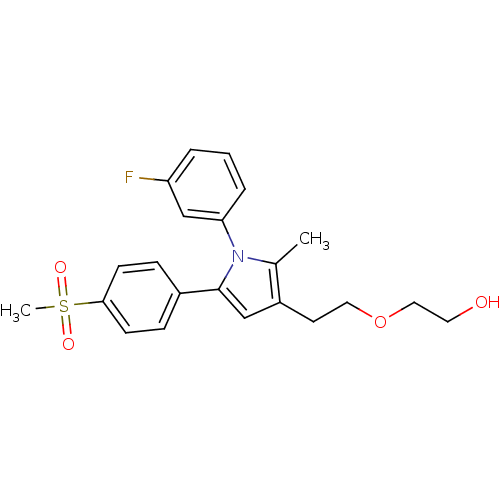 (CHEMBL2337408 | US9162979, 38-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041508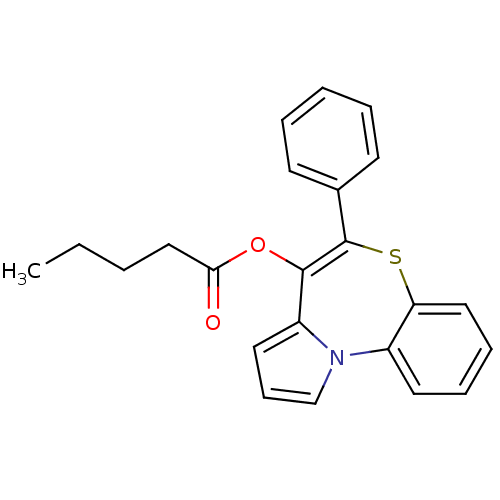 (CHEMBL416040 | Pentanoic acid 5-phenyl-6-thia-10b-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50004704 ((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221482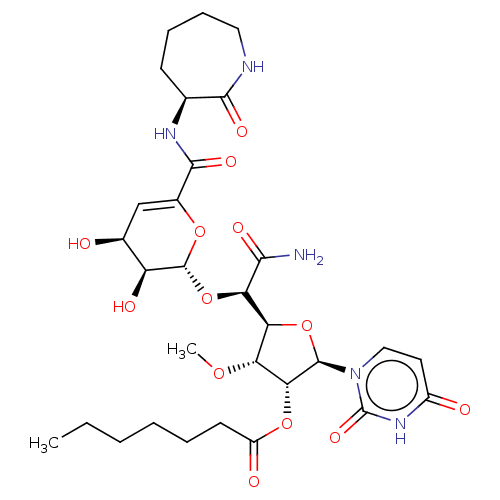 (CHEMBL94673) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 58.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against translocase-I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221499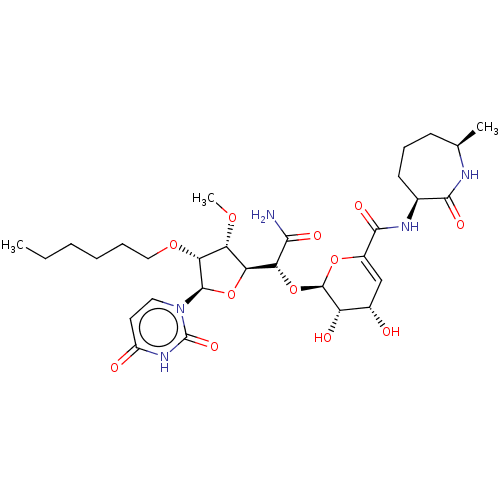 (CHEMBL318555) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 59.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221512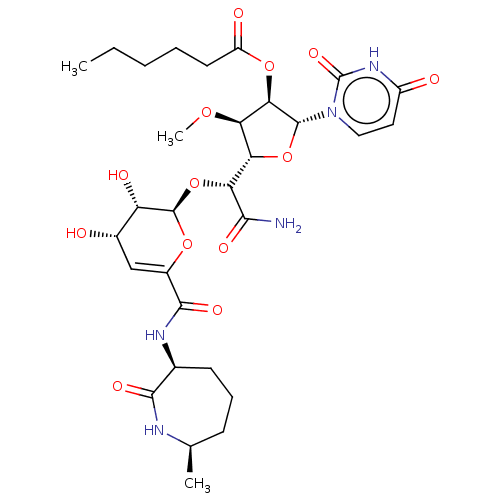 (CHEMBL318366) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221493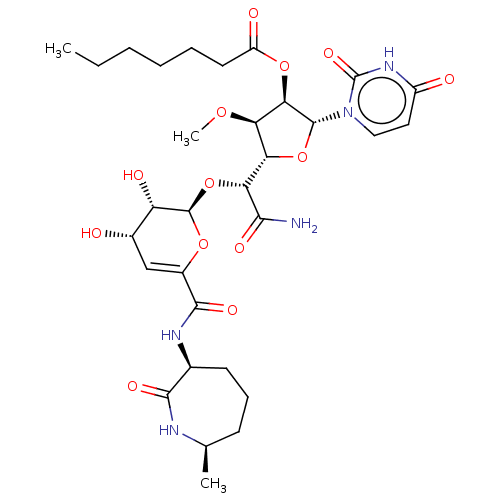 (CHEMBL95078) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 64.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221486 (CHEMBL94445) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 76.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221504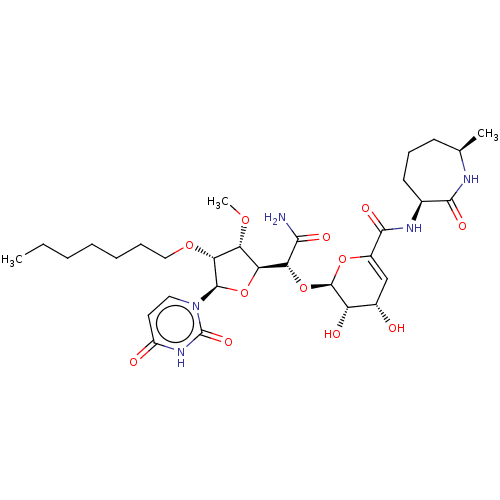 (CHEMBL318623) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430947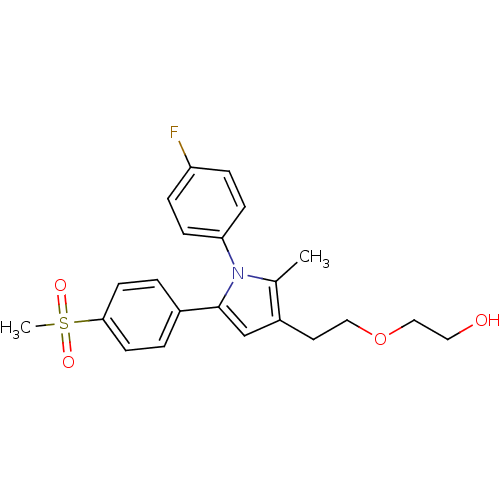 (CHEMBL2337409 | US9162979, 37-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50052317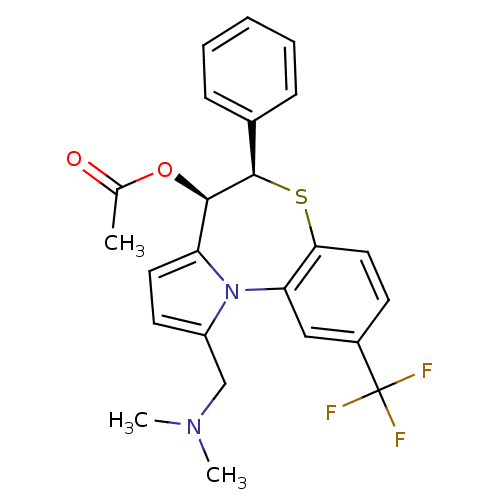 (Acetic acid (4R,5R)-1-dimethylaminomethyl-5-phenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50041496 (Butyric acid 5-(4-methoxy-phenyl)-6-thia-10b-aza-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50041485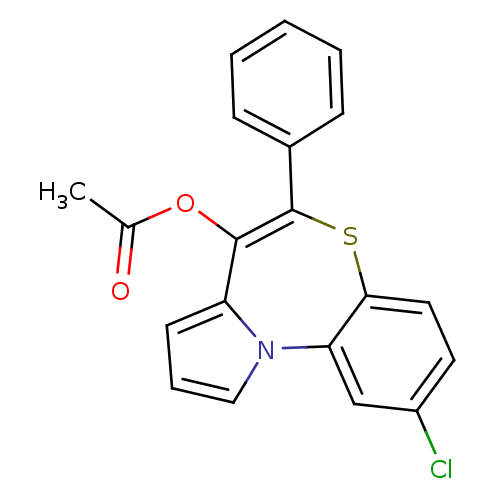 (Acetic acid 9-chloro-5-phenyl-6-thia-10b-aza-benzo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]-PK 11195 binding to peripheral-type benzodiazepine receptor(PBR) in rat cerebral cortex homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221506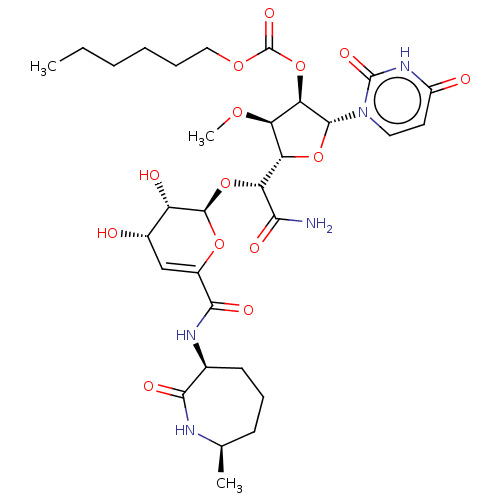 (CHEMBL428285) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430955 (CHEMBL2337406) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221484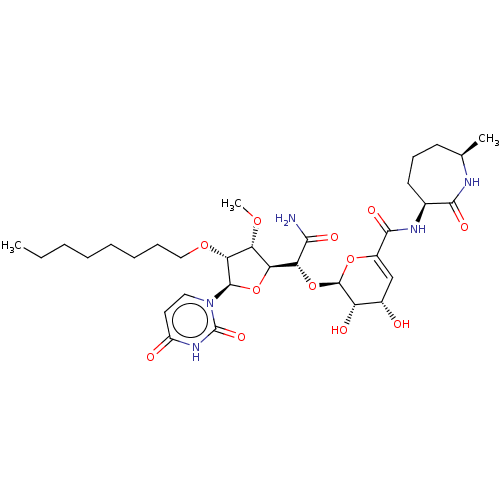 (CHEMBL98456) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50041493 (Acetic acid 5-phenyl-6-thia-10b-aza-benzo[e]azulen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221497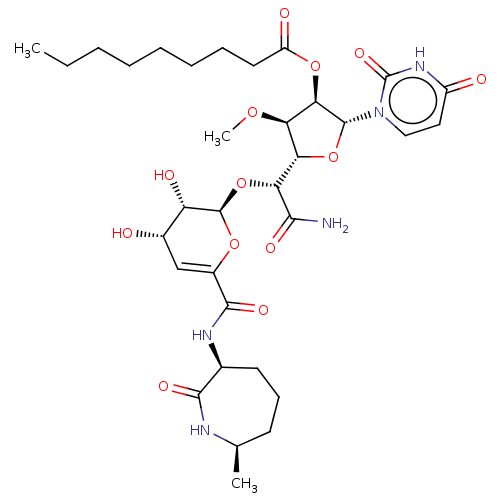 (CHEMBL314508) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50430950 (CHEMBL2337398 | US9162979, 42-II) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Escherichia coli lipopolysaccharide-induced COX2 activity in mouse J774 cells assessed as decrease in PGE2 levels after 24 hrs by RIA | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221483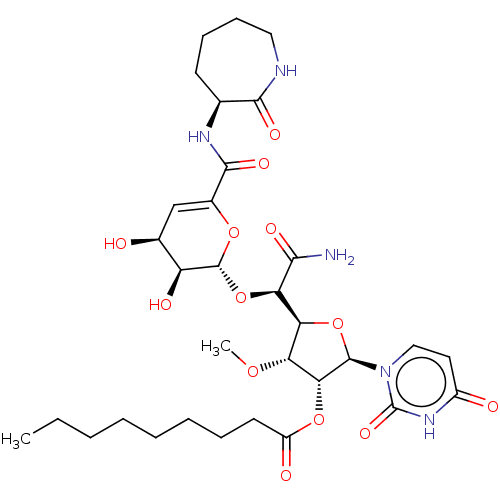 (CHEMBL95124) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 253 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration against translocase-I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221496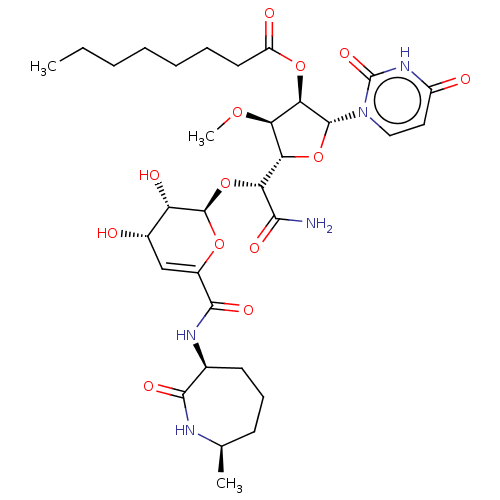 (CHEMBL94295) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221503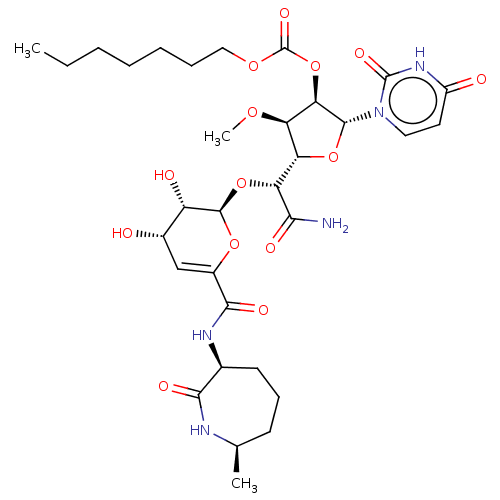 (CHEMBL329218) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50041502 (Acetic acid 5-(4-methoxy-phenyl)-6-thia-10b-aza-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Siena Curated by ChEMBL | Assay Description Ability to inhibit [3H]nitrendipine binding to the L-type calcium channel receptor(CCR) in rat heart homogenate. | J Med Chem 39: 2922-38 (1996) Article DOI: 10.1021/jm960162z BindingDB Entry DOI: 10.7270/Q2HH6KQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospho-N-acetylmuramoyl-pentapeptide-transferase (Escherichia coli (strain K12)) | BDBM50221492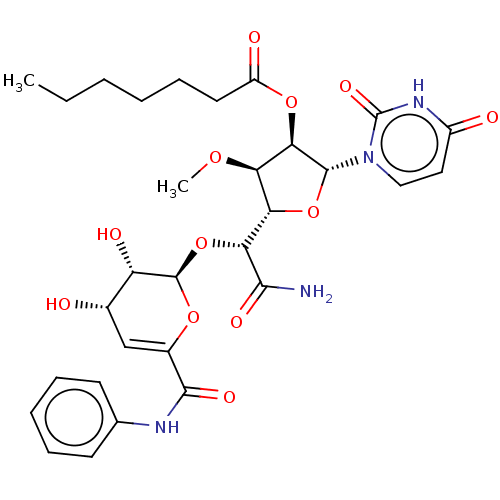 (CHEMBL320600) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 371 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration required against Translocase I | Bioorg Med Chem Lett 13: 2833-6 (2003) BindingDB Entry DOI: 10.7270/Q2VX0JP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50430947 (CHEMBL2337409 | US9162979, 37-II) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of COX2 in human whole blood assessed as reduction in LPS-induced PGE2 generation pre-treated prior to LPS-challenge | J Med Chem 56: 3191-206 (2013) Article DOI: 10.1021/jm301370e BindingDB Entry DOI: 10.7270/Q2JS9RSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |